Open Access Article
Open Access ArticleNovel encapsulation of water soluble inorganic or organic ingredients in melamine formaldehyde microcapsules to achieve their sustained release in an aqueous environment†
Cong Sui ab,
Jon A. Preece
ab,
Jon A. Preece c,
Shu-Hong Yu
c,
Shu-Hong Yu b and
Zhibing Zhang
b and
Zhibing Zhang *a
*a
aSchool of Chemical Engineering, University of Birmingham, UK. E-mail: Z.Zhang@bham.ac.uk
bDivision of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, Collaborative Innovation of Suzhou Nano Science and Technology, Department of Chemistry, CAS Centre for Excellence in Nanoscience, Hefei Science Centre of CAS, University of Science and Technology of China, Hefei 230026, China
cSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
First published on 20th August 2018
Abstract
A novel type of melamine formaldehyde microcapsule with a desirable barrier has been used to encapsulate water soluble ingredients, including potassium chloride (KCl) and allura red (dye) as models of an inorganic salt and organic molecule, respectively, via a facile method, and it has shown a sustained release of KCl and allura red for 12 h and 10 days in aqueous environment, respectively.
Microencapsulation is an important technology to stabilise active ingredients and/or control their release for a range of industrial sectors including healthcare, household care, cosmetics and agrochemicals.1–3 Various techniques including interfacial polymerisation, in situ polymerisation, solvent evaporation and coacervation, have been developed to encapsulate different types of active ingredients, such as oil-soluble, water-soluble, amphiphilic, powders or cells.4–8 Encapsulation of water-soluble active ingredients is usually achieved by forming a water in oil (W/O) emulsion, initially stabilised by the self-assembly of molecular and/or polymolecular surfactants at the liquid/liquid interface, followed by further consolidation through chemical cross-linking of the surfactants at the interface, leading to robust isolatable capsules.2,9 The actives that have been encapsulated range from biomacromolecules, such as proteins,3 polysaccharides,10 and enzymes11,12 to small molecules, such as doxorubicin,10,13 peroxide,14 polyphenols,15 and inorganic salts.16,17
Melamine formaldehyde (MF) is a versatile chemical cross-linking agent and has been broadly used in a number of encapsulation applications, due to its polycondensates providing a tight seal, thermal and mechanical stability and acid/alkaline resistance.18–20 MF polycondensates have been used as shell material to encapsulate oils in applications in the area of carbonless copy paper,21 fragrant oil22,23 and herbicide/insecticide delivery.24 However, it has not been possible to form microcapsules, which can encapsulate water soluble ingredients and achieve a sustained release. Herein, we have developed a novel way to synthesise MF microcapsules via an in situ polymerisation process (Scheme 1) with a water soluble inorganic salt (KCl) and an organic dye (allura red), which displayed prolonged release times of 12 h and 10 days, respectively.
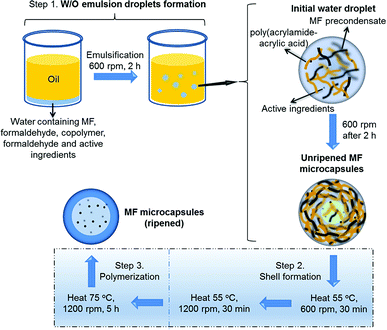 | ||
| Scheme 1 Illustration of MF microcapsules synthesised via an in situ polymerisation process with water soluble ingredients (KCl salt/allura red molecules) encapsulated. | ||
An aqueous phase containing the MF precondensate solution, formaldehyde, poly(acrylamide-acrylic acid) copolymer and the active (KCl or allura red) with the pH adjusted to 4.3, was emulsified within an oil (sunflower oil) phase at a stirring speed of 600 rpm (Scheme 1, Step 1) to form a Pickering emulsion (unripened (low-level cross-linking) microcapsules), with the MF and copolymer acting as the stabilising surfactants at the liquid/liquid interface.25,26 The unripened MF microcapsules were then heated at 55 °C at 600 rpm for 30 min, which partially solidified the outer shell,27 and the stirring speed was then increased to 1200 rpm, which was maintained for a further 30 min, to prevent aggregation between the unripened microcapsules (Step 2). The fully ripened MF microcapsules were finally formed by heating at 75 °C at 1200 rpm for a further 5 h (Step 3). The MF-KCl microcapsules (ripened) formed with different volumes of formaldehyde solution (200 μl and 600 μl, 37% (aq.) w/w) were named as MF-KCl (200 F) and MF-KCl (600 F), respectively.
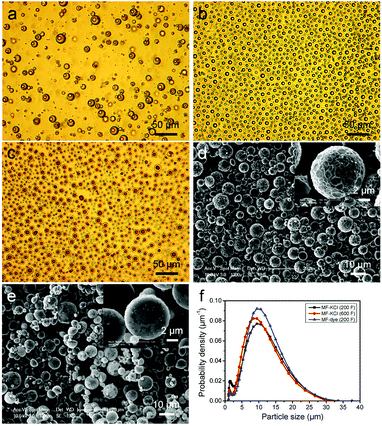
The W/O emulsion droplets were stabilised by the pre-cross-linked MF precondensate and copolymer at pH 4.3, which adsorbed at the interface of the emulsion as shown in the optical micrograph (Fig. 1a), forming the unripened MF microcapsules with a core–shell structure. After preheated at 55 °C for 30 min, the unripened MF microcapsules partly solidified and they were strong enough to survive when the stirring speed increased to 1200 rpm, to avoid aggregation between microcapsules (Fig. S1†). The unripened microcapsules maintained their shapes and sizes after the stirring speed was increased as shown in Fig. S1b.† The formed ripened MF-KCl and MF-dye microcapsules showed spherical shapes on the microscale, and the MF-dye revealed a red core under the optical microscope, indicating the encapsulated dye in the MF microcapsules (Fig. 1b and c). The MF-KCl (600 F) revealed a smoother surface than the MF-KCl (200 F) one, which resulted from the increasing amount of formaldehyde integrated into the MF polymerisation process (Fig. 1d and e). The size distributions exhibited in Fig. 1f show similar mean size [D (4, 3)] which were around 13 μm (Table 1). Clearly, the mean size varied with the viscosity of the prepared aqueous solution, and it slightly decreased with the decreasing viscosity of solution. The three batches of MF samples thus showed similar size distributions.
| Sample | Mean size [D (4, 3)] (μm) | SPAN | Viscosity (Pa s) |
|---|---|---|---|
| MF-KCl (200 F) | 13 (±1) | 1.26 (±0.01) | 0.0187 (±7 × 10−4) |
| MF-KCl (600 F) | 12 (±1) | 1.26 (±0.02) | 0.0169 (±7 × 10−4) |
| MF-dye (200 F) | 13 (±1) | 1.21 (±0.05) | 0.0188 (±8 × 10−4) |
The cross-sections of MF-KCl (200 F) and MF-KCl (600 F) microcapsules are shown in Fig. 2, indicating clear shell structures. The MF-KCl (200 F) microcapsules displayed a thicker shell (0.65 (±0.04) μm to 1.30 (±0.09) μm) with a uniformly distributed core than MF-KCl (600 F) microcapsules (0.59 (±0.03) μm to 1.09 (±0.05) μm) with an inhomogeneously distributed core. The high concentration of formaldehyde in MF-KCl (600 F) led to the excess amount of formaldehyde in the water phase and might increase the polymerisation rate between MF precondensate and free formaldehyde, forming the unevenly distributed self-assembled solid MF core. Meanwhile, it led to higher local concentrations of formaldehyde, forming a smoother and more compact surface (Fig. 1e). The shell thicknesses of MF-KCl (600 F) microcapsules were thus thinner than those of MF-KCl (200 F) microcapsules due to the less amount of MF assembled in the shell. The elemental compositions of shell and core of MF-KCl (200 F) microcapsules confirmed by the energy dispersive X-ray (EDX) analysis, demonstrate that both of the shell and core contained C, N, Na and Cl, revealing the existing of assembled MF precondensate and copolymer (Fig. S2†). Meanwhile, the detectable K element in the core indicates that the K+ ions were encapsulated in the MF microcapsules. The formed MF microcapsules were characterised by the Fourier-transform infrared (FT-IR) spectrometry. The absorption peaks of N–H stretching vibration at around 3340, 1559 and 813 cm−1, the C–N stretching at around 1358 cm−1, the C–H stretching at around 2938 and 1180 cm−1 and the methylol at roughly 1010 cm−1, are the characteristics of MF and copolymer.28–30 The slight shift of carboxyl group at 1671 cm−1 for MF-KCl, might be due to the interactions between the carboxyl groups of copolymer and K+ ions, relative to the peak at 1663 cm−1 for the neat MF as shown in Fig. S3.†31
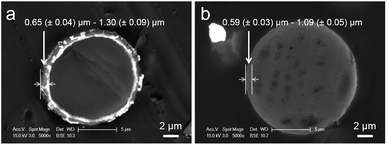 | ||
| Fig. 2 TEM images of the cross-section of (a) MF-KCl (200 F) and (b) MF-KCl (600 F) microcapsules embedded in epon/araldite resin. (Each data was calculated from at least 30 microcapsules). | ||
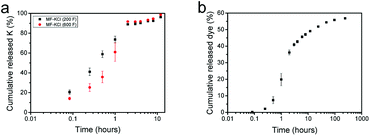
The microcapsules were dispersed in water and the release of K+ ions or dye molecules from the microcapsules were monitored, via removal of aliquots at extended time intervals over 12 hours and 10 days, and analysis with flame photometry (K+) and UV/Vis spectroscopy (dye), respectively. The cumulative release data of K+ ions in Fig. 3a from two batches of MF-KCl microcapsules demonstrates a sustained release within 2 hours, reaching roughly 90% of the encapsulated ions released out, and then followed by a slow release up to 12 h, which might be due to the interaction between K+ and carboxyl groups of copolymer as indicated by FT-IR (Fig. S3†). Interestingly, the release of K+ ions from MF-KCl (600 F) with more content of formaldehyde revealed a slower release rate, which may be attributed to the formed more compact MF, providing a barrier to the encapsulated ions, compared to MF-KCl (200 F). The release rate of allura red (dye) molecules from MF (200 F) displayed a sustained release, revealing a fast release leaking 36 ± 1% within 2 h and then slowed down, leaking 56.8 ± 0.4% for 10 days (Fig. 3b). The chemical structure of the released allura red (dye) molecules was not checked, since it had been demonstrated to be stable in acidic pH and at temperatures up to 80 °C.32 The payloads and encapsulation efficiencies of KCl and dye in the MF microcapsules were calculated using the equations in Experimental section of the ESI† as shown in Table 2. Clearly, the ones for dye in MF microcapsules were higher than for KCl ions, which probably resulted from the larger molar mass of allura red, and its functional groups (Fig. S4†) might interact with MF and the copolymer. Regarding encapsulation of small water soluble ingredients, the commonly used liposomes also exhibited their sustained release for <24 h in water,33,34 but the obvious drawbacks are the fast release of the ingredients, high cost, low payload, low reproducibility and instability during storage.35 In contrast, the MF microcapsules fabricated here are cheap and reproducible, and they display stable structure for 2 months in ethanol (Fig. S5†).
| Sample | F/M molar ratio | Payload (%) | Encapsulation efficiency (%) | Young's modulus (GPa) |
|---|---|---|---|---|
| MF-KCl (200 F) | 0.7 | 7.7 (±0.3) | 70 (±2) | 3.3 (±0.2) |
| MF-KCl (600 F) | 1.7 | 7.2 (±0.1) | 69 (±2) | 2.8 (±0.2) |
| MF-dye (200 F) | 0.7 | 8.4 (±0.1) | 82 (±1) | 4.9 (±0.2) |
The mechanical properties of MF microcapsules were measured by a micromanipulation technique.36 A single MF microcapsule was compressed by two parallel flat surfaces (Fig. 4a and b), and a force versus displacement curve for compression of the single MF-KCl (200 F) microcapsule was obtained as shown in Fig. S6.† The relationship of force (F) versus displacement (δ) is assumed to represent the elastic behavior, when the nominal deformation calculated by eqn (1) is ≤ 10%, described by the Hertz model as given in eqn (2).37 d is the diameter of the microcapsule; E is the Young's modulus of the microcapsules and v is the Poisson's ratio.
 | (1) |
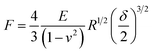 | (2) |
In this way, a force vs. (δ/2)3/2 curve was fitted with the Hertz model [eqn (2)], see Fig. 4c, and they are in good agreements with a correlation coefficient of 0.99, which justifies the assumption of the elastic behavior when the nominal deformation is ≤10%. The Young's modulus values of the three batches of MF microcapsules are showed in Table 2, and the MF-KCl (200 F) microcapsules formed with the less amount of formaldehyde indicates a higher Young's value due to the thicker shells and uniform cores, relative to the MF-KCl (600 F) microcapsules with an interstitial hollow core. In general, the Young's modulus of the formed MF microcapsules with a range of 2.8–4.9 GPa displayed a similar value, relative to previous researches, and the larger shell thickness could enhance the stiffness of the microcapsule.38,39
In conclusion, a novel method to synthesise MF microcapsules with aqueous phase encapsulated was developed in this study, and the water soluble ingredients, including an ion (K+) and small molecule (allura red) were encapsulated, indicating a sustained release in water environment for 12 h and 10 days, respectively. In addition, the formed MF microcapsules displayed Young's modulus values in the order of GPa, revealing comparatively strong stiffness. Therefore, the developed MF formation method may provide a new way to deliver different kinds of water soluble ingredients except those sensitive to acidic pH, which can have applications in different areas of scientific research and industry.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. S. was fully sponsored by College of Engineering & Physical Sciences, the University of Birmingham, UK.Notes and references
- R. Ciriminna and M. Pagliaro, Chem. Soc. Rev., 2013, 42, 9243–9250 RSC.
- R. Ciriminna, M. Sciortino, G. Alonzo, A. de Schrijver and M. Pagliaro, Chem. Rev., 2011, 111, 765–789 CrossRef PubMed.
- S. Mitragotri, P. A. Burke and R. Langer, Nat. Rev. Drug Discovery, 2014, 13, 655–672 CrossRef PubMed.
- F. Casanova and L. Santos, J. Microencapsulation, 2016, 33, 1–17 CrossRef PubMed.
- N. V. N. Jyothi, P. M. Prasanna, S. N. Sakarkar, K. S. Prabha, P. S. Ramaiah and G. Y. Srawan, J. Microencapsulation, 2010, 27, 187–197 CrossRef PubMed.
- B. J. Kim, T. Park, H. C. Moon, S. Y. Park, D. Hong, E. H. Ko, J. Y. Kim, J. W. Hong, S. W. Han, Y. G. Kim and I. S. Choi, Angew. Chem., Int. Ed., 2014, 53, 14443–14446 CrossRef PubMed.
- H. Lee, C. H. Choi, A. Abbaspourrad, C. Wesner, M. Caggioni, T. Zhu and D. A. Weitz, ACS Appl. Mater. Interfaces, 2016, 8, 4007–4013 CrossRef PubMed.
- M. Windbergs, Y. Zhao, J. Heyman and D. A. Weitz, J. Am. Chem. Soc., 2013, 135, 7933–7937 CrossRef PubMed.
- J. Zhang, R. J. Coulston, S. T. Jones, J. Geng, O. A. Scherman and C. Abell, Science, 2012, 335, 690–694 CrossRef PubMed.
- C. Sui, Y. Lu, H. L. Gao, L. Dong, Y. Zhao, L. Ouali, D. Benczedi, H. Jerri and S. H. Yu, Cryst. Growth Des., 2013, 13, 3201–3207 CrossRef.
- M. J. Abdekhodaie, J. Cheng and X. Y. Wu, Chem. Eng. Sci., 2015, 125, 4–12 CrossRef.
- P. H. R. Keen, N. K. H. Slater and A. F. Routh, Langmuir, 2014, 30, 1939–1948 CrossRef PubMed.
- Y. Zhao, Z. Luo, M. H. Li, Q. Y. Qu, X. Ma, S. H. Yu and Y. L. Zhao, Angew. Chem., Int. Ed., 2015, 54, 919–922 CrossRef PubMed.
- E. M. Dogan, F. Sudur Zalluhoglu and N. Orbey, AIChE J., 2017, 63, 409–417 CrossRef.
- A. Elabbadi, N. Jeckelmann, O. P. Haefliger and L. Ouali, J. Microencapsulation, 2011, 28, 1–9 CrossRef PubMed.
- J. Bhaumik, N. S. Thakur, P. K. Aili, A. Ghanghoriya, A. K. Mittal and U. C. Banerjee, ACS Biomater. Sci. Eng., 2015, 1, 382–392 CrossRef.
- C. Sui, J. A. Preece and Z. Zhang, RSC Adv., 2017, 7, 478–481 RSC.
- D. Chen, H. Zhu, S. Yang, N. Li, Q. Xu, H. Li, J. He and J. Lu, Adv. Mater., 2016, 28, 10443–10458 CrossRef PubMed.
- J. Ge, H. Y. Zhao, H. W. Zhu, J. Huang, L. A. Shi and S. H. Yu, Adv. Mater., 2016, 28, 10459–10490 CrossRef PubMed.
- C. P. Ruan, K. L. Ai, X. B. Li and L. H. Lu, Angew. Chem., Int. Ed., 2014, 53, 5556–5560 CrossRef PubMed.
- M. A. White, J. Chem. Educ., 1998, 75, 1119 CrossRef.
- A. Ghaemi, A. Philipp, A. Bauer, K. Last, A. Fery and S. Gekle, Chem. Eng. Sci., 2016, 142, 236–243 CrossRef.
- R. Mercade-Prieto, R. Allen, Z. B. Zhang, D. York, J. A. Preece and T. E. Goodwin, AIChE J., 2012, 58, 2674–2681 CrossRef.
- M. Pretzl, M. Neubauer, M. Tekaat, C. Kunert, C. Kuttner, G. Leon, D. Berthier, P. Erni, L. Ouali and A. Fery, ACS Appl. Mater. Interfaces, 2012, 4, 2940–2948 CrossRef PubMed.
- A. Schrade, K. Landfester and U. Ziener, Chem. Soc. Rev., 2013, 42, 6823–6839 RSC.
- Y. Long, B. Vincent, D. York, Z. B. Zhang and J. A. Preece, Chem. Commun., 2010, 46, 1718–1720 RSC.
- D. J. Merline, S. Vukusic and A. A. Abdala, Polym. J., 2013, 45, 413 CrossRef.
- X. D. He, X. W. W. Ge, M. Z. Wang and Z. C. Zhang, Polymer, 2005, 46, 7598–7604 CrossRef.
- K. Hong and S. Park, Mater. Chem. Phys., 1999, 58, 128–131 CrossRef.
- N. Seetapan, N. Limparyoon and S. Kiatkamjornwong, Polym. Degrad. Stab., 2011, 96, 1927–1933 CrossRef.
- P. Lanthong, R. Nuisin and S. Kiatkamjornwong, Carbohydr. Polym., 2006, 66, 229–245 CrossRef.
- K. Rovina, S. Siddiquee and S. M. Shaarani, Front Microbiol., 2016, 7, 798 Search PubMed.
- J.-Y. Fang, W.-R. Lee, S.-C. Shen and Y.-L. Huang, J. Dermatol. Sci., 2006, 42, 101–109 CrossRef PubMed.
- Q. Lu, D.-C. Li and J.-G. Jiang, J. Agric. Food Chem., 2011, 59, 13004–13011 CrossRef PubMed.
- A. Munin and F. Edwards-Lévy, Pharmaceutics, 2011, 3, 793–829 CrossRef PubMed.
- Z. Zhang, R. Saunders and C. R. Thomas, J. Microencapsulation, 1999, 16, 117–124 CrossRef PubMed.
- J. Hu, H.-Q. Chen and Z. Zhang, Mater. Chem. Phys., 2009, 118, 63–70 CrossRef.
- J. F. Su, J. Qiu, E. Schlangen and Y. Y. Wang, Constr. Build. Mater., 2015, 74, 83–92 CrossRef.
- R. Mercade-Prieto, R. Allen, D. York, J. A. Preece, T. E. Goodwin and Z. B. Zhang, Chem. Eng. Sci., 2011, 66, 1835–1843 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c8ra05533e |
| This journal is © The Royal Society of Chemistry 2018 |