Open Access Article
Open Access Article8-Hydroxy-2-methylquinoline-modified H4SiW12O40: a reusable heterogeneous catalyst for acetal/ketal formation
Li-jun Liua,
Qing-jie Luana,
Jing Lua,
Dong-mei Lva,
Wen-zeng Duana,
Xu Wangb and
Shu-wen Gong *a
*a
aInstitute of Functional Organic Molecules and Materials, School of Chemistry and Chemical Engineering, Liaocheng University, No. 1 Hunan Road, Liaocheng, 252000, People's Republic of China. E-mail: gongshw@lcu.edu.cn
bCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, P. R. China
First published on 20th July 2018
Abstract
A heteropoly acid based organic hybrid heterogeneous catalyst, HMQ-STW, was prepared by combining 8-hydroxy-2-methylquinoline (HMQ) with Keggin-structured H4SiW12O40 (STW). The catalyst was characterized via elemental analysis, X-ray diffractometry (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), thermogravimetric analysis (TG) and potentiometric titration analysis. The catalytic performance of the catalyst was assessed in the ketalization of ketones with glycol or 1,2-propylene glycol. Various reaction parameters, such as the glycol to cyclohexanone molar ratio, catalyst dosage, reaction temperature and time, were systematically examined. HMQ-STW exhibited a relatively high yield of corresponding ketal, with 100% selectivity under the optimized reaction conditions. Moreover, catalytic recycling tests demonstrated that the heterogeneous catalyst exhibited high potential for reusability, and it was revealed that the organic modifier HMQ plays an important role in the formation of a heterogeneous system and the improvement of structural stability. These results indicated that the HMQ-STW catalyst is a promising new type of heterogeneous acid catalyst for the ketalization of ketones.
1. Introduction
The formation of acetals/ketals is valuable as a common method for the protection and isolation of carbonyl and polyol compounds.1 And acetals/ketals are often used as synthetic intermediates, and can be used as starting materials in the production of flavours, disinfectants and surfactants.1,2 Some glycerol acetals may also find applications as beneficial fuel additives, especially to boost the low temperature properties of biodiesel fuels.3,4 Mota5,6 once reported that solketal, when used as a fuel additive, can effectively increase the octane number of gasoline and reduce emissions resulting from fuel combustion.Acetals and ketals are generally prepared from carbonyl compounds and alcohols in the presence of acid catalysts. Commonly used catalysts generally include sulfuric acid, phosphoric acid, hydrochloric acid and p-toluenesulfonic acid.7,8 Although these homogeneous catalysts have remarkable catalytic activities, corrosion problems are inevitable and the synthetic process can hardly meet requirements to protect the environment. Therefore, it is desirable to investigate solid acid catalysts as an alternative to homogeneous catalysts with the above shortcomings.9 Several heterogeneous catalysts have been reported for the acetalization and ketalization of different carbonyl compounds, including montmorillonite clay,7 zeolites,10 metal(IV) phosphates,11 activated carbons,12 acidic resins such as Amberlyst,13 etc. Rat et al.14 once reported a comparison between the catalytic effects of AS-MES (arene sulfonic acid ethane-silica) and AS-SBA-15, indicating that the AS-MES material was more efficient than AS-SBA-15 for the acetalization of heptanal by 1-butanol at 75 °C. Recently, in order to meet the requirements of high activity and environmental protection, new types of catalysts for the formation of acetals and ketals have constantly sprung up.3,9,15
As a kind of eco-friendly material, heteropolyacids with a Keggin structure have been widely studied in recent decades. They can be used as a substitute for conventional acid catalysts in acid catalyzed reactions in the liquid phase, owing to their strong Brønsted acidity and uniform acidic sites.16–19 However, efficient heterogeneous catalysts based on heteropolyacids have to be obtained through “immobilization” or “solidification”, since they are highly soluble in polar compounds.16–20 Supporting heteropolyacids on suitable supports is a useful method for improving the catalytic performance in a heterogeneous reaction, and it also can resolve the problem of the small surface areas (<10 m2 g−1) of heteropolyacids.16,21 Ivanov22 once pointed out that neutral or acidic carriers were more suitable for the preparation of supported heteropolyacid-based catalysts. Therefore, neural alumina was selected to prepare a supported tungstophosphoric acid (HPW) catalyst, which was applied to the synthesis of acetals and/or ketals, and the yields of ketals and acetals could reach up to 60.5–86.7%.23 Ion exchange, i.e. the partial or total substitution of H+ in heteropolyacids with large cation ions such as Cs+ and Ag+, is another effective technique in heterogenisation.24–26 The obtained salts of heteropolyacids are obviously changed from the pore structures of pristine acids, with improved surface area and reusability properties. In addition, co-doped heteropolyacids and mixed salts of heteropolyacids have been developed for acid catalyzed reactions.27,28 Besides the inorganic cations mentioned above, many organic compounds, such as cyclodextrin, pyridine, 4,4′-bipyridine, aliphatic amines and quaternary ammonium salts, have been utilized to modify heteropolyacids.29–32 Park33 prepared 12-phosphotungstic acid-based complex catalysts modified via nitrogen-containing heterocycles, including imidazole, pyrazole and 1,2,4-triazole. And these three organic–inorganic hybrid catalysts exhibited good catalytic performance in propylene epoxidation. However, few studies are found to focus on the application of organic–inorganic heterogeneous catalysts in acetalization or ketalization.32
In this work, a new organic–inorganic material was prepared through the combination of 8-hydroxy-2-methylquinoline with H4SiW12O40. The material was systematically characterized and its potential was assessed as a heterogeneous catalyst for the synthesis of acetals or ketals. The reaction conditions were optimized and the catalyst reusability was also studied.
2. Experimental
2.1. Materials
Silicotungstic acid (H4SiW12O40, STW, AR), 8-hydroxy-2-methylquinoline (HMQ, AR, 98%), quinaldic acid (QA, AR, >98%), and nicotinic acid N-oxide (NAN, AR, >98%) were purchased from Energy Chemical Co., Ltd. All other organic reagents were of analytical grade and used as received without further purification.2.2. Material preparation
The preparation of HMQ modified STW was carried out in aqueous solution. In a typical procedure, STW (0.001 mol, 2.878 g) was dissolved in 20 mL of distilled water with vigorous stirring at 50 °C. Then HMQ solution (0.004 mol, 0.636 g, 0.4 mol L−1) was added dropwise, and the resulting solution was kept under stirring conditions for over 12 h at 50 °C. The resulting green-yellow precipitate was filtered and dried at 100 °C to obtain the final modified catalyst (HMQ-STW).Meanwhile, the other control materials were synthesized, changing HMQ to QA or NAN. The obtained catalysts were named QA-STW and NAN-STW, respectively.
2.3. Characterization
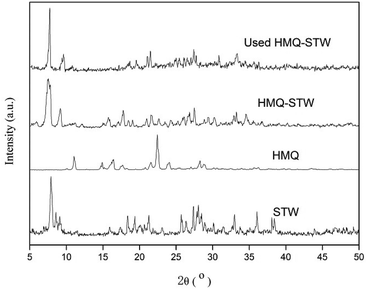
Elemental analysis (C, H and N) was performed using a PerkinElmer 2400 elemental analyzer.The XRD patterns of the catalyst were obtained using a XD-3 diffractometer (Beijing Purkinje General Instrument Co. Ltd.) with Cu Kα (1.542 Å) radiation at a scanning rate of 4° min−1 over a 2θ range from 5° to 50°.
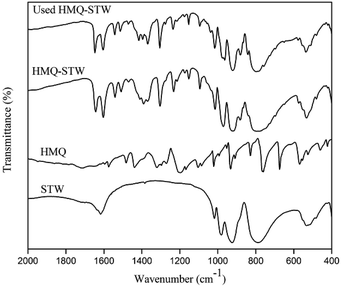
FT-IR spectra were scanned using a Thermo Nicolet 6700 FT-IR spectrometer using the KBr disc technique (4000–400 cm−1).
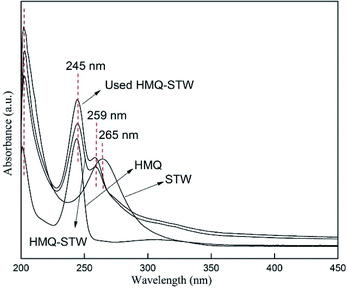
UV-vis absorption spectra were recorded using a T9CS UV-vis spectrophotometer (Persee Instrument Co., Ltd. Beijing, China), using a matched pair of quartz cells with a 1 cm path length. Ethanol was used as the background.
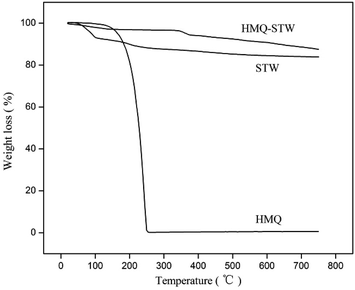
Thermogravimetric (TG) analysis was performed on a PerkinElmer-7 instrument at a heating rate of 5 °C min−1.
Scanning electron microscopy (SEM) images were obtained using a JSM6380LV type scanning electron microscope.
The mean pore diameter and pore volume of HMQ-STW were determined via N2 adsorption–desorption techniques performed at −196 °C using a Quantachrome Autosorb IQ-C instrument. Before measurements, samples were evacuated at 200 °C for 2 h.
The potentiometric titration method was used to test the acidity of the solid samples through an automatic potentiometric titrator with a pH composite electrode.34,35 The catalyst (0.05 g) was suspended in 15 mL of methanol and stirred for 2 h at room temperature. The turbid liquid was titrated with a solution of 0.05 mol L−1 n-butylamine in acetonitrile.
2.4. Catalytic tests
The catalytic properties of the catalyst were studied for the ketalization of glycol by cyclohexanone. The reaction was carried out in a three-neck flask equipped with a magnetic stirrer, a reflux condenser and Dean–Stark apparatus. In a typical procedure, certain amounts of cyclohexanone, glycol, cyclohexane and catalyst were mixed, where cyclohexane was the water-carrying agent. Under vigorous stirring, the reaction mixture was heated at different reaction temperatures (75 °C, 85 °C, 95 °C, 105 °C and 115 °C) over a certain time. Upon completion, the catalytic system was cooled down to room temperature immediately, and the catalyst was separated from the reaction mixture via filtration. The composition of the products was identified using gas chromatography-mass spectrometry (GC-MS, HP-5MS UI capillary column, 30 m × 0.25 mm, 0.25 μm film thickness).3. Results and discussion
3.1. Characterization
Elemental analysis (C, H and N) of an HMQ-STW sample was performed to confirm the element content. The analysis results show 13.35% as the weight percentage of C, with 1.53% for N and 1.18% for H; these are close to the calculated values of C, 13.67%, N, 1.59%, and H, 1.15%, according to the used amounts of HMQ and STW. Therefore, it could be preliminarily speculated that the theoretical formula of HMQ-STW is [(C10H9NO)]4H4SiW12O40.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in HMQ-STW heterocycles.39 The latter could be related to the protonated quinolone ring, which was based on the fact that this band has often been ascribed to the chemisorption of pyridine on Brønsted acid centers and that there is similarity between nitrogen atoms in quinoline rings and pyridine rings.38 These observations not only revealed the co-existence of HMQ and Keggin-structured SiW12O404− anions in HMQ-STW, but also illustrated the existence of electronic interaction between the metal oxygen clusters in the anions and HMQ. So it could be tentatively proposed that the combination of protonated HMQ as a cation with the SiW12O404− anion produces a new organic STW salt family.
C bonds in HMQ-STW heterocycles.39 The latter could be related to the protonated quinolone ring, which was based on the fact that this band has often been ascribed to the chemisorption of pyridine on Brønsted acid centers and that there is similarity between nitrogen atoms in quinoline rings and pyridine rings.38 These observations not only revealed the co-existence of HMQ and Keggin-structured SiW12O404− anions in HMQ-STW, but also illustrated the existence of electronic interaction between the metal oxygen clusters in the anions and HMQ. So it could be tentatively proposed that the combination of protonated HMQ as a cation with the SiW12O404− anion produces a new organic STW salt family.
3.2. Catalytic activity
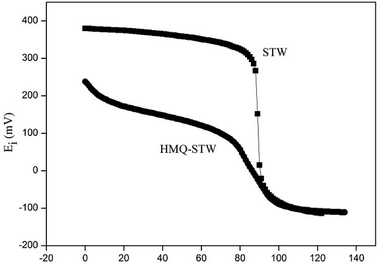
The reaction of cyclohexanone with glycol in the presence of the HMQ-STW catalyst was chosen as a model reaction of ketal formation. Parallel tests were performed using several control samples, including STW, HMQ, NAN-STW and QA-STW, under the same conditions as for HMQ-STW (Table 1). It was shown that the yield of cyclohexanone glycol ketal was only 38.2% in the absence of catalyst, and the ketal was the uniquely detected product. HMQ gave a low yield near to that of the blank experiment, indicating that HMQ has extremely low catalytic activity. In contrast, a high yield of 99.1% was obtained when STW was used as a catalyst. Nonetheless, HMQ and STW were homogeneous samples due to their good solubility in the reaction media. The yield of the modified sample, HMQ-STW, was 96.0%, which was slightly lower than that of pure STW. However, its advantage was that it was not a homogeneous catalyst any longer, which will simplify the recycling process for this catalyst. For the other two modified catalyst, NAN-STW and QA-STW, although they exhibited high catalytic activity with strong acidity, they were still homogeneous catalysts in the model reaction. So, HMQ-STW was selected as a heterogeneous catalyst to systematically study the catalytic performance under different conditions.| Catalyst | Catalyst solubility in the reaction | Potentiometric titration (Ei) | Yielda (%) |
|---|---|---|---|
a Reaction conditions: catalyst content = 7 wt% of cyclohexanone; mole ratio of glycol to cyclohexanone = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; reaction temperature = 105 °C; reaction time = 60 min; 5 mL of cyclohexane. 1; reaction temperature = 105 °C; reaction time = 60 min; 5 mL of cyclohexane. |
|||
| None | 38.2 | ||
| HMQ | Soluble | 150 | 40.5 |
| STW | Soluble | 380 | 99.1 |
| HMQ-STW | Insoluble | 238 | 96.0 |
| NAN-STW | Soluble | 376 | 98.6 |
| QA-STW | Soluble | 352 | 97.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
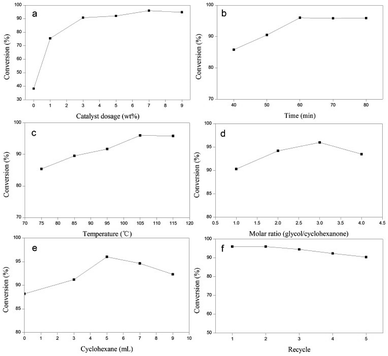
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 60 min at 105 °C with 5 mL of the water-carrying agent cyclohexane. As shown in Fig. 7a, the yield of ketal significantly increased with the addition of catalyst into the reaction system and a yield of 75.5% was obtained when the catalyst dosage was only 1 wt% (based on the quantity of cyclohexanone), which further indicated that HMQ-STW has excellent catalytic activity. With an increase in the catalyst dosage from 1 wt% to 7 wt%, the yield increased slightly and eventually reached 96.0% with a catalyst dosage of 7 wt%. However, the conversion decreased when the catalyst dosage increased to above 9 wt%, which may be related to excess catalyst resulting in the higher availability of active sites, which may facilitate the reverse reaction.44 Therefore, 7 wt% was chosen as the optimum catalyst dosage and was used for further investigation in the present research.
1 for 60 min at 105 °C with 5 mL of the water-carrying agent cyclohexane. As shown in Fig. 7a, the yield of ketal significantly increased with the addition of catalyst into the reaction system and a yield of 75.5% was obtained when the catalyst dosage was only 1 wt% (based on the quantity of cyclohexanone), which further indicated that HMQ-STW has excellent catalytic activity. With an increase in the catalyst dosage from 1 wt% to 7 wt%, the yield increased slightly and eventually reached 96.0% with a catalyst dosage of 7 wt%. However, the conversion decreased when the catalyst dosage increased to above 9 wt%, which may be related to excess catalyst resulting in the higher availability of active sites, which may facilitate the reverse reaction.44 Therefore, 7 wt% was chosen as the optimum catalyst dosage and was used for further investigation in the present research.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which indicated that HMQ-STW has the advantage of obtaining a high yield even at a lower molar ratio of glycol to cyclohexanone. With the molar ratio increasing to 3
1, which indicated that HMQ-STW has the advantage of obtaining a high yield even at a lower molar ratio of glycol to cyclohexanone. With the molar ratio increasing to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield slightly increased to 96.0%. However, higher ratios (e.g. 4
1, the yield slightly increased to 96.0%. However, higher ratios (e.g. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were found to negatively influence the reaction, which could possibly be attributed to insufficient mixing of the reactants with the heterogeneous catalyst when there was a lot of excessive glycol, as well as there being a decrease in catalyst concentration. Therefore, in order to obtain a high yield, the optimum molar ratio of glycol to cyclohexanone for this system was 3
1) were found to negatively influence the reaction, which could possibly be attributed to insufficient mixing of the reactants with the heterogeneous catalyst when there was a lot of excessive glycol, as well as there being a decrease in catalyst concentration. Therefore, in order to obtain a high yield, the optimum molar ratio of glycol to cyclohexanone for this system was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.3.3. Catalytic performance of HMQ-STW for other ketalization reactions
Since HMQ-STW had superior catalytic activity for the reaction of cyclohexanone with glycol, reactions with other substrates (Table 2) were attempted to evaluate the application of HMQ-STW. The results were inspiring. When benzaldehyde and acetophenone were respectively subjected to ketalization with glycol, high yields of the corresponding ketals were obtained as well (entries 1–2). Furthermore, the yields were even higher when there was an electron withdrawing group, such as nitro-, in the benzene ring of the aromatic aldehydes and ketones (entries 3–6). For studying the effects of diol variation, glycol was substituted with 1,2-propylene glycol (entries 7–13). High yields still were obtained for all the ketones used in these experiments. And it was worth pointing out that ketals were the only product, attesting to high selectivity in all these reactions. These results demonstrated that the HMQ-STW catalyst is a suitable catalyst for ketalization and has great potential for the catalytic synthesis of many types of ketals.| Entry | Ketone/aldehyde | Diol | Product | Yielda/% |
|---|---|---|---|---|
a Reaction conditions: catalyst content = 7 wt% of ketone; mole ratio of diol to ketone = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; reaction temperature = 105 °C; reaction time = 60 min; 5 mL of cyclohexane.b Amount of [PPSH]2.0HPW12O40 catalyst = 5 wt%; glycol/benzaldehyde ratio = 1.8; 12 mL of cyclohexane; reflux reaction for 3 h.c Amount of 15 wt% PW/MCM-41 catalyst = 0.3 g; pentaerythritol/benzaldehyde ratio = 1 1; reaction temperature = 105 °C; reaction time = 60 min; 5 mL of cyclohexane.b Amount of [PPSH]2.0HPW12O40 catalyst = 5 wt%; glycol/benzaldehyde ratio = 1.8; 12 mL of cyclohexane; reflux reaction for 3 h.c Amount of 15 wt% PW/MCM-41 catalyst = 0.3 g; pentaerythritol/benzaldehyde ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 20 mL of toluene/benzene; reflux reaction for 2 h.d Amount of Fe(HSO4)3 catalyst = 8 mol%; neopentyl glycol/benzaldehyde ratio = 1.2 3; 20 mL of toluene/benzene; reflux reaction for 2 h.d Amount of Fe(HSO4)3 catalyst = 8 mol%; neopentyl glycol/benzaldehyde ratio = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 3 mL of anhydrous CH2Cl2; reflux reaction for 45 min. 1; 3 mL of anhydrous CH2Cl2; reflux reaction for 45 min. |
||||
| 1 | Benzaldehyde | Glycol | 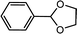 |
97.7 |
| 2 | Acetophenone | Glycol | 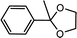 |
94.3 |
| 3 | p-Nitrobenzaldehyde | Glycol | 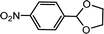 |
100 |
| 4 | m-Nitrobenzaldehyde | Glycol | 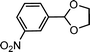 |
98.6 |
| 5 | p-Chlorobenzaldehyde | Glycol | 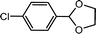 |
99.5 |
| 6 | p-Nitroacetophenone | Glycol | 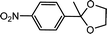 |
100 |
| 7 | Benzaldehyde | 1,2-Propylene glycol | 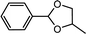 |
99.7 |
| 8 | Acetophenone | 1,2-Propylene glycol | 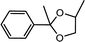 |
91.3 |
| 9 | Cyclohexanone | 1,2-Propylene glycol | 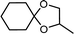 |
94.6 |
| 10 | p-Chlorobenzaldehyde | 1,2-Propylene glycol | 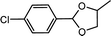 |
98.6 |
| 11 | p-Nitrobenzaldehyde | 1,2-Propylene glycol |  |
100 |
| 12 | p-Nitroacetophenone | 1,2-Propylene glycol |  |
100 |
| 13 | Acetone | 1,2-Propylene glycol |  |
100 |
| 14 (ref. 45) | Benzaldehyde | Glycol |  |
85.0b |
| 15 (ref. 46) | Benzaldehyde | Pentaerythritol | 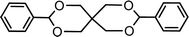 |
92.0c |
| 16 (ref. 47) | Benzaldehyde | Neopentyl glycol | 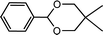 |
95.0d |
Compared with reported catalysts, including heteropolyacid-based ionic liquids and supported heteropolyacids (entries 14–16), HMQ-STW exhibited comparable catalytic activity under similar reaction conditions in the formation of acetals/ketals.
4. Conclusions
In summary, the facile synthesis of a novel organic salt of H4SiW12O40 with a Keggin structure was described in this paper. This material displayed excellent catalytic performance in the preparation of ketals from ketones with glycol or 1,2-propylene glycol. In all the reactions, not only could a high yield of the corresponding ketal be obtained, but HMQ-STW also exhibited excellent reusability and stability. The catalyst could be easily recycled via filtration, and its fine structure, including the Keggin unit structure, was not damaged during the reaction. Therefore, HMQ-STW was a promising catalyst for the ketalization reaction, and it can be preliminarily forecast that the catalyst will have potential for future development as an environmentally friendly heterogeneous solid acid catalyst.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the support of the Shandong Provincial Natural Science Foundation, China (ZR2017MB060, ZR2017MB052), the National Natural Science Foundation of China (21101086) and the Foundation of Liaocheng University (318011406).References
- K. Sato, T. Kishimoto, M. Morimoto, H. Saimotob and Y. Shigemasa, Tetrahedron Lett., 2003, 44, 8623–8625 CrossRef.
- V. Kannan, K. Sreekumar, A. Gil and M. A. Vicente, Catal. Lett., 2011, 141, 1118–1122 CrossRef.
- M. R. Nanda, Y. Zhang, Z. Yuan, W. Qin, H. S. Ghaziaskar and C. B. (C.) Xu, Renewable Sustainable Energy Rev., 2016, 56, 1022–1031 CrossRef.
- G. Chen, Y. Li, W. Zhao, K. Qu, Y. Ning and J. Zhang, Fuel Process. Technol., 2015, 133, 64–68 CrossRef.
- P. H. R. Silva, V. L. C. Gonçalves and C. J. A. Mota, Bioresour. Technol., 2010, 101, 6225–6229 CrossRef PubMed.
- C. J. A. Mota, C. X. A. da Silva, N. Rosenbach Jr, J. Costa and F. da Silva, Energy Fuels, 2010, 24, 2733–2736 CrossRef.
- M. N. Timofeeva, V. N. Panchenko, V. V. Krupskaya, A. Gil and M. A. Vicente, Catal. Commun., 2017, 90, 65–69 CrossRef.
- M. Gonçalves, R. Rodrigues, T. S. Galhardo and W. A. Carvalho, Fuel, 2016, 181, 46–54 CrossRef.
- A. R. Trifoi, P. Ş. Agachi and T. Pap, Renewable Sustainable Energy Rev., 2016, 62, 804–814 CrossRef.
- J. Kowalska-Kus, A. Held, M. Frankowski and K. Nowinska, J. Mol. Catal. A: Chem., 2017, 426, 205–212 CrossRef.
- S. M. Patel, U. V. Chudasama and P. A. Ganeshpure, J. Mol. Catal. A: Chem., 2003, 194, 267–271 CrossRef.
- R. Rodrigues, M. Goncalves, D. Mandelli, P. P. Pescarmona and W. A. Carvalho, Catal. Sci. Technol., 2014, 4, 2293–2301 RSC.
- J. Deutsch, A. Martin and H. Lieske, J. Catal., 2007, 245, 428–435 CrossRef.
- M. Rat, M. H. Zahedi-Niaki, S. Kaliaguine and T. O. Do, Microporous Mesoporous Mater., 2008, 112, 26–31 CrossRef.
- L. Chen, B. Nohair and S. Kaliaguine, Appl. Catal., A, 2016, 509, 143–152 CrossRef.
- L. C. Liu, B. Wang, Y. Y. Du and A. Borgna, Appl. Catal., A, 2015, 489, 32–41 CrossRef.
- W. H. Zhang, Y. Leng, D. R. Zhu, Y. J. Wu and J. Wang, Catal. Commun., 2009, 11, 151–154 CrossRef.
- Y. Leng, J. Wang, D. R. Zhu, X. Q. Ren, H. Q Ge and L. Shen, Angew. Chem., Int. Ed., 2009, 48, 168–171 CrossRef PubMed.
- Y. Zhou, G. J. Chen, Z. Y. Long and J. Wang, RSC Adv., 2014, 4, 42092–42113 RSC.
- J. Li, D. F. Li, J. Y. Xie, Y. Q. Liu, Z. J. Guo, Q. Wang, Y. Lyu, Y. Zhou and J. Wang, J. Catal., 2016, 339, 123–134 CrossRef.
- S. K. Bhorodwaj and D. K. Dutta, Appl. Catal., A, 2010, 378, 221–226 CrossRef.
- A. V. Ivanov, T. V. Vasina and V. D. Nissenbaum, Appl. Catal., A, 2004, 259, 65–72 CrossRef.
- J. Z. Gao, Y. X. Wei, X. M. Wang and W. Yang, Rare Met., 2007, 26, 152–157 CrossRef.
- K. M. Parida, S. Rana, S. Mallick and D. Rath, J. Colloid Interface Sci., 2010, 350, 132–139 CrossRef PubMed.
- S. H. Zhu, X. Q. Gao, F. Dong, Y. L. Zhu, H. Y. Zheng and Y. W. Li, J. Catal., 2013, 306, 155–163 CrossRef.
- M. Safariamin, S. Paul, K. Moonen, D. Ulrichts, F. Dumeignil and B. Katryniok, Catal. Sci. Technol., 2016, 6, 2129–2135 RSC.
- Q. Y. Zhang, F. F. Wei, Q. Li, J. S. Huang, Y. M. Fenga and Y. T. Zhang, RSC Adv., 2017, 7, 51090–51095 RSC.
- J. S. Santos, J. A. Dias, S. C. L. Dias, J. L. de Macedo, F. A. C. Garcia, L. S. Almeida and E. N. C. B. de Carvalho, Appl. Catal., A, 2012, 443–444, 33–39 CrossRef.
- In K. Song, M. S. Kaba and M. A. Barteau, J. Phys. Chem., 1996, 100, 17528–17534 CrossRef.
- Z. Y. Long, Y. Zhou, G. J. Chen, P. P. Zhao and J. Wang, Chem. Eng. J., 2014, 239, 19–25 CrossRef.
- H. Ge, Y. Leng, F. M. Zhang, C. J. Zhou and J. Wang, Catal. Lett., 2008, 124, 250–255 CrossRef.
- S. Sandesh, A. B. Halgeri and G. V. Shanbhag, J. Mol. Catal. A: Chem., 2015, 401, 73–80 CrossRef.
- H. J. Kim, Y. Jeon, J.-I. Park and Y.-G. Shul, J. Mol. Catal. A: Chem., 2013, 378, 232–237 CrossRef.
- R. Cid and G. Pecchi, Appl. Catal., 1985, 14, 15–21 CrossRef.
- L. R. Pizzio, P. G. Vázquez, C. V. Cáceres and M. N. Blanco, Appl. Catal., A, 2003, 256, 125–139 CrossRef.
- G. F. Chen, J. Li, X. G. Yang and Y. Wu, Appl. Catal., A, 2006, 310, 16–23 CrossRef.
- K. Yan, G. S. Wu, J. L. Wen and A. C. Chen, Catal. Commun., 2013, 34, 58–63 CrossRef.
- L. S. Felices, P. Vitoria, J. M. Gutiérrez-Zorrilla, S. Reinoso, J. Etxebarria and L. Lezama, Chem.–Eur. J., 2004, 10, 5138–5146 CrossRef PubMed.
- Z. Y. Long, G. J. Chen, S. Liu, F. M. Huang, L. M. Sun, Z. L. Qin, Q. Wang, Y. Zhou and J. Wang, Chem. Eng. J., 2018, 334, 873–881 CrossRef.
- J. Gong, X. J. Cui, Z. W. Xie, S. G. Wang and L. Y. Qu, Synth. Met., 2002, 129, 187–192 CrossRef.
- S. Shanmugam, B. Viswanathan and T. K. Varadarajan, J. Membr. Sci., 2006, 275, 105–109 CrossRef.
- Y. H. Kong, X. M. Cheng, H. L. An, X. Q. Zhao and Y. J. Wang, Chin. J. Chem. Eng., 2018, 26, 330–336 CrossRef.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef PubMed.
- A. N. Chermahini and M. Nazeri, Fuel Process. Technol., 2017, 167, 442–450 CrossRef.
- X. X. Han, W. Yan, K. K. Chen, C. T. Hung, L. L. Liu, P. H. Wu, S. J. Huang and S. B. Liu, Appl. Catal., A, 2014, 485, 149–156 CrossRef.
- B. R. Jermy and A. Pandurangan, Appl. Catal., A, 2005, 295, 185–192 CrossRef.
- H. Eshghi, M. Rahimizadeh and S. Saberi, Catal. Commun., 2008, 9, 2460–2466 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |