Open Access Article
Open Access ArticleAl(III)-responsive “off–on” chemosensor based on rhodamine derivative and its application in cell imaging†
Chunwei Yua,
Li Jian*a,
Yuxiang Jia and
Jun Zhang *ab
*ab
aDepartment of Environmental Sciences, School of Tropical and Laboratory Medicine, Hainan Medical University, Haikou 571199, P. R. China. E-mail: jianli0622@163.com
bSchool of International Education, Hainan Medical University, Haikou 571199, P. R. China. E-mail: jun_zh1979@163.com
First published on 4th September 2018
Abstract
In this work, a new rhodamine chemosensor (P) with excellent photochromic properties upon vis irradiation was designed and synthesized. The fabricated chemosensor P could detect Al3+ via the opening of the spirolactam ring of the rhodamine unit with high selectivity and sensitivity. The spirolactam ring opening was confirmed by NMR and infrared spectroscopy. Upon binding with Al3+, the generated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 P-Al3+ complex, confirmed by Job's plot titrations and mass spectrometry analysis, could exhibit a remarkable fluorescence enhancement with a limit of detection (LOD) of 0.16 μM. Furthermore, the sensing of P to Al3+ in vivo was also studied quantitatively and qualitatively in detail, and the results showed that the coordination between P with Al3+ was reversible in living cells.
1 P-Al3+ complex, confirmed by Job's plot titrations and mass spectrometry analysis, could exhibit a remarkable fluorescence enhancement with a limit of detection (LOD) of 0.16 μM. Furthermore, the sensing of P to Al3+ in vivo was also studied quantitatively and qualitatively in detail, and the results showed that the coordination between P with Al3+ was reversible in living cells.
1. Introduction
As the most abundant (8.3% by weight) metallic element and the third most abundant of all elements (after oxygen and silicon), aluminum has been widely used in many industrial and domestic fields.1 Nowadays, because of acidic rain and human activities in the environment, increasing exposure to free aluminum ions (Al3+) poses a severe threat to biospheres and human health. According to the WHO report, the average daily human intake of aluminium is approx. 3–10 mg d−1, and the tolerable weekly aluminium intake in the human body is estimated to be 7 mg kg−1 body weight.2 Excessive aluminum not only hampers plant and animals, but also damages the central nervous system to cause human illnesses like Alzheimer's and Parkinson's diseases.3 In this regard, detection of Al3+ in living biosystems is of great importance, not only for monitoring aluminum contamination but also for understanding its biological functions.Fluorescent chemosensors have been proven to be a useful tool for sensing biologically important species in vitro and/or in vivo because of their non-destructive character, instantaneous response, simplicity and high sensitivity.4 In particular, for a certain purpose, it is highly demanding to selectively sense metal ions. Nevertheless, compared to other metal ions, effective design of Al3+-specific fluorescent chemosensors has always been problematic due to the lack of spectroscopic characteristics and poor coordination ability.5 Recently, based on the photophysical processes, number of signal mechanisms like photo-induced electron transfer (PET),6 intramolecular charge transfer (ICT),7 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization8 and fluorescence resonance energy transfer (FRET) have been emerged for the development of Al3+ fluorescent chemosensors.9 Unfortunately, their selectivity and sensitivity is limited due to the interference of other trivalent ions, such as Fe3+ and Cr3+,10 moreover, visible light excitable chemosensors, highly desirable for in vivo intracellular recognition and trace level determination of Al3+ are lacking too,11 and sensing materials for Al3+ detection in aqueous media are also still rare.12 These limitations have severely prevented their further applications in environmental and biological systems.
N isomerization8 and fluorescence resonance energy transfer (FRET) have been emerged for the development of Al3+ fluorescent chemosensors.9 Unfortunately, their selectivity and sensitivity is limited due to the interference of other trivalent ions, such as Fe3+ and Cr3+,10 moreover, visible light excitable chemosensors, highly desirable for in vivo intracellular recognition and trace level determination of Al3+ are lacking too,11 and sensing materials for Al3+ detection in aqueous media are also still rare.12 These limitations have severely prevented their further applications in environmental and biological systems.
Rhodamine derivatives possess excellent spectroscopic properties such as a large molar extinction coefficient, high fluorescence quantum yields, and visible light excitation as well as long wavelength emission, which is more optimal for visualizing subcellular distribution of analytes in physiological processes. Moreover, rhodamine derivatives are nonfluorescent and colorless, but ring-opening of the corresponding spirolactam via coordination with metal ions or irreversible chemical reactions gives rise to a strong fluorescence emission and a pink color.13 This response system has confirmed the great potential for environmental and biological studies in terms of selectivity and sensitivity. In the context of long-term interests in searching for fluorescent chemosensors for Al3+, rhodamine spirolactam-based chemosensors for “turn-on” detection of Al3+ have been designed.14 Paul et al. designed a rhodamine–naphthalene conjugate for the ratiometric fluorescent detection of Al3+.14a In another example, the combination of rhodamine and C-dots units has been employed for the detection of Al3+.14b However, these reported rhodamine fluorogens usually work in pure organic or organic-aqueous solvents (with high content of organic reagents), resulting in the insufficient biocompatibility.14c–e Hence, novel rhodamine chemosensors with high selectivity and sensitivity is more desirable for Al3+ quantitation and bioimaging detection.
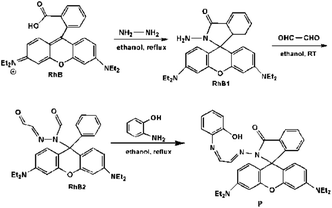
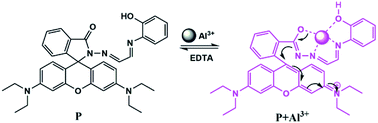
Additionally, based on our previous research, it is necessary to consider the geometry of coordination sites for a certain metal ion. Introduction of O and N donor atoms to the structure of compounds proved the coordination ability of the proposed chemosensors to Al3+.15 Herein, a new and simple Al3+-selective fluorescent chemosensor P based on rhodamine derivatives was synthesized and characterized (Scheme 1). The introduction of 2-aminophenol group into rhodamine not only enhanced the water solubility, more importantly, it also enabled the specific complexation of Al3+. The results demonstrated that the particular molecular structure gives rise to the high selectivity toward Al3+. Taking advantage of the rhodamine ring-opening reaction, fluorescence turn-on bioimaging of Al3+ in living cells confirms the great potential of chemosensor P in analyzing living biosystems.
2. Results and discussion
2.1 pH effect on the fluorescent response of P and P-Al3+
Fluoroionophores are usually disturbed by proton in the detection of metal ions. Fig. 1 presents the fluorescence spectra of chemosensor P in ethanol–water solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v, 20 mM HEPES) at different pH values ranging from 4 to 10. The fluorescence intensity of P is relatively stable when pH was higher than 5.0. However, the emission of Al3+ complex maintained fairly intense maximum emission between pH 4–7. While at pH > 6, the fluorescence intensity decrease may be due to the formation of Al(OH)3, thereby reducing the concentration of Al3+ complex. This result reveals that P exhibits satisfactory Al3+ sensing ability in the medium pH range. Thereby, the following experiments were carried out in solution at pH 5.8.
v, 20 mM HEPES) at different pH values ranging from 4 to 10. The fluorescence intensity of P is relatively stable when pH was higher than 5.0. However, the emission of Al3+ complex maintained fairly intense maximum emission between pH 4–7. While at pH > 6, the fluorescence intensity decrease may be due to the formation of Al(OH)3, thereby reducing the concentration of Al3+ complex. This result reveals that P exhibits satisfactory Al3+ sensing ability in the medium pH range. Thereby, the following experiments were carried out in solution at pH 5.8.
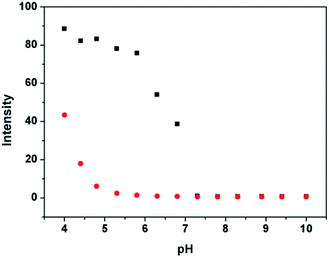 | ||
Fig. 1 pH-dependence of P (10 μM) (●) and P (10 μM) plus 4 μM Al3+ (■) in HEPES buffers as a function of different pH values in ethanol–water solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v 1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v, 20 mM HEPES). v, 20 mM HEPES). | ||
2.2 Fluorescent properties of P
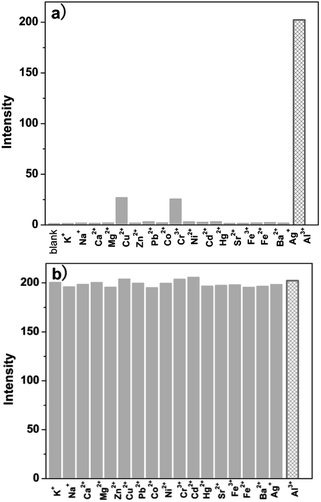
Usually, a highly selective chemosensor for Al3+ that gives a positive response rather than fluorescent quenching upon analyst binding is preferred to promote the sensitivity factor. To study the selectivity of P, 17 metal ions with different valences were examined as the alternative of Al3+ for the incubation with P. As shown in Fig. 2a and S1a, (ESI†), none of these ions can turn on the fluorescence of P under the same conditions, except Cu2+ and Cr3+ have a negligible interference. Under this condition, only Al3+ can induce distinct blue fluorescence variation at 578 nm, the intensity was 250% of that induced by P (Φ = 0.45, see Cal. 1 in ESI†), the emission band is reasonably assigned to the delocalized xanthene tautomer of the rhodamine group. Meanwhile, compared with Al3+ that caused a remarkable increase in the fluorescence intensity at 578 nm, the presence of other competing anions and ROS or RNS (ClO−, NO3−, CO32−, Cl−, Ac−, ClO4−, Br−, I−, HPO42−, H2O2, ONOO−, ·OH and ·O2−) had no significant fluorescence signal as shown in Fig. S1b (ESI†).Furthermore, to verify the ability of P to discriminate Al3+ in complex samples, Al3+ was analyzed in the mixture of the above mentioned 17 metal ions (as shown in Fig. 2b). Without any pretreatment, when selected metal ions (50 μM) were added into ethanol–water solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v, pH 5.8, 20 mM HEPES) of P (10 μM) containing Al3+ ions (10 μM), the mixed ion solution containing Al3+ releases fluorescence was similar to that with Al3+ ions alone. Also, it was investigated that the fluorescence response of P toward Al3+ in the presence of various coexistent anions and ROS or RNS such as ClO−, NO3−, CO32−, Cl−, Ac−, ClO4−, Br−, I−, HPO42−, H2O2, ·OH, ONOO− and ·O2−. It is gratifying to note that all the tested competitive analytes have no interference (Fig. S2, ESI†). For the Al3+ chemosensor P, neither cross-sensitivity to the other metal ions nor to the commonly present anions, ROS and RNS was observed. These results clearly indicate the high selectivity of P for Al3+ over other species, which can facilitate the practical detection of Al3+ in complex biological samples.
v, pH 5.8, 20 mM HEPES) of P (10 μM) containing Al3+ ions (10 μM), the mixed ion solution containing Al3+ releases fluorescence was similar to that with Al3+ ions alone. Also, it was investigated that the fluorescence response of P toward Al3+ in the presence of various coexistent anions and ROS or RNS such as ClO−, NO3−, CO32−, Cl−, Ac−, ClO4−, Br−, I−, HPO42−, H2O2, ·OH, ONOO− and ·O2−. It is gratifying to note that all the tested competitive analytes have no interference (Fig. S2, ESI†). For the Al3+ chemosensor P, neither cross-sensitivity to the other metal ions nor to the commonly present anions, ROS and RNS was observed. These results clearly indicate the high selectivity of P for Al3+ over other species, which can facilitate the practical detection of Al3+ in complex biological samples.
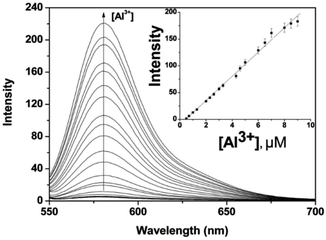
The dependence of fluorescence emission of P on the concentration of Al3+ was investigated by incubation with Al3+ at concentrations ranging from 0.1 to 10 μM. As shown in Fig. 3, the fluorescence at 578 nm enhances gradually as the Al3+ concentration increases. The titration curve shows a steady and smooth increase until a plateau is reached. A linear dependence of fluorescence at 578 nm can be determined as a function of Al3+ concentration within 0.5–9 μM (inset of Fig. 3), with the detection limit of P toward Al3+ to be 0.16 μM based on 3 × δblank/k (where δblank is the standard deviation of the blank solution and k is the slope of the calibration plot), which is enough to satisfy the U.S. EPA and FDA guidelines of 7.41 μM Al3+ for bottled drinking water. The results indicated that P could sensitively detect environmentally relevant levels of Al3+.
2.3 Recognition mechanism of P to Al3+
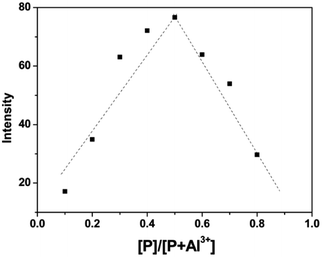
The Job's plot was carried out to prove the complex ratio of P with Al3+ (Fig. 4), total concentration of P and Al3+ was kept at a fixed 10 μM. The results showed that the maximum fluorescence emission intensity of P-Al3+ complex appeared at 0.5, which indicated that P and Al3+ formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. If P binds with Al3+ to form a complex with a complexing ratio of 1
1 complex. If P binds with Al3+ to form a complex with a complexing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, one can describe the equilibrium as follows (eqn (1)):
1, one can describe the equilibrium as follows (eqn (1)):
 | (1) |
The binding constant for the formation of P-Al3+ complex was evaluated using the Benesi–Hildebrand (B–H) plot (eqn (2), with details in Cal. 2, ESI†).16
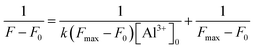 | (2) |
F0 is the fluorescence intensity of P without Al3+, F is the fluorescence intensity of P obtained with Al3+, Fmax is the fluorescence intensity of P in the presence of excess amount of Al3+, k is the binding constant (M−1) and was determined from the slope of the linear plot. The Benesi–Hildebrand analysis of the emission data gives a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the P-Al3+ complexation species, with an association constant (k) being calculated as 6.9 × 104 M−1 (Fig. S3, ESI†), corresponding to a stronger binding capability toward Al3+ in comparison with a pyrazoline derivative based chemosensor for Al3+ (with a k value of 2.13 × 103 M−1),17 or a naphthadehyde derivative based chemosensor for Al3+ (with a k value of 2.5 × 103 M−1).18
1 stoichiometry for the P-Al3+ complexation species, with an association constant (k) being calculated as 6.9 × 104 M−1 (Fig. S3, ESI†), corresponding to a stronger binding capability toward Al3+ in comparison with a pyrazoline derivative based chemosensor for Al3+ (with a k value of 2.13 × 103 M−1),17 or a naphthadehyde derivative based chemosensor for Al3+ (with a k value of 2.5 × 103 M−1).18
To better understanding their binding behavior, ESI-MS of P-Al3+ characterization was performed. m/z: 649.21 corresponding to [P + Al3+ + Cl− − H+]+ confirmed the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. S4, ESI†). To further strengthen the explanation, the IR spectra of P and P-Al3+ were given in Fig. S5.† The carbonyl stretching frequency of spirolactam ring in free chemosensor P and the P-Al3+ complex are 1732.8 cm−1 and 1700.8 cm−1, respectively. This shifting (32.0 cm−1) indicates the spirolactam ring opening phenomena where carbonyl oxygen make a bond towards Al3+ ion as reported earlier.19 Meanwhile, 1H NMR titrations were performed to strengthen the proposed reaction mechanism and plausible binding mode as shown in Fig. S6a (ESI†). Peak of –OH proton of P at 9.27 ppm disappears on addition of 1.0 equiv. of Al3+, supporting the deprotonation of P, and a new peak at 10.99 ppm indicates the spiro-cycle opening and formation of a new –OH group. The above results indicate that a plausible binding mode of P-Al3+ includes a coordinated ‘N’, spirocarbonyl ‘O’ and –OH. Additionally, the spirolactam ring opening mechanism was also established from the disappearance of the tertiary carbon signal appeared at δ = 65.18 ppm and appearance of a new signal at δ = 137.09 ppm upon addition 1.0 equiv. of Al3+ (Fig. S6b), ESI.† This is attributed to the conversion of sp3 spiro carbon of rhodamine to sp2 carbon upon binding with Al3+ ion.20
1 complex (Fig. S4, ESI†). To further strengthen the explanation, the IR spectra of P and P-Al3+ were given in Fig. S5.† The carbonyl stretching frequency of spirolactam ring in free chemosensor P and the P-Al3+ complex are 1732.8 cm−1 and 1700.8 cm−1, respectively. This shifting (32.0 cm−1) indicates the spirolactam ring opening phenomena where carbonyl oxygen make a bond towards Al3+ ion as reported earlier.19 Meanwhile, 1H NMR titrations were performed to strengthen the proposed reaction mechanism and plausible binding mode as shown in Fig. S6a (ESI†). Peak of –OH proton of P at 9.27 ppm disappears on addition of 1.0 equiv. of Al3+, supporting the deprotonation of P, and a new peak at 10.99 ppm indicates the spiro-cycle opening and formation of a new –OH group. The above results indicate that a plausible binding mode of P-Al3+ includes a coordinated ‘N’, spirocarbonyl ‘O’ and –OH. Additionally, the spirolactam ring opening mechanism was also established from the disappearance of the tertiary carbon signal appeared at δ = 65.18 ppm and appearance of a new signal at δ = 137.09 ppm upon addition 1.0 equiv. of Al3+ (Fig. S6b), ESI.† This is attributed to the conversion of sp3 spiro carbon of rhodamine to sp2 carbon upon binding with Al3+ ion.20
According to the experimental results, the reaction mechanism was proposed as shown in Scheme 2. Namely, without Al3+, P existed in a non-fluorescence spirocyclic form. Addition of Al3+ led to the formation of five-membered ring by coordination with the ligand groups (oxygen and nitrogen atoms on the phenolic hydroxyl group and rhodamine unit, respectively), resulting in spiro-cycle opening along with an appearance of pink fluorescence. It is believed that this process is reversible, which has been proved by the test using EDTA-Al3+ (Fig. S7, ESI†). Thus, a reversible “off–on” based fluorescent chemosensor for Al3+ was constructed.
2.4 Application study of P
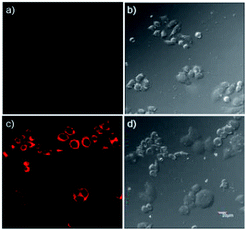
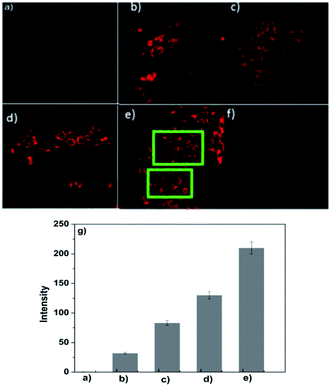
The good stability of the fluorescence switched on by Al3+ in the pH range of 4.0–7.0 provides the chemosensor with good suitability for the application in bioimaging of living cells. Confocal microscopy experiments were carried out to demonstrate the practical applicability of P, and the fluorescence images of HepG2 cells were recorded before and after the addition of Al3+ (Fig. 5). When the cells incubated with P for 30 min at 37 °C, no background fluorescence was observed from HepG2 cells themselves, suggesting that autofluorescence from the cells can be avoided and no fluorescence signal was detected in cells only treated with P (Fig. 5a). Under this condition, for the P-loaded HepG2 cells, a strong fluorescence was detected after incubation with Al3+ (Fig. 5c), demonstrating the membrane penetrability of P and the complexation with Al3+ inside the cells, clearly demonstrating that Al3+ and its interaction with P are essential for the fluorescence turn-on. The bright field images of Fig. 5a and c were shown as Fig. 5b and d, respectively, whose shapes of cells indicated that P has low toxicity, revealing good biocompatibility of P for bioanalysis.As a control, the cells not treated with Al3+ were also imaged, which showed faint fluorescence (Fig. 6a), but with the increase of the concentration of Al3+ (0.1, 0.5, 1.0, 2.0 μM) in living cells, the intensity of fluorescence was enhanced regularly (Fig. 6b–e). The reversibility of P with Al3+ in living cells was also conducted using N, N, N′, N′-Tetrakis (2-pyridylmethyl)ethylenediamine (TPEN) as chelating agent, and results show that the coordination of P with Al3+ was reversible (Fig. 6f). The cell body regions in the visual field (Fig. 6b–e) were selected as the regions of interest (ROI), and the average fluorescence intensity was determined via confocal laser scanning microscopy with various Al3+ concentrations (Fig. 6g). The results suggested that P had good membrane permeability, and these data also established that P could respond to intracellular Al3+ level changes within living cells.
We also applied P to the subcellular locations of Al3+ in the HepG2 cells using confocal fluorescence microscopy. The cells, with the same condition used in Fig. 6d, were co-stained with P (10 μM) and Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (1 μg mL−1) for 30 min. Fig. S8 (ESI†) further reveals that P primarily locates in the cytoplasm of these living HepG2 cells.
342 (1 μg mL−1) for 30 min. Fig. S8 (ESI†) further reveals that P primarily locates in the cytoplasm of these living HepG2 cells.
2.5 Methods comparison
Many fluorescent chemosensors have been reported for the determination of Al3+. The performance comparison of P and others reported chemosensors are listed in Table S1 (ESI†). It is can be seen from Table S1† that most fluorescent chemosensors present good selectivity and reversibility for Al3+,17,18,21–24,26 high fluorescence quantum yield,21–23,26 even down to 0.001 μM LOD,18 and satisfactory qualitative analysis of cell imaging,17,22,26 high binding constants at the level of 104 M−1,21–23,26 but some of them have more or less disadvantages, such as big background fluorescence,21,23,25 and unsuitable for cell imaging,18,21,23–25 irreversibility,25 pure organic solvent.18,21,25 Our newly developed chemosensor P based on rhodamine spirolactame derivative presents a number of attractive analytical features such as excellent detection limit, high association constant, good selectivity, high fluorescence quantum yield, good reproducibility, and wide qualitative and quantitative analysis of ultra-trace level Al3+ in living cells.3. Experimental
All reagents and solvents are of analytical grade and used without further purification. The metal ions salts employed were NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O, Zn(NO3)2·6H2O, PbCl2, FeCl3·6H2O, CrCl3·6H2O, CoCl2·6H2O, NiCl2·6H2O, CuCl2·2H2O, CdCl2, Hg(NO3)2, AgNO3 and AlCl3·6H2O, respectively.Fluorescence emission spectra were conducted on a Hitachi 4600 spectrofluorometer. UV-Vis spectra were obtained on a Hitachi U-2910 spectrophotometer. Nuclear magnetic resonance (NMR) spectra were measured with a Brucker AV 400 instrument and chemical shift were given in ppm from tetramethylsilane (TMS). Mass (MS) spectra were recorded on a Thermo TSQ Quantum Access Agilent 1100.
3.1 Synthesis of P
2-Aminophenol (0.120 g, 1.1 mmol) and RhB2 (ref. 27) (0.496 g, 1.0 mmol) were mixed in ethanol (40 mL). The reaction mixture was stirred at 80 °C for 4 h and then cooled to room temperature, the solution was removed under reduced pressure. The precipitate so obtained was filtered and purified with silica gel column chromatography (petroleum ether/acetic ether = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) to afford P as yellow solid. Yields: 83.4%. MS (ES+) m/z: 588.30 [M + H]+. IR (KBr, cm−1): ν = 3404, 2968, 1732, 1700, 1634, 1615, 1515, 1466, 1308, 1263, 1219, 1118, 1078, 820, 753; 1H NMR ([d6]DMSO): δ = 9.40 (s, 1H), 9.26 (d, 1H, J = 7.48), 8.17 (d, 1H, J = 8.24), 8.00 (d, 1H, J = 7.56), 7.94 (d, 1H, J = 7.48), 7.67 (t, 1H, J = 7.48), 7.61 (d, 1H, J = 7.00), 7.45 (d, 1H, J = 7.48), 7.08 (t, 1H, J = 8.98), 7.02 (d, 1H, J = 7.40), 6.84 (d, 1H, J = 8.08), 6.73 (t, 1H, J = 7.62), 6.46 (d, 1H, J = 8.16), 6.44 (s,1H), 6.40 (t, 1H, J = 10.52), 6.37 (d, 1H, J = 6.40), 6.34 (d, 1H, J = 6.04), 3.32 (m, 8H, J = 8.55), 1.08 (t, 12H, J = 6.90); 13C NMR ([d6]DMSO): δ = 164.53, 157.96, 152.34, 152.13, 151.17, 148.64, 136.75, 128.40, 127.34, 123.69, 123.38, 120.74, 119.42, 116.39, 108.22, 104.39, 97.52, 65.18, 43.63, 12.40. (Fig. S9–11†) anal. calcd (%) for C36H37N5O3 (MW 539.68): C 73.57; H 6.35; N 11.92. Found C 72.89; H 6.38; N 11.95.
v) to afford P as yellow solid. Yields: 83.4%. MS (ES+) m/z: 588.30 [M + H]+. IR (KBr, cm−1): ν = 3404, 2968, 1732, 1700, 1634, 1615, 1515, 1466, 1308, 1263, 1219, 1118, 1078, 820, 753; 1H NMR ([d6]DMSO): δ = 9.40 (s, 1H), 9.26 (d, 1H, J = 7.48), 8.17 (d, 1H, J = 8.24), 8.00 (d, 1H, J = 7.56), 7.94 (d, 1H, J = 7.48), 7.67 (t, 1H, J = 7.48), 7.61 (d, 1H, J = 7.00), 7.45 (d, 1H, J = 7.48), 7.08 (t, 1H, J = 8.98), 7.02 (d, 1H, J = 7.40), 6.84 (d, 1H, J = 8.08), 6.73 (t, 1H, J = 7.62), 6.46 (d, 1H, J = 8.16), 6.44 (s,1H), 6.40 (t, 1H, J = 10.52), 6.37 (d, 1H, J = 6.40), 6.34 (d, 1H, J = 6.04), 3.32 (m, 8H, J = 8.55), 1.08 (t, 12H, J = 6.90); 13C NMR ([d6]DMSO): δ = 164.53, 157.96, 152.34, 152.13, 151.17, 148.64, 136.75, 128.40, 127.34, 123.69, 123.38, 120.74, 119.42, 116.39, 108.22, 104.39, 97.52, 65.18, 43.63, 12.40. (Fig. S9–11†) anal. calcd (%) for C36H37N5O3 (MW 539.68): C 73.57; H 6.35; N 11.92. Found C 72.89; H 6.38; N 11.95.
3.2 General spectroscopic methods
Metal ions and P were dissolved in deionized water and DMSO to obtain 1.0 mM stock solutions, respectively. Before spectroscopic measurements, the solution was freshly prepared by diluting the high concentration stock solution to the corresponding solution. For all measurements, excitation and emission slit widths were 5/10 nm, excitation wavelength was 540 nm.3.3 Cell culture
HepG2 cells were purchased from the Committee on Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). HepG2 cells were incubated in DMEM (Dulbecco's Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS).4. Conclusions
A novel Al3+-selective rhodamine B fluorescent chemosensor was synthesized and characterized. Al3+ could induce spirolactam ring opening of the rhodamine unit and achieved “off–on” effect. In addition, the proposed P has been successfully used to detect Al3+ in living cells quantitatively and qualitatively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81560347, 81660356, 81760387), Key Research and Development Projects of Hainan Province (No. ZDYF2017103).Notes and references
- (a) Z. C. Liu, Z. Y. Yang, Y. X. Li, T. R. Li, B. D. Wang, Y. Li and X. L. Jin, Inorg. Chim. Acta, 2013, 395, 77 CrossRef; (b) J. Lee, H. Kim, S. Kim, J. Y. Noh, E. J. Song, C. Kim and J. Kim, Dyes Pigm., 2013, 96, 590 CrossRef; (c) J. C. Qin, L. Fan, T. R. Li and Z. Y. Yang, Synth. Met., 2015, 199, 179 CrossRef.
- (a) J. Barceló and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75 CrossRef; (b) Z. Krejpcio and R. W. Wójciak, Pol. J. Environ. Stud., 2002, 11, 251 Search PubMed.
- (a) S. W. King, J. Savory and M. R. Willis, Crit. Rev. Clin. Lab. Sci., 1981, 13, 1 Search PubMed; (b) I. S. Parkinson, M. K. Ward and D. N. Kerr, J. Clin. Pathol., 1981, 34, 1285 CrossRef PubMed; (c) A. M. Pierdes, Int. J. Artif. Organs, 1978, 1, 206 Search PubMed.
- (a) S. Z. Pu, Q. Sun, C. B. Fan, R. J. Wang and G. Liu, J. Mater. Chem., 2016, 4, 3075 RSC; (b) Y. L. Fu, Y. Y. Tu, C. B. Fan, C. H. Zheng, G. Liu and S. Z. Pu, New J. Chem., 2016, 40, 8579 RSC; (c) S. Z. Pu, C. C. Zhang, C. B. Fan and G. Liu, Dyes Pigm., 2016, 129, 24 CrossRef; (d) Y. L. Fu, C. B. Fan, G. Liu, S. Q. Cui and S. Z. Pu, Dyes Pigm., 2016, 126, 121 CrossRef; (e) H. C. Ding, B. Q. Li, S. Z. Pu, G. Liu, D. C. Jia and Z. Yu, Sens. Actuators, B, 2017, 247, 26 CrossRef; (f) Y. L. Fu, C. B. Fan, G. Liu and S. Z. Pu, Sens. Actuators, B, 2017, 239, 295 CrossRef.
- (a) X. Sun, Y. W. Wang and Y. Peng, Org. Lett., 2012, 14, 3420 CrossRef PubMed; (b) K. K. Upadhyay and A. Kumar, Org. Biomol. Chem., 2010, 8, 4892 RSC.
- (a) O. Alici and S. Erdemir, Sens. Actuators, B, 2015, 208, 159 CrossRef; (b) J. Kumar, M. J. Sarma, P. Phukan and D. K. Das, Dalton Trans., 2015, 44, 4576 RSC; (c) C. A. Huerta-Aguilar, P. Raj, P. Thangarasu and N. Singh, RSC Adv., 2016, 6, 37944 RSC; (d) S. Mukherjee, P. Mal and H. Stoeckli-Evans, J. Lumin., 2016, 172, 124–130 CrossRef.
- (a) Y. Li, C. Liao, S. Huang, H. Xu, B. Zheng, J. Du and D. Xiao, RSC Adv., 2016, 6, 25420 RSC; (b) S. M. Hossain, K. Singh, A. Lakma, R. N. Pradhan and A. K. Singh, Sens. Actuators, B, 2017, 239, 1109 CrossRef; (c) B. K. Datta, D. Thiyagarajan, A. Ramesh and G. Das, Dalton Trans., 2015, 44, 13093 RSC; (d) L. Kang, Z. Y. Xing, X. Y. Ma, Y. T. Liu and Y. Zhang, Spectrochim. Acta, Part A, 2016, 167, 59 CrossRef PubMed.
- (a) I. H. Hwang, Y. W. Choi, K. B. Kim, G. J. Park, J. J. Lee, L. Nguyen, I. Noh and C. Kim, New J. Chem., 2016, 40, 171 RSC; (b) J. Ma, W. Shi, L. Feng, Y. Chen, K. Fan, Y. Hao, Y. Hui and Z. Xie, RSC Adv., 2016, 6, 28034 RSC; (c) C. Liang, W. Bu, C. Li, G. Men, M. Deng, Y. J. Yao, H. Sun and S. Jiang, Dalton Trans., 2015, 44, 11352 RSC; (d) R. Alam, T. Mistri, R. Bhowmick, A. Katarkar, K. Chaudhuri and M. Ali, RSC Adv., 2016, 6, 1268 RSC; (e) V. K. Gupta, S. K. Shoora, L. K. Kumawat and A. K. Jain, Sens. Actuators, B, 2015, 209, 15 CrossRef.
- (a) J. C. Qin, J. Yan, B. D. Wang and Z. Y. Yang, Tetrahedron Lett., 2016, 57, 1935 CrossRef; (b) S. Paul, S. Goswami and A. Manna, Dalton Trans., 2015, 44, 11805 RSC.
- (a) Y. M. Xue, R. J. Wang, C. H. Zheng, G. Liu and S. Z. Pu, Tetrahedron Lett., 2016, 57, 1877 CrossRef; (b) S. Goswami, K. Aich, S. Das, A. K. Das, D. Sarkar, S. Panja, T. K. Mondal and S. Mukhopadhyay, Chem. Commun., 2013, 49, 10739 RSC.
- (a) E. T. Feng, C. B. Fan, N. S. Wang, G. Liu and S. Z. Pu, Dyes Pigm., 2018, 151, 22 CrossRef; (b) E. Oliveira, H. M. Santos, J. L. Capelo and C. Lodeiro, Inorg. Chim. Acta, 2012, 381, 203 CrossRef; (c) R. Azadbakht and J. Khanabadi, Inorg. Chem. Commun., 2013, 30, 21 CrossRef.
- (a) P. Torawane, K. Tayade, S. Bothra, S. K. Sahoo, N. Singh and A. Borse, Sens. Actuators, B, 2016, 222, 562 CrossRef; (b) C. R. Li, J. C. Qin, G. Q. Wang, B. D. Wang, A. K. Fu and Z. Y. Yang, Inorg. Chim. Acta, 2015, 430, 91 CrossRef; (c) L. K. Kumawat, N. Mergu, M. Asif and V. K. Gupta, Sens. Actuators, B, 2016, 231, 847 CrossRef; (d) C. R. Li, Z. C. Liao, J. C. Qin, B. D. Wang and Z. Y. Yang, J. Lumin., 2015, 168, 330 CrossRef.
- (a) K. Tiensomjitr, R. Noorat, K. Wechakorn, S. Prabpai, K. Suksen, P. Kanjanasirirat, Y. Pewkliang, S. Borwornpinyo and P. Kongsaeree, Spectrochim. Acta, Part A, 2017, 185, 228 CrossRef PubMed; (b) M. J. Liu, J. Yuan, J. Tao, Y. Q. Zhang, C. M. Liu and H. Z. Kou, Inorg. Chem., 2018, 57, 4061 CrossRef PubMed; (c) Z. Q. Mao, H. Jiang, X. J. Song, W. Hu and Z. H. Liu, Anal. Chem., 2017, 89, 9620 CrossRef PubMed; (d) R. J. Iwatate, M. Kamiya, K. Umezawa, H. Kashima, M. Nakadate, R. Kojima and Y. Urano, Bioconjugate Chem., 2018, 29, 241 CrossRef PubMed; (e) A. Rai, A. K. Singh, K. Tripathi, A. K. Sonkar and B. S. Chauhan, Sens. Actuators, B, 2018, 266, 95 CrossRef; (f) J. Tang, S. G. Ma, D. Zhang, Y. Q. Liu, Y. F. Zhao and Y. Ye, Sens. Actuators, B, 2016, 236, 109 CrossRef; (g) Z. Q. Mao, H. Jiang, X. J. Song, W. Hu and Z. H. Liu, Anal. Chem., 2017, 89, 9620 CrossRef PubMed; (h) S. Li, D. Zhang, X. Y. Xie, S. G. Ma, Y. Liu, Z. H. Xu, Y. F. Gao and Y. Ye, Sens. Actuators, B, 2016, 224, 661 CrossRef; (i) F. Yan, R. Zhang, Y. Huang, D. Kong and Q. Ye, RSC Adv., 2016, 6, 50732 RSC; (j) J. Y. Cheng, E. B. Yang, P. G. Ding, J. Tang, D. Zhang, Y. F. Zhao and Y. Ye, Sens. Actuators, B, 2015, 221, 688 CrossRef.
- (a) S. Paul, S. Goswami and A. Manna, Dalton Trans., 2015, 44, 11805 RSC; (b) Y. Kim, G. Jang and T. S. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 15649 CrossRef PubMed; (c) M. Yu, R. Yuan, C. Shi, W. Zhou, L. Wei and Z. Li, Dyes Pigm., 2013, 99, 887 CrossRef; (d) A. Rai, N. Kumari, A. K. Srivastava, S. K. Singh, S. Srikrishna and L. Mishra, J. Photochem. Photobiol., A, 2016, 319, 78 CrossRef; (e) H. S. Kim, S. Angupillai and Y. A. Son, Sens. Actuators, B, 2016, 222, 447 CrossRef; (f) S. Chemate and N. Sekar, Sens. Actuators, B, 2015, 220, 1196 CrossRef; (g) P. G. Ding, J. H. Wang, J. Y. Cheng, Y. F. Zhao and Y. Ye, New J. Chem., 2015, 39, 342 RSC; (h) S. Dey, S. Sarkar, D. Maity and P. Roy, Sens. Actuators, B, 2017, 246, 518 CrossRef.
- (a) D. Maity and T. Govindaraju, Inorg. Chem., 2010, 49, 7229 CrossRef PubMed; (b) D. Maity and T. Govindaraju, Chem. Commun., 2010, 46, 4499 RSC.
- (a) M. I. Rodriguez-Caceres, R. A. Agbaria and I. M. Warner, J. Fluoresc., 2005, 15, 185 CrossRef PubMed; (b) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef; (c) F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, J. Fan, Y. Wu and S. Sun, Chem.–Eur. J., 2007, 13, 2880 CrossRef PubMed.
- R. Manjunath and K. Palaninathan, New J. Chem., 2018, 42, 10891 RSC.
- C. Y. Sun, X. Miao, L. J. Zhang, W. J. Li and Z. D. Chang, Inorg. Chim. Acta, 2018, 478, 112 CrossRef.
- P. Mahato, S. Saha, E. Suresh, R. Di Liddo, P. P. Parnigotto, M. T. Conconi, M. K. Kesharwani, B. Ganguly and A. Das, Inorg. Chem., 2012, 51, 1769 CrossRef PubMed.
- B. Sen, S. Pal, S. Lohar, M. Mukherjee, S. K. Mandal, A. R. Khuda-Bukhshb and P. Chattopadhyay, RSC Adv., 2014, 4, 21471 RSC.
- D. Maity and T. Govindaraju, Chem. Commun., 2010, 46, 4499 RSC.
- A. Rai, A. K. Singh, K. Tripathi, A. K. Sonkar, B. S. Chauhan, S. Srikrishna, T. D. James and L. Mishra, Sens. Actuators, B, 2018, 266, 95 CrossRef.
- K. Shen, S. Mao, X. Shi, F. Wang, Y. L. Xu, S. O. Aderinto and H. L. Wu, Luminescence, 2017, 33, 54 CrossRef PubMed.
- S. Mabhai, M. Dolai, S. Dey, A. Dhara, B. Das and A. Jana, New J. Chem., 2018, 42, 10191 RSC.
- G. Kumar, K. Paul and V. Luxami, Sens. Actuators, B, 2018, 263, 585 CrossRef.
- A. Rai, N. Kumari, A. K. Srivastava, S. K. Singh, S. Srikrishna and L. Mishra, J. Photochem. Photobiol., A, 2016, 319, 78 CrossRef.
- X. F. Yang, X. Q. Guo and Y. B. Zhao, Talanta, 2002, 57, 883 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental characterizations (PDF). See DOI: 10.1039/c8ra05359f |
| This journal is © The Royal Society of Chemistry 2018 |