Open Access Article
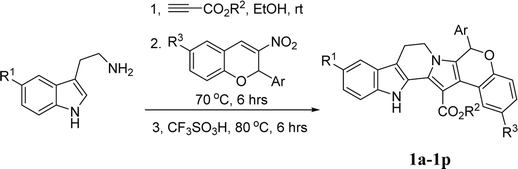
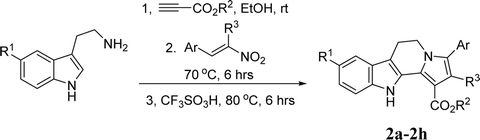
Open Access ArticleConvenient construction of tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles and tetrahydroindolizino[8,7-b]indoles via one-pot domino reaction†
Jing Sun,
Wang Jiang and
Chao-Guo Yan *
*
College of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou 225002, China. E-mail: cgyan@yzu.edu.cn; Fax: +86-514-87975244; Tel: +86-514-87975531
First published on 14th August 2018
Abstract
The functionalized tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles were conveniently synthesized in high yields by one-pot domino reaction of tryptamines, alkyl propiolates and 2-aryl-3-nitro-2H-chromenes. Under similar conditions, the one-pot reaction of tryptamines, alkyl propiolates and β-nitroalkenes resulted in functionalized tetrahydroindolizino[8,7-b]indoles. The reaction mechanism involved sequential generation of β-enamino ester, Michael addition, Pictet–Spengler reaction and annulation process. The reaction showed high atomic economy and met the goals of sustainable chemistry.
Introduction
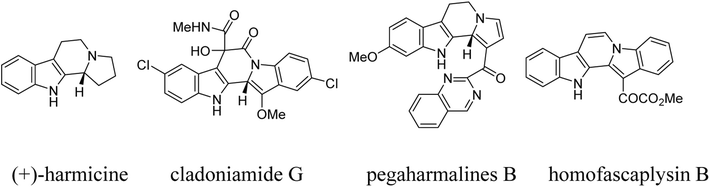
Indolizino[8,7-b]indole is one of the most important nitrogen-containing heterocyclic cores, which not only widely exists in various naturally occurring alkaloids such as (+)-harmicine, cladoniamide G, pegaharmalines B, and homofascaplysin B (Scheme 1), but also is represented in many synthetic pharmacologically active compounds.1,2 Additionally, dihydroindolizino[8,7-b]indole was also employed as a useful synthetic precursor for the preparation of complex heterocyclic systems, due to its piperidyl ring easily undergoing rapid nucleophilic attack in a ring-opening process.3 Therefore, the development of elegant methodologies for the preparation of diverse indolizino[8,7-b]indole derivatives has attracted continual attention in organic and medicinal chemistry.4,5 Among various useful synthetic methods,6 the Pictet–Spengler reaction has been known as one of most efficient methods for the construction of indolizino[8,7-b]indole framework.7,8 In this respect, the β-enamino esters generated from addition reaction of tryptamines and alkyl propiolates were widely used as the valuable building blocks for sequential Pictet–Spengler reaction to construct versatile indole-annulated heterocyclics.9–11 Recently, we have successfully developed a facile synthetic procedure for the functionalized hexahydroindolo[2,3-a]quinolizines by Lewis acid catalyzed one-pot domino reactions of tryptamines, alkyl propiolates and α,β-unsaturated aldehydes as well as arylideneacetones.12a Similarly, we also provided domino reaction of tryptamine, alkyl propiolates and 3-phenacylideneoxindoles for convenient synthesis of functionalized 6,11-dihydro-5H-indolizino[8,7-b]indoles.12b In order to develop the potential synthetic values of this one-pot domino reaction and to hunt for new efficient domino reactions based on the reactive β-enamino esters,13,14 herein we wish to report the convenient construction of tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles and tetrahydroindolizino[8,7-b]indoles via one-pot domino reaction of tryptamine, alkyl propiolate and 2-aryl-3-nitro-2H-chromenes as well as β-nitrostyrenes.Results and discussion
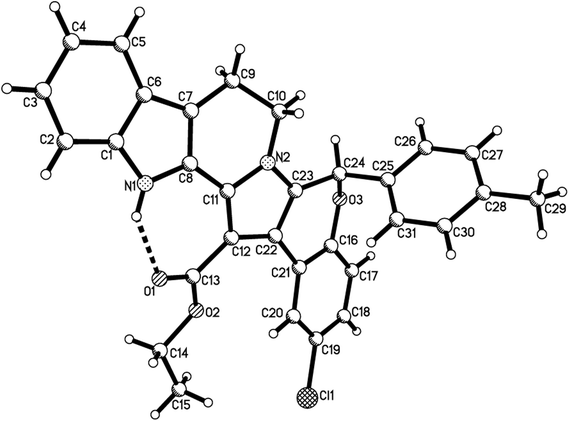
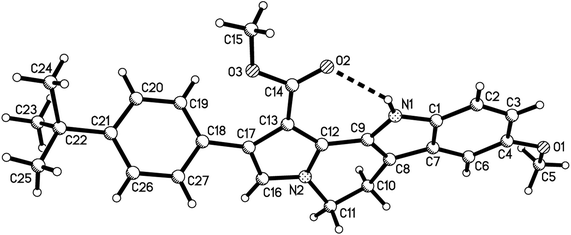
According to our previously established reaction conditions for the domino reaction of arylamine, methyl propiolate and 2-aryl-3-nitro-2H-chromenes,13 a one-pot step-by-step reaction procedure was employed. Firstly, addition reaction of tryptamine to methyl propiolate in ethanol at room temperature can be finished in about half hour to give the expected β-enamino ester. Then, the reaction of the generated in situ β-enamino ester with 2-aryl-3-nitro-2H-chromenes was carried out at 70 °C to give an adduct through Michael addition reaction, which structure has been previously characterized.14 TLC monitor indicated that this chain product cannot converted further to the cyclized product after heating its ethanol solution for longer time. However, it converted smoothly to the desired polycyclic compound 1a in 84% yield by refluxing in ethanol in presence of strong acid TfOH as acid catalyst for eight hours. It should be pointed out that the three-component reaction of tryptamine, methyl propiolate and 2-aryl-3-nitro-2H-chromene in ethanol in the presence of TfOH resulted in a complicate mixture of products. Thus, the novel tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole was only prepared in satisfactory yield by employing one-pot step-by-step reaction procedure. Then, the scope to the reaction was developed by using various substituted substrates. The results are summarized in Table 1. The reaction usually afforded the polycyclic tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles 1a–1p in high yields. The substituent on the 2-aryl-3-nitrochromenes showed little effect on the yields of products. 5-Methoxytryptamine and ethyl propiolate also showed high reactivity in the reaction. The structures of the obtained compounds 1a–1p were fully characterized by IR, HRMS, 1H NMR and 13C NMR spectra. The single crystal structures of the compounds 1e (Fig. 1), 1h, 1i and 1p (Fig. s1–s3†) were successfully determined, which unambiguously confirmed the structures of the prepared polycyclic products. From the single crystal structure, it can be clearly seen that a linear polycyclic compound was actually formed by domino annulation reaction, in which only nitro group was eliminated from the starting material.| Entry | Compd | R1 | R2 | R3 | Ar | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction condition: (1) tryptamine (1.0 mmol), propiolate (1.2 mmol) in EtOH (5.0 mL), r.t., 0.5 h; (2) 2-aryl-3-nitrochromene (1.0 mmol), 70 °C, 6 h; (3) TfOH (25% mol), 80 °C, 6 h.b Isolated yield. | ||||||
| 1 | 1a | H | CH3 | Br | p-CH3C6H4 | 81 |
| 2 | 1b | H | CH3 | H | m-FC6H4 | 84 |
| 3 | 1c | H | CH3 | Cl | m-CH3OC6H4 | 62 |
| 4 | 1d | H | CH3 | Br | m-CH3OC6H4 | 65 |
| 5 | 1e | H | C2H5 | Cl | p-CH3C6H4 | 79 |
| 6 | 1f | H | C2H5 | Br | p-CH3C6H4 | 84 |
| 7 | 1g | H | C2H5 | Br | o-NO2C6H4 | 81 |
| 8 | 1h | OCH3 | CH3 | Cl | p-CH3C6H4 | 83 |
| 9 | 1i | OCH3 | CH3 | Br | p-CH3C6H4 | 87 |
| 10 | 1j | OCH3 | C2H5 | Br | p-CH3C6H4 | 82 |
| 11 | 1k | OCH3 | CH3 | Cl | C6H5 | 75 |
| 12 | 1l | OCH3 | CH3 | Cl | p-ClC6H4 | 76 |
| 13 | 1m | OCH3 | CH3 | H | o-ClC6H4 | 72 |
| 14 | 1n | OCH3 | CH3 | Cl | p-BrC6H4 | 89 |
| 15 | 1o | OCH3 | CH3 | Br | o-NO2C6H4 | 82 |
| 16 | 1p | OCH3 | CH3 | Br | p-ClC6H4 | 87 |
In order to demonstrate the synthetic values of this domino reaction, the common β-nitroalkenes were also employed in the reaction under same reaction conditions. The results are summarized in Table 2. The reaction proceeded smoothly to give the corresponding tetrahydroindolizino[8,7-b]indoles 2a–2d in good yields. The reactions with 1-methyl-1-nitroalkenes derived from condensation reaction of aromatic aldehydes with nitroethane afforded the methyl-substituted products in good yields 2e–2g. This result showed that this one-pot domino reaction has a widely variety of scope and is an efficient synthetic protocol for diverse indolizino[8,7-b]indole derivatives. The structure of the polycyclic compounds 2a–2h were established on various spectroscopy. The single crystal structures of the compounds 2b (Fig. 2) and 2e (Fig. s4†) were also successfully determined.
| Entry | Compd | R1 | R2 | R3 | Ar | Yieldb |
|---|---|---|---|---|---|---|
| a Reaction condition: (1) tryptamine (1.0 mmol), propiolate (1.2 mmol) in EtOH (5.0 mL), r.t., 0.5 h; (2) β-nitroalkene (1.0 mmol), 70 °C, 6 h; (3) TfOH (25% mol), 80 °C, 6 h.b Isolated yield. | ||||||
| 1 | 2a | H | CH3 | H | p-ClC6H4 | 79 |
| 2 | 2b | OCH3 | CH3 | H | p-BuC6H4 | 91 |
| 3 | 2c | OCH3 | CH3 | H | p-CH3C6H4 | 85 |
| 4 | 2d | OCH3 | CH2CH3 | H | p-BuC6H4 | 83 |
| 5 | 2e | H | CH3 | CH3 | p-CH3C6H4 | 69 |
| 6 | 2f | OCH3 | CH3 | CH3 | p-CH3C6H4 | 82 |
| 7 | 2h | OCH3 | CH3 | CH3 | p-BrC6H4 | 77 |
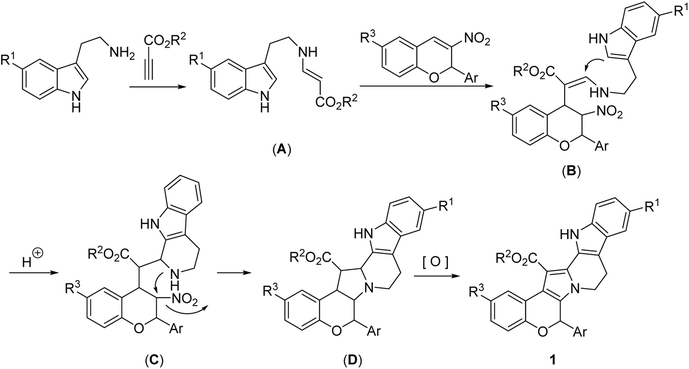
For explaining the formation of the polycyclic compounds, a plausible domino reaction mechanism was briefly proposed on the basis of the previously reported similar reactions.12,13,15 Firstly, addition of tryptamine to methyl propiolate resulted in the expected β-enamino ester (A). Secondly, Michael addition of β-enamino ester (A) to 2-aryl-3-nitrochromene afforded intermediate (B). Thirdly, the acid catalyzed intramolecular Pictet–Spengler cyclization gave the intermediate (C). Then, the intramolecular substitution of amino group to nitro group yielded the intermediate (D), which in turn converted to the final product 1 by dehydrogenation process in air.15 The formation of indolizino[8,7-b]indole 2 obviously proceeded with similar reaction mechanism, in which 2-aryl-3-nitrochromene was replaced by β-nitroalkene (Scheme 2).
Conclusion
In summary, we have investigated the one-pot domino reaction of tryptamines, alkyl propiolates and 2-aryl-3-nitrochromenes and successfully developed a convenient protocol for synthesis of functionalized tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles. Additionally, the functionalized tetrahydroindolizino[8,7-b]indoles can be also efficiently prepared by similar reaction with normal β-nitroalkenes. The advantages of this protocol included using easily accessible starting materials, wide range of substrates, high yields and high molecular diversity. This reaction not only provided a practical synthetic method for cyclic fused indolizino[8,7-b]indoles, but also developed the synthetic values of the reactive β-enamino ester in synthetic and medicinal chemistry.Experimental section
General procedure for the synthesis of tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indoles
A solution of tryptamine (1.0 mmol) and alkyl propiolate (1.2 mmol) in absolute ethanol (10.0 mL) was stirred at room temperature for about half hour. Then, 2-aryl-3-nitrochromane (1.0 mmol) was added. The solution was heated at 60–70 °C for six hours. After cooling, trifluoromethanesulfonic acid (25% mol) was added. The resulting solution was refluxed at 80 °C for additional six hour. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to chromatography with ethyl acetate and light petroleum (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give pure product for analysis.
5) as eluent to give pure product for analysis.
Methyl-2-bromo-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1a)
Yellow solid, 81%, mp. 235–237 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.90 (s, 1H, NH), 8.19 (d, J = 2.4 Hz, 1H, CH), 7.62 (d, J = 8.4 Hz, 1H, ArH), 7.53 (d, J = 8.0 Hz, 1H, ArH), 7.17–7.03 (m, 7H, ArH), 6.77–6.75 (m, 2H, ArH), 4.33–4.29 (m, 1H, CH), 3.97 (s, 3H, OCH3), 3.55–3.52 (m, 1H, CH), 3.10–3.06 (m, 2H, CH), 2.23 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.9, 149.7, 138.9, 136.6, 134.9, 131.1, 130.7, 129.8, 129.4, 128.0, 127.8, 125.9, 125.6, 123.5, 122.9, 119.9, 119.7, 118.8, 113.7, 112.8, 112.2, 109.6, 104.9, 72.6, 52.1, 42.6, 21.1, 20.0; IR (KBr) ν: 3366, 3019, 2939, 1686, 1583, 1494, 1447, 1326, 1206, 968, 849, 784 cm−1. MS (m/z): HRMS (ESI) calcd for C30H23BrN2O3Na ([M + Na]+): 561.0784. Found: 561.0790.Methyl-6-(3-fluorophenyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1b)
Yellow solid, 84%, mp. 219–220 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.92 (s, 1H, NH), 7.95 (d, J = 7.2 Hz, 1H, ArH), 7.63 (d, J = 8 Hz, 1H, ArH), 7.55 (d, J = 8 Hz, 1H, ArH), 7.36 (dd, J1 = 10 Hz, J2 = 4 Hz, 1H, ArH), 7.17–7.12 (m, 2H, ArH), 7.07–6.94 (m, 5H, ArH), 6.87 (d, J = 7.6 Hz, 1H, ArH), 6.81 (s, 1H, CH), 4.30–4.34 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.67–3.59 (m, 1H, CH), 3.14–3.10 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 166.3, 163.8, 161.3, 150.3, 141.2, 141.2, 136.5, 131.3, 131.2, 131.0, 129.7, 127.3, 126.1, 125.7, 125.7, 123.8, 123.7, 122.8, 122.3, 121.1, 119.9, 118.7, 117.8, 116.2, 116.0, 114.6, 114.4, 113.3, 112.8, 109.3, 105.1, 71.7, 52.1, 42.6, 20.0; IR (KBr) ν: 3391, 2957, 2928, 1680, 1625, 1563, 1490, 1016, 825, 806, 666 cm−1. MS (m/z): HRMS (ESI) calcd for C29H22FN2O3 ([M + H]+): 465. 1609. Found: 465.1612.Methyl-2-chloro-6-(3-methoxyphenyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1c)
Yellow solid, 62%, mp. 227–228 °C; 1H NMR (400 MHz, CDCl3) δ: 10.96 (s, 1H, NH), 7.90–7.89 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.23–7.19 (m, 2H, ArH), 7.14–7.10 (m, 1H, ArH), 6.98–6.95 (m, 1H, ArH), 6.84–6.82 (m, 1H, ArH), 6.79–6.77 (m, 3H, ArH), 6.32 (s, 1H, CH), 4.07–4.00 (m, 1H, CH), 3.73–3.67 (m, 4H, OCH3, CH), 3.19–3.07 (m, 2H, CH); 13C NMR (100 MHz, CDCl3) δ: 166.9, 159.9, 149.0, 138.7, 136.0, 132.4, 129.9, 129.7, 126.7, 126.3, 126.1, 125.6, 125.4, 123.0, 122.4, 119.9, 119.8, 118.7, 118.2, 114.3, 113.5, 113.3, 111.9, 108.3, 105.1, 73.7, 55.2, 51.4, 42.8, 20.2; IR (KBr) ν: 3395, 2988, 2894, 1698, 1625, 1578, 1527, 1280, 825, 867, 725 cm−1. MS (m/z): HRMS (ESI) calcd for C30H24ClN2O4 ([M + H]+): 511.1419. Found: 511.1441.Methyl-2-bromo-6-(3-methoxyphenyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1d)
Yellow solid, 65%, mp. 229–230 °C; 1H NMR (400 MHz, CDCl3) δ: 10.97 (s, 1H, NH), 8.05–8.05 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.23–7.19 (m, 2H, ArH), 7.14–7.09 (m, 2H, ArH), 6.84–6.82 (m, 1H, ArH), 6.78–6.77 (m, 2H, ArH), 6.74–6.72 (m, 1H, ArH), 6.33 (s, 1H, CH), 4.07–4.00 (m, 1H, CH), 3.73–3.66 (m, 4H, OCH3, CH), 3.15–3.07 (m, 2H, CH); 13C NMR (100 MHz, CDCl3) δ: 166.9, 159.9, 149.5, 138.6, 136.0, 132.5, 129.9, 129.7, 129.2, 128.3, 126.1, 125.6, 123.0, 122.8, 119.9, 119.8, 119.1, 118.2, 114.3, 114.2, 113.5, 113.1, 111.9, 108.3, 105.1, 73.7, 55.2, 51.4, 42.8, 20.2; IR (KBr) ν: 3385, 2972, 2954, 1690, 1680, 1547, 1437, 1290, 1025, 921, 831 cm−1. MS (m/z): HRMS (ESI) calcd for C30H24BrN2O4 ([M + H]+): 555.0914. Found: 555.0911.Ethyl-2-chloro-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1e)
Yellow solid, 79%, mp. 232–233 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.99 (s, 1H, NH), 8.09 (d, J = 2.0 Hz, 1H, CH), 7.63 (d, J = 8.0 Hz, 1H, ArH), 7.54 (d, J = 7.6 Hz, 1H, ArH), 7.17–7.01 (m, 7H, ArH), 6.82–6.78 (m, 2H, ArH), 4.57–4.52 (m, 1H, CH), 4.41–4.31 (m, 2H, OCH2), 3.58–3.50 (m, 1H, CH), 3.10–3.09 (m, 2H, CH), 2.23 (s, 3H, CH3), 1.42 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.7, 149.3, 138.8, 136.4, 134.9, 131.4, 130.9, 129.8, 127.8, 126.4, 125.9, 125.7, 125.6, 125.3, 123.0, 122.9, 119.9, 119.3, 118.8, 112.8, 112.2, 109.4, 105.3, 72.6, 61.2, 42.7, 21.1, 19.9, 14.3; IR (KBr) ν: 3453, 3362, 1679, 1639, 1567, 1387, 1156, 1093, 978, 853, 818, 740 cm−1. MS (m/z): HRMS (ESI) calcd for C31H25ClN2O3Na ([M + Na]+): 531.1446. Found: 531.1456.Ethyl-2-bromo-11-methoxy-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1f)
Yellow solid, 84%, mp. 204–206 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.98 (s, 1H, NH), 8.21 (d, J = 2.0 Hz, 1H, CH), 7.63 (d, J = 8.4 Hz, 1H, ArH), 7.54 (d, J = 8.0 Hz, 1H, ArH), 7.17–7.03 (m, 7H, ArH), 6.77–6.75 (m, 2H, ArH), 4.57–4.50 (m, 1H, CH), 4.40–4.30 (m, 2H, OCH2), 3.58–3.51 (m, 1H, CH), 3.10–3.06 (m, 2H, CH), 2.23 (s, 3H, CH3), 1.44 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.7, 149.7, 138.9, 136.4, 134.8, 131.5, 130.9, 129.8, 129.3, 128.1, 127.8, 125.9, 125.6, 123.5, 122.9, 119.9, 119.8, 118.8, 113.7, 112.8, 112.0, 109.4, 105.2, 72.6, 61.3, 42.7, 21.1, 19.9, 14.4; IR (KBr) ν: 3453, 3355, 2980, 2929, 1679, 1564, 1492, 978, 850, 816, 785 cm−1. MS (m/z): HRMS (ESI) calcd for C31H25BrN2O3Na ([M + Na]+): 575.0941. Found: 575.0947.Ethyl-2-bromo-6-(2-nitrophenyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1g)
Yellow solid, 81%, mp. 239–240 °C; 1H NMR (400 MHz, DMSO-d6) δ: 11.05 (s, 1H, NH), 8.18 (s, 1H, CH), 7.99 (d, J = 8.0 Hz, 1H, ArH), 7.66 (d, J = 8.4 Hz, 1H, ArH), 7.58–7.50 (m, 3H, ArH), 7.35 (s, 1H, ArH), 7.19–7.15 (m, 2H, ArH), 7.09–7.07 (m, 1H, ArH), 6.82 (d, J = 7.6 Hz, 1H, ArH), 6.62 (d, J = 8.4 Hz, 1H, ArH), 4.56–4.53 (m, 1H, CH), 4.44–4.37 (m, 2H, OCH2), 3.77–3.70 (m, 1H, CH), 3.16–3.12 (m, 2H, CH), 1.43 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.6, 149.1, 149.0, 136.5, 133.6, 132.1, 131.1, 130.6, 129.6, 129.5, 128.4, 128.3, 125.8, 125.6, 125.3, 123.4, 123.1, 120.0, 119.4, 118.9, 114.5, 113.2, 112.8, 109.8, 105.3, 68.4, 61.3, 42.8, 20.0, 14.4; IR (KBr) ν: 3458, 3299, 2977, 1674, 1574, 1491, 1364, 1081, 885, 787, 735 cm−1. MS (m/z): HRMS (ESI) calcd for C30H22BrN3O5Na ([M + Na]+): 606.0635. Found: 606.0649.Methyl-2-chloro-11-methoxy-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1h)
Yellow solid, 83%, mp. 208–210 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.82 (s, 1H, NH), 8.05 (d, J = 2.8 Hz, 1H, ArH), 7.55 (d, J = 8.8 Hz, 1H, ArH), 7.13–7.07 (m, 4H, ArH), 7.03–7.00 (m, 2H, ArH), 6.82–6.78 (m, 3H, ArH), 4.34–4.31 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.52–3.48 (m, 1H, CH), 3.08–3.04 (m, 2H, CH), 2.23 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.9, 154.1, 149.2, 138.8, 134.9, 131.8, 131.3, 130.7, 129.8, 127.8, 126.4, 126.3, 125.9, 125.8, 125.1, 123.0, 119.2, 113.6, 113.6, 112.3, 109.4, 104.7, 99.9, 72.6, 55.7, 52.1, 42.7, 21.1, 20.0; IR (KBr) ν: 3387, 2944,1679, 1623, 1567, 1488, 1452, 1367, 1325, 1283, 1082, 871, 81, 839, 623 cm−1. MS (m/z): HRMS (ESI) calcd for C31H25ClN2O4Na ([M + Na]+): 547.1395. Found: 547.1401.Methyl-2-bromo-11-methoxy-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1i)
Yellow solid, 87%, mp. 213–215 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.80 (s, 1H, NH), 8.18 (d, J = 2.4 Hz, 1H, CH), 7.53 (d, J = 9.2 Hz, 1H, ArH), 7.15–7.07 (m, 5H, ArH), 7.02 (d, J = 2.0 Hz, 1H, ArH), 6.82–6.79 (m, 1H, ArH), 6.76–6.74 (m, 2H, ArH), 4.32–4.29 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.53–3.50 (m, 1H, CH), 3.08–3.04 (m, 2H, CH), 2.23 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.9, 154.2, 149.7, 138.8, 134.9, 131.7, 131.3, 130.7, 129.8, 129.3, 128.0, 127.8, 126.3, 125.9, 123.5, 119.7, 113.7, 113.6, 113.6, 112.2, 109.4, 104.7, 99.9, 72.6, 55.7, 52.0, 42.7, 21.1, 20.0; IR (KBr) ν: 3381, 2943, 1679, 1621, 1566, 1450, 1283, 1075, 870, 810 cm−1. MS (m/z): HRMS (ESI) calcd for C31H25BrN2O4Na ([M + Na]+): 591.0890. Found: 591.0891.Ethyl-2-bromo-11-methoxy-6-(p-tolyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1j)
Yellow solid, 82%, mp. 217–218 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.89 (s, 1H, NH), 8.19 (d, J = 2.4 Hz, 1H, CH), 7.54 (d, J = 9.2 Hz, 1H, ArH), 7.15–7.07 (m, 5H, ArH), 7.03–7.02 (m, 1H, ArH), 6.81–6.75 (m, 3H, ArH), 4.56–4.52 (m, 1H, CH), 4.39–4.30 (m, 2H, OCH2), 3.77 (s, 3H, OCH3), 3.54–3.49 (m, 1H, CH), 3.08–3.04 (m, 2H, CH), 2.23 (s, 3H, CH3), 1.43 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 165.7, 154.2, 149.7, 138.8, 134.8, 131.7, 131.6, 130.8, 129.8, 129.3, 128.0, 127.8, 126.3, 125.9, 123.6, 119.8, 113.6, 113.6, 113.6, 112.0, 109.2, 105.0, 99.9, 72.6, 61.2, 55.7, 42.7, 21.1, 20.0, 14.4; IR (KBr) ν: 3327, 2944, 1682, 1529, 1487, 1286, 1078, 790, 739 cm−1. MS (m/z): HRMS (ESI) calcd for C32H28BrN2O4 ([M + H]+): 583.1227. Found: 583.1211.Methyl-2-chloro-11-methoxy-6-phenyl-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1k)
Yellow solid, 75%, mp. 218–220 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.80 (s, 1H, NH), 8.05 (d, J = 2.4 Hz, 1H, CH), 7.53 (d, J = 8.8 Hz, 1H, ArH), 7.36–7.31 (m, 3H, ArH), 7.23–7.21 (m, 2H, ArH), 7.04–7.02 (m, 2H, ArH), 6.85–6.79 (m, 3H, ArH), 4.37–4.31 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.58–3.52 (m, 1H, CH), 3.09–3.04 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 165.9, 154.2, 149.2, 137.9, 131.8, 131.4, 130.6, 129.3, 129.2, 127.8, 126.5, 126.3, 125.9, 125.9, 125.2, 123.0, 119.2, 113.6, 113.6, 112.3, 109.4, 104.8, 100.0, 72.8, 55.7, 52.1, 42.7, 20.0; IR (KBr) ν: 3448, 3352, 2944, 1678, 1623, 1564, 1490, 1368, 1083, 806, 743 cm−1. MS (m/z): HRMS (ESI) calcd for C30H23ClN2O4Na ([M + Na]+): 533.1239. Found: 533.1243.Methyl-2-chloro-6-(4-chlorophenyl)-11-methoxy-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1l)
Yellow solid, 76%, mp. 231–232 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.80 (s, 1H, NH), 8.06 (d, J = 2.4 Hz, 1H, CH), 7.53 (d, J = 9.2 Hz, 1H, ArH), 7.40 (d, J = 8.4 Hz, 2H, ArH), 7.22 (d, J = 8.4 Hz, 2H, ArH), 6.86–6.79 (m, 3H, ArH), 4.35–4.32 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.59–3.55 (m, 1H, CH), 3.10–3.05 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 165.8, 154.2, 149.0, 136.8, 134.1, 131.8, 131.5, 130.1, 129.7, 129.3, 129.3, 126.6, 126.3, 126.1, 125.9, 125.3, 122.9, 119.3, 113.7, 112.3, 109.5, 104.8, 99.9, 71.9, 55.7, 52.1, 42.7, 20.0; IR (KBr) ν: 3385, 2897, 1678, 1621, 1568, 1489, 1325, 871, 813, 733 cm−1. MS (m/z): HRMS (ESI) calcd for C30H22Cl2N2O4Na ([M + Na]+): 567.0849. Found: 567.0861.Methyl-6-(2-chlorophenyl)-11-methoxy-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1m)
Yellow solid, 72%, mp. 224–226 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.81 (s, 1H, NH), 7.97–7.94 (m, 1H, CH), 7.59–7.53 (m, 2H, ArH), 7.36–7.32 (m, 1H, ArH), 7.17–7.13 (m, 1H, ArH), 7.06–6.94 (m, 4H, ArH), 6.81–6.74 (m, 2H, ArH), 4.25–4.19 (m, 1H, CH), 3.97 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 3.44–3.39 (m, 1H, CH), 3.07–3.04 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 166.3, 154.2, 149.9, 134.3, 133.5, 131.7, 131.4, 130.7, 129.9, 128.7, 127.9, 127.2, 126.4, 126.0, 125.6, 122.4, 121.1, 117.6, 114.3, 113.6, 113.5, 109.1, 104.8, 99.9, 69.8, 55.7, 52.1, 42.6, 20.0; IR (KBr) ν: 3325, 2940, 1680, 1621, 1574, 1357, 1261, 1079, 811, 759, 630 cm−1. MS (m/z): HRMS (ESI) calcd for C30H24ClN2O4 ([M + H]+): 511.1419. Found: 511.1417.Methyl-6-(4-bromophenyl)-2-chloro-11-methoxy-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1n)
Yellow solid, 89%, mp. 237–239 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.80 (s, 1H, NH), 8.05 (d, J = 2.4 Hz, 1H, CH), 7.53 (d, J = 9.2 Hz, 1H, ArH), 7.17–7.15 (m, 2H, ArH), 7.06–7.03 (m, 2H, ArH), 6.86–6.79 (m, 3H, ArH), 4.36–4.30 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 3.61–3.53 (m, 1H, CH), 3.10–3.05 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 165.7, 154.2, 149.7, 138.8, 134.8, 131.7, 131.6, 130.8, 129.8, 129.3, 128.0, 127.8, 126.3, 125.9, 123.6, 119.8, 113.6, 113.6, 113.6, 112.0, 109.2, 105.0, 99.9, 72.6, 61.2, 55.7, 42.7, 21.1, 20.0, 14.4; IR (KBr) ν: 3385, 2939, 1678, 1623, 1568, 1488, 1325, 1284, 870, 810, 619 cm−1. MS (m/z): HRMS (ESI) calcd for C30H23BrClN2O4 ([M + H]+): 589.0524. Found: 589.0528; found: 605.0717.Methyl-2-bromo-11-methoxy-6-(2-nitrophenyl)-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1o)
Yellow solid, 82%, mp. 231–233 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.85 (s, 1H, NH), 8.14 (d, J = 2.8 Hz, 1H, CH), 7.99 (d, J = 7.6 Hz, 1H, ArH), 7.59–7.49 (m, 3H, ArH), 7.35 (s, 1H, ArH), 7.15 (d, J = 8.4 Hz, 1H, ArH), 7.06 (s, 1H, ArH), 6.82 (d, J = 7.6 Hz, 2H, ArH), 6.61 (d, J = 8.4 Hz, 1H, ArH), 4.44–4.40 (m, 1H, CH), 3.96 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.72–3.68 (m, 1H, CH), 3.12–3.09 (m, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 165.7, 154.2, 149.1, 149.0, 133.6, 132.0, 131.8, 131.1, 130.7, 129.6, 129.5, 128.3, 128.2, 126.2, 125.9, 125.3, 123.4, 119.4, 114.6, 113.8, 113.7, 113.3, 109.7, 104.8, 100.0, 68.4, 55.7, 52.1, 42.8, 20.0; IR (KBr) ν: 3327, 2944, 1682, 1619, 1529, 1487, 1356, 1078, 790, 739, 624 cm−1. MS (m/z): HRMS (ESI) calcd for C30H23BrN3O6 ([M + H]+): 600.0765. Found: 600.0749.Methyl-2-bromo-6-(4-chlorophenyl)-11-methoxy-6,8,9,14-tetrahydrochromeno[4′,3′:2,3]indolizino[8,7-b]indole-15-carboxylate (1p)
Yellow solid, 87%, mp. 227–228 °C; 1H NMR (400 MHz, CDCl3) δ: 10.87 (s, 1H, NH), 8.05 (s, 1H, ArH), 7.37–7.35 (m, 1H, ArH), 7.28–7.26 (m, 2H, ArH), 7.15–7.09 (m, 3H, ArH), 6.91–6.90 (m, 2H, ArH), 6.71–6.69 (m, 1H, ArH), 6.32 (s, 1H, CH), 4.03 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.73–3.63 (m, 2H, CH2), 3.12–3.05 (m, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ: 166.8, 154.3, 149.1, 135.5, 135.2, 132.7, 131.2, 129.3, 129.1, 129.0, 128.4, 126.5, 125.8, 122.8, 119.2, 114.4, 113.7, 113.2, 112.7, 108.0, 105.0, 99.6, 72.9, 55.7, 51.5, 42.8, 20.2; IR (KBr) ν: 3321, 2939, 1682, 1619, 1487, 1346, 1077, 739 cm−1. MS (m/z): HRMS (ESI) calcd for C30H23BrClN2O4 ([M + H]+): 589.0524. Found: 589.0527.General procedure for the synthesis of tetrahydroindolizino[8,7-b]indoles
A solution of tryptamine (1.0 mmol) and alkyl propiolate (1.2 mmol) in absolute ethanol (10.0 mL) was stirred at room temperature for about half hour. Then, β-nitroalkene (1.0 mmol) was added. The solution was heated at 60–70 °C for six hours. After cooling trifluoromethanesulfonic acid (25% mol) was added. The resulting solution was refluxed at 80 °C for additional six hour. After removing the solvent by rotatory evaporation at reduced pressure, the residue was subjected to chromatography with ethyl acetate and light petroleum (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give pure product for analysis.
5) as eluent to give pure product for analysis.
Methyl-2-(4-chlorophenyl)-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2a)
Light yellow solid, 79%, mp. 176–178 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.93 (s, 1H, NH), 7.64 (d, J = 8.0 Hz, 1H, ArH), 7.55 (d, J = 7.6 Hz, 1H, ArH), 7.42–7.37 (m, 4H, ArH), 7.16–7.12 (m, 2H, ArH), 7.08–7.04 (m, 1H, ArH), 4.24 (t, J = 7.2 Hz, 2H, CH), 3.68 (s, 3H, OCH3), 3.12 (t, J = 7.2 Hz, 2H, CH); 13C NMR (100 MHz, DMSO-d6) δ: 166.3, 136.3, 134.6, 131.2, 131.0, 130.3, 128.0, 126.4, 125.9, 124.2, 123.6, 122.6, 119.9, 118.6, 112.8, 108.8, 106.9, 51.5, 45.5, 20.4; IR (KBr) ν: 3374, 2932, 2839, 1688, 1611, 1529, 1456, 1364, 1127, 1033, 927, 822, 747 cm−1; MS (m/z): HRMS (ESI) calcd for C22H18ClN2O2 ([M + H]+): 377.1051. Found: 377.1063.Methyl-2-(4-(tert-butyl)phenyl)-8-methoxy-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2b)
Light yellow solid, 91%, mp. 183–185 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.79 (s, 1H, NH), 7.53 (d, J = 8.8 Hz, 1H, ArH), 7.36 (d, J = 8.0 Hz, 2H, ArH), 7.29 (d, J = 8.0 Hz, 2H, ArH), 7.05–7.03 (m, 2H, ArH), 6.77 (d, J = 8.8 Hz, 1H, ArH), 4.22 (t, J = 7.2 Hz, 2H, CH), 3.79 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 3.12 (t, J = 7.2 Hz, 2H, CH), 1.32 (s, 9H, C(CH3)3); 13C NMR (100 MHz, DMSO-d6) δ: 166.6, 154.1, 148.8, 132.7, 131.4, 130.2, 128.9, 127.1, 126.2, 125.4, 124.7, 123.2, 113.6, 113.0, 108.3, 106.8, 99.9, 55.7, 51.5, 45.5, 34.6, 31.6, 20.5; MS (m/z): HRMS (ESI) calcd for C27H29N2O3 ([M + H]+): 429.2173. Found: 429.2185; IR (KBr) ν: 3390, 3013, 2968, 1685, 1529, 1456, 1364, 1306, 1250, 1033, 927, 822, 747 cm−1.Methyl-8-methoxy-2-(p-tolyl)-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2c)
Grew solid, 85%, mp. 196–198 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.79 (s, 1H, NH), 7.53 (d, J = 8.8 Hz, 1H, ArH), 7.24 (d, J = 8.0 Hz, 2H, ArH), 7.15 (d, J = 8.0 Hz, 2H, ArH), 7.04–7.02 (m, 2H, ArH), 6.79–6.76 (m, 1H, ArH), 4.22 (t, J = 7.2 Hz, 2H, CH), 3.79 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.12 (t, J = 7.2 Hz, 2H, CH), 2.33 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.6, 154.1, 135.5, 132.7, 131.4, 130.2, 129.1, 128.6, 127.0, 126.2, 125.4, 123.1, 113.6, 113.0, 108.3, 106.8, 99.9, 55.7, 51.4, 45.5, 21.1, 20.5; IR (KBr) ν: 3386, 2932, 2839, 1688, 1611, 1529, 1456, 1364, 1306, 1250, 1176, 1033, 927, 822, 747 cm−1. MS (m/z): HRMS (ESI) calcd for C24H23N2O3 ([M + H]+): 387.1703. Found: 387.1698.Ethyl-2-(4-(tert-butyl)phenyl)-8-methoxy-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2d)
Yellow solid, 83%, mp. 182–184 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.81 (s, 1H, NH), 7.52 (d, J = 8.8 Hz, 1H, ArH), 7.35 (d, J = 8.0 Hz, 2H, ArH), 7.28 (d, J = 8.0 Hz, 2H, ArH), 7.03 (s, 2H, ArH), 6.77 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H, ArH), 4.22 (t, J = 7.2 Hz, 2H, CH), 4.14 (dd, J1 = 14 Hz, J2 = 7.6 Hz, 2H, OCH2), 3.79 (s, 3H, OCH3), 3.12 (t, J = 7.2 Hz, 2H, CH), 1.31 (s, 9H, C(CH3)3), 1.01 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.2, 154.1, 148.8, 132.8, 131.4, 130.1, 129.3, 127.1, 126.2, 125.6, 124.5, 122.9, 113.6, 112.9, 108.1, 107.3, 99.9, 60.1, 55.7, 45.5, 34.6, 31.6, 20.5, 14.0; IR (KBr) ν: 3452, 3027, 2987, 2968, 1679, 1580, 1364, 1306, 1250, 1176, 1127, 1033, 927, 822, 747 cm−1. MS (m/z): HRMS (ESI) calcd for C28H31N2O3 ([M + H]+): 443.2329. Found: 443.2431.Methyl-3-methyl-2-(p-tolyl)-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2e)
Yellow solid, 69%, mp. 193–195 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.97 (s, 1H, NH), 7.61 (d, J = 8.0 Hz, 1H, ArH), 7.54 (d, J = 7.6 Hz, 1H, ArH), 7.18–7.10 (m, 5H, ArH), 7.07–7.03 (m, 2H, ArH), 4.14 (t, J = 7.2 Hz, 2H, CH), 3.55 (s, 3H, OCH3), 3.15 (t, J = 7.2 Hz, 2H, CH), 2.35 (s, 1H, CH3), 2.13 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 166.7, 136.2, 135.3, 133.0, 130.6, 129.1, 128.7, 128.4, 126.9, 125.9, 122.4, 122.3, 119.8, 118.5, 112.6, 107.9, 107.5, 51.3, 42.5, 21.2, 20.2, 10.7; IR (KBr) ν: 3381, 3019, 2922, 1688, 1608, 1540, 1484, 1359, 1233, 1034, 929, 853, 816, 754 cm−1. MS (m/z): HRMS (ESI) calcd for C24H23N2O2 ([M + H]+): 371.1754. Found: 371.1765.Methyl-8-methoxy-3-methyl-2-(p-tolyl)-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2f)
Yellow solid, 82%, mp. 223–225 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.84 (s, 1H, NH), 7.51 (d, J = 8.8 Hz, 1H, ArH), 7.17 (d, J = 7.6 Hz, 1H, ArH), 7.11 (d, J = 8.0 Hz, 1H, ArH), 7.04 (s, 1H, ArH), 6.77–6.75 (m, 5H, ArH), 4.14 (t, J = 7.2 Hz, 2H, CH), 3.79 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.13 (t, J = 7.2 Hz, 2H, CH), 2.35 (s, 3H, CH3), 2.13 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 166.7, 154.1, 135.3, 133.0, 131.3, 130.6, 129.0, 128.9, 128.4, 127.4, 126.2, 122.3, 113.4, 112.8, 107.7, 107.3, 99.9, 55.7, 51.3, 42.6, 21.2, 20.2, 10.7; IR (KBr) ν: 3376, 3071, 3019, 2919, 2841, 1669, 1591, 1356, 1226, 1106, 946, 889, 837, 743 cm−1. MS (m/z): HRMS (ESI) calcd for C25H25N2O3 ([M + H]+): 401.1860. Found: 401.1871.Methyl-2-(4-bromophenyl)-8-methoxy-3-methyl-6,11-dihydro-5H-indolizino[8,7-b]indole-1-carboxylate (2g)
Yellow solid, 77%, mp. 208–210 °C; 1H NMR (400 MHz, DMSO-d6) δ: 10.84 (s, 1H, NH), 7.56–7.51 (m, 3H, ArH), 7.19 (d, J = 8.4 Hz, 2H, ArH), 7.05–7.04 (m, 1H, ArH), 6.78–6.75 (m, 1H, ArH), 4.15 (t, J = 7.2 Hz, 2H, CH2), 3.79 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.14 (t, J = 7.2 Hz, 2H, CH), 2.14 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 166.4, 154.1, 135.4, 132.9, 131.4, 130.7, 129.2, 129.2, 127.1, 126.1, 121.1, 119.7, 113.5, 113.0, 108.0, 107.0, 99.9, 55.7, 51.4, 42.6, 20.2, 10.6; MS (m/z): HRMS (ESI) calcd for C24H22BrN2O3 ([M + H]+): 465.0808. Found: 465.0816; IR (KBr) ν: 3382, 3068, 2982, 2930, 2899, 1732, 1578, 1538, 1459, 1408, 1376, 1230, 1171, 905, 882, 817, 747 cm−1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank Analysis and Test Center of Yangzhou University providing instruments for analysis.References
- (a) J. Yang, X. Z. Wearing, P. W. Le Quesne, J. R. Deschamps and J. M. Cook, J. Nat. Prod., 2008, 71, 1431–1440 CrossRef PubMed; (b) C. Sánchez, C. Méndez and J. A. Salas, Nat. Prod. Rep., 2006, 23, 1007–1045 RSC; (c) M. Martín-Martínez, N. De la Figuera, M. Latorre, R. Herranz, M. T. García-López, E. Cenarruzabeitia, J. Del Río and R. González-Muñiz, J. Med. Chem., 2000, 43, 3770–3777 CrossRef; (d) M. Martín-Martínez, N. De la Figuera, M. LaTorre, M. T. García-López, E. Cenarruzabeitia, J. Del Río and R. González-Muñiz, J. Med. Chem., 2005, 48, 7667–7674 CrossRef PubMed; (e) A. Hormann, B. Chaudhuri and H. Fretz, Bioorg. Med. Chem., 2001, 9, 917–921 CrossRef PubMed.
- (a) R. Chaniyara, S. Tala, C. W. Chen, X. G. Zang, R. Kakadiya, L. F. Lin, C. H. Chen, S. I. Chien, T. C. Chou and T. H. Tsai, et al., J. Med. Chem., 2013, 56, 1544–1563 CrossRef PubMed; (b) R. Skouta, M. Hayano, K. Shimada and B. R. Stockwell, Bioorg. Med. Chem. Lett., 2012, 22, 5707–5713 CrossRef PubMed; (c) H. Dueckert, V. Pries, V. Khedkar, S. Menninger, H. Bruss, A. W. Bird, Z. Maliga, A. Brockmeyer, P. Janning and A. Hyman, et al., Nat. Chem. Biol., 2012, 8, 179–184 CrossRef PubMed; (d) W. L. Huang, T. Zuo, H. W. Jin, Z. M. Liu, Z. J. Yang, X. H. Yu, L. R. Zhang and L. H. Zhang, Mol. Diversity, 2013, 17, 221–243 CrossRef PubMed; (e) S. M. Chang, W. Christian, M. H. Wu, T. L. Chen, Y. W. Lin, C. S. Suen, H. B. Pidugu, D. Detroja, A. Shah and M. J. Hwang, et al., Eur. J. Med. Chem., 2017, 127, 235–249 CrossRef PubMed.
- (a) J. P. Kutney, N. Abdurahman, C. Gletsos, P. Le Quesne, E. Piers and I. Vlattas, J. Am. Chem. Soc., 1970, 92, 1727–1735 CrossRef PubMed; (b) H.-J. Knölker and S. Agarwal, Synlett, 2004, 1767–1768 CrossRef; (c) M. E. Zhidkov, O. V. Baranova, N. S. Kravchenko and S. V. Dubovitskii, Tetrahedron Lett., 2010, 51, 6498–6499 CrossRef; (d) Y. Dai, W. Zhang, K. Wang, W. Wang and W. Zhang, Tetrahedron, 2013, 69, 1912–1918 CrossRef.
- (a) S. Agarwal and H.-J. Knoelker, Org. Biomol. Chem., 2004, 2, 3060–3062 RSC; (b) H.-J. Knoelker and S. Agarwal, Synlett, 2004, 1767–1768 CrossRef; (c) J. Schuette, F. Kilgenstein, M. Fischer and U. Koert, Eur. J. Org. Chem., 2014, 5302–5311 CrossRef; (d) D. Chandrasekhar, S. Borra, J. S. Kapure, G. S. Shivaji, G. Srinivasulu and R. A. Maurya, Org. Chem. Front., 2015, 2, 1308–1312 RSC; (e) S. Nekkanti, N. P. Kumar, P. Sharma, A. Kamal, F. M. Nachtigall, O. Forero-Doria, L. S. Santos and N. Shankaraiah, RSC Adv., 2016, 6, 2671–2677 RSC; (f) M. D. Matveeva, T. N. Borisova, A. A. Titov, L. V. Anikina, S. V. Dyachenko, G. S. Astakhov, A. V. Varlamov and L. G. Voskressensky, Synthesis, 2017, 49, 5251–5257 CrossRef.
- (a) R. Crigg, P. Myers, A. Somasunderam and V. Sridharan, Tetrahedron, 1992, 48, 9735–9744 CrossRef; (b) M. E. Zhidkov, O. V. Baranova, N. S. Kravchenko and S. V. Dubovitskii, Tetrahedron Lett., 2010, 51, 6498–6499 CrossRef; (c) I. Deb and D. Seidel, Tetrahedron Lett., 2010, 51, 2945–2947 CrossRef; (d) Y. S. Dai, W. X. Zhang, K. C. Wang, W. X. Wang and W. Zhang, Tetrahedron, 2013, 69, 1912–1918 CrossRef; (e) P. Ngernmeesri, S. Soonkit, A. Konkhum and B. Kongkathip, Tetrahedron Lett., 2014, 55, 1621–1624 CrossRef; (f) D. Basavaiah, B. Lingaiah, G. C. Reddy and B. C. Sahu, Eur. J. Org. Chem., 2016, 2398–2403 CrossRef.
- (a) H. Wasserman, R. Frechette, T. Oida and J. Van Duzer, J. Org. Chem., 1989, 54, 6012–6014 CrossRef; (b) G. Poissonnet, M. Theret-Bettiol and R. Dodd, J. Org. Chem., 1996, 61, 2273–2282 CrossRef; (c) B. V. S. Reddy, M. R. Reddy, Y. G. Rao, J. S. Yadav and B. Sridhar, Org. Lett., 2013, 15, 464–467 CrossRef PubMed; (d) B. C. Loosley, R. J. Andersen and G. R. Dake, Org. Lett., 2013, 15, 1152–1154 CrossRef PubMed; (e) Q. Cai, D. K. Li, R. R. Zhou, W. M. Shu, Y. D. Wu and A. X. Wu, Org. Lett., 2016, 18, 1342–1345 CrossRef PubMed.
- (a) E. D. Cox and J. M. Cook, Chem. Rev., 1995, 95, 1797–1842 CrossRef; (b) B. E. Maryanoff, H. Zhang, J. H. Cohen, I. J. Turchi and C. A. Maryanoff, Chem. Rev., 2004, 104, 1431–1628 CrossRef PubMed; (c) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311–2352 CrossRef PubMed.
- (a) X. Y. Wu, X. Y. Dai, L. L. Nie, H. H. Fang, J. Chen, Z. J. Ren, W. G. Cao and G. Zhao, Chem. Commun., 2010, 46, 2733–2735 RSC; (b) H. H. Fang, X. Y. Wu, L. , L. Nie, X. Y. Dai, J. Chen, W. G. Cao and G. Zhao, Org. Lett., 2010, 12, 5366–5369 CrossRef PubMed; (c) Z. C. Jin, H. C. Huang, W. J. Li, X. Y. Luo, X. M. Liang and J. X. Ye, Adv. Synth. Catal., 2011, 353, 343–348 CrossRef; (d) H. L. Zhou, J. B. Ling and P. F. Xu, J. Org. Chem., 2012, 77, 7737–7743 CrossRef PubMed.
- (a) U. Rosentreter, L. Born and J. Kurz, J. Org. Chem., 1986, 51, 1165–1171 CrossRef; (b) H. H. Wasserman, R. Frechette, T. Oida and J. H. Van Duzer, J. Org. Chem., 1989, 54, 6012–6014 CrossRef; (c) P. D. Bailey, I. D. Collier, S. P. Hollinshead, M. H. Moore, K. M. Morgan, D. I. Smith and J. M. Vernon, J. Chem. Soc., Chem. Commun., 1994, 1559–1560 RSC; (d) X. Y. Wu, X. Y. Dai, H. H. Fang, L. L. Nie, J. Chen, W. G. Cao and G. Zhao, Chem.–Eur. J., 2011, 17, 10510–10514 CrossRef PubMed; (e) V. Eschenbrenner-Lux, H. Dückert, V. Khedkar, H. Bruss, H. Waldmann and K. V. Frank, Chem.–Eur. J., 2013, 19, 2294–2304 CrossRef PubMed.
- (a) S. P. Govek and L. E. Overman, J. Am. Chem. Soc., 2001, 123, 9468–9469 CrossRef PubMed; (b) G. C. Condie and J. Bergman, Eur. J. Org. Chem., 2004, 1286–1297 CrossRef; (c) A. S. Karpov, F. Rominger, T. J. J. Mueller and A. S. Karpov, Org. Biomol. Chem., 2005, 3, 4382–4391 RSC; (d) S. P. Chavan, P. Sharma, S. Rasapalli and U. R. Kalkote, Tetrahedron Lett., 2006, 47, 9301–9303 CrossRef; (e) F. Volz and N. Krause, Org. Biomol. Chem., 2007, 5, 1519–1521 RSC.
- (a) L. Zhang, L. Chang, H. W. Hu, H. Q. Wang, Z. J. Yao and S. Z. Wang, Chem.–Eur. J., 2014, 20, 2925–2932 CrossRef PubMed; (b) L. G. Voskressensky, T. N. Borisova, T. M. Chervyakova, A. A. Titov, A. V. Kozlov, E. A. Sorokina, R. Samavati and A. V. Varlamov, Chem. Heterocycl. Compd., 2014, 50, 658–669 CrossRef; (c) S. Mantenuto, S. Lucarini, M. De Santi, G. Piersanti, G. Brandi, G. Favi and F. Mantellini, Eur. J. Org. Chem., 2016, 3193–3199 CrossRef; (d) D. Singh, N. Devi, V. Kumar, C. C. Malakar, S. Mehra, R. K. Rawal, B. S. Kaith and V. Singh, RSC Adv., 2016, 6, 88066–88076 RSC; (e) Y. G. Suh, C. J. Lim, J. H. Sim, J. K. Lee, Y. J. Surh and S. M. Paek, J. Org. Chem., 2017, 82, 1464–1470 CrossRef PubMed; (f) S. Mantenuto, C. Ciccolini, S. Lucarini, G. Piersanti, G. Favi and F. Mantellini, Org. Lett., 2017, 19, 608–611 CrossRef PubMed.
- (a) L. L. Zhang, J. Sun and C. G. Yan, Tetrahedron, 2013, 69, 5451–5459 CrossRef; (b) D. Zhu, J. Sun and C. G. Yan, RSC Adv., 2014, 4, 62817–62826 RSC.
- L. L. Zhang, J. Sun and C. G. Yan, Chin. J. Chem., 2013, 31, 1546–1550 CrossRef.
- (a) J. Sun, Y. Sun, H. Gong, Y. J. Xie and C. G. Yan, Org. Lett., 2012, 14, 5172–5175 CrossRef PubMed; (b) Y. Han, Y. Sun, J. Sun and C. G. Yan, Tetrahedron, 2012, 68, 8256–8260 CrossRef; (c) J. Sun, D. Zhu, H. Gong and C. G. Yan, Tetrahedron, 2013, 69, 10565–10572 CrossRef; (d) H. Gong, J. Sun and C. G. Yan, Synthesis, 2014, 46, 489–495 CrossRef; (e) H. Gao, J. Sun and C. G. Yan, J. Org. Chem., 2014, 79, 4131–4136 CrossRef PubMed; (f) J. Sun, L. Chen, H. Gong and C. G. Yan, Org. Biomol. Chem., 2015, 13, 5905–5917 RSC; (g) W. J. Yang, J. Zhang, J. Sun and C. G. Yan, Eur. J. Org. Chem., 2016, 5423–5428 CrossRef; (h) Y. Zhang, J. Sun, G. L. Shen and C. G. Yan, Org. Biomol. Chem., 2017, 15, 8072–8077 RSC.
- (a) J. W. Xie, L. P. Fan, H. Su, X. S. Li and D. C. Xu, Org. Biomol. Chem., 2010, 8, 2117–2122 RSC; (b) Z. W. Guo, X. S. Li, W. D. Zhu and J. W. Xie, Eur. J. Org. Chem., 2012, 6924–6932 CrossRef; (c) Y. Jia and D. M. Du, RSC Adv., 2013, 3, 1970–1975 RSC; (d) J. N. S. Rao and R. Raghunathan, Tetrahedron Lett., 2013, 54, 6568–6573 CrossRef; (e) V. Yu. Korotaev, A. Yu. Barkov, V. S. Moshkin, E. G. Matochkina, M. I. Kodess and V. Ya. Sosnovskikh, Tetrahedron, 2013, 69, 8602–8608 CrossRef; (f) Z. K. Fu, J. Y. Pan, D. C. Xu and J. W. Xie, RSC Adv., 2014, 4, 51548–51557 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all new compounds are available. CCDC 1e (1833962), 1h (1833963), 1i (1833964), 1p (1836518), 2b (1833965), 2e (1833966). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra05138k |
| This journal is © The Royal Society of Chemistry 2018 |