 Open Access Article
Open Access ArticleCombing δ15N and δ18O to identify the distribution and the potential sources of nitrate in human-impacted watersheds, Shandong, China†
Baoshan Yangab,
Hui Wang*ab,
Yingkui Jiangab,
Fang Dongab,
Xinhua Hec and
Xiaoshuang Laia
aSchool of Water Conservancy and Environment, University of Jinan, Jinan 250022, China. E-mail: hwang_118@163.com; Fax: +86 053182769233; Tel: +86 82767237
bKey Laboratory of Water Resources and Environmental Engineering in Universities of Shandong, Jinan 250022, China
cSchool of Plant Biology, University of Western Australia, Crawley, WA 6009, Australia
First published on 26th June 2018
Abstract
Identifying the anthropogenic and natural sources of nitrate emissions contributing to surface water continues to be an enormous challenge. It is necessary to control the water quality in the watershed impacted by human disturbance. In this study, water chemical parameters including nitrate (NO3−) concentrations, δ15N–NO3−, δ18O–NO3−, and δ18O–H2O were analyzed to investigate the contamination and sources of NO3− in two watershed rivers (Jinyun, JYN and Jinyang, JYA), Jinan, Shandong, China. Results indicated NO3− concentrations in the JYN were significantly higher than those in the JYA (P < 0.05), probably because of high N input of the extensive farmlands or orchards in the drainage basin. δ15N–NO3− and δ18O–NO3−, associated with Cl−, indicated that nitrate-nitrogen (NO3−–N) was not derived from atmospheric deposition but came principally from manure/sewage and soil organic matter in these two watersheds. The microbial nitrification took place in the nitrate of manure/sewage and soil nitrate. The combination of NO3− concentration and nitrogen and oxygen isotope suggested that NO3− had undergone microbial denitrification after entering the rivers. Furthermore, NO3− concentrations had significant temporal and spatial variation highlighting differential sources and fates. These results expand our understanding of mechanisms driving NO3− retention and transport and provide strategies in managing NO3− contamination in different land use watersheds around the world.
1. Introduction
Nitrate (NO3−) is a widespread contamination source which often poses potential adverse health effects to humans through surface and underground water systems.1,2 Higher concentrations have occurred due to mixed land use and anthropogenic activities, resulting in acidification, eutrophication, drinking water degradation and human health risks.3–6 There are complicated sources of NO3− under different watershed conditions. Anthropogenic N is becoming the dominant contributor to the water quality impairments with potential sources of NO3− contamination from N deposition, septic decomposition, animal waste, synthetic fertilizer, and decaying organic matter.7,8 A worldwide analysis suggests that NO3− exports from watersheds are highly variable, and that a broader array of studies is more important for global-scale analyses.9–11 Identifying the distribution and sources of NO3− in rivers across land use and hydrologic duration will be critical in prioritizing effective NO3− reduction methods and providing an effective watershed management strategy.Although the concentration of NO3− and the N-isotopic signature in NO3− have been successfully applied in various case studies of surface and ground waters in widespread watersheds around the world to identify the sources of nitrate-nitrogen (NO3−–N), the wide range and overlapping values for some sources make it difficult to identify the source and transformation of NO3− in surface water and groundwater.12–15 It is promising to employ δ18O–NO3− and δ18O–H2O associated with δ15N–NO3− to differentiate the sources according to the well-established linear model between δ18O–NO3− ranges and microbial nitrification/denitrification.16–18
The rivers flow through different human disturbed watersheds, which could result in the different nitrate level. In this research, the stable isotopes of 15N and 18O in NO3− and δ18O–H2O, in conjunction with land use information and water quality under different land use and anthropogenic activities, were used to characterize the source and transformation of NO3− that delivered to the one important reservoir from two rivers in pairwised watersheds (Jinyun and Jinyang watersheds). The objectives of this research were to determine: (1) if the dissolved NO3− displays significantly seasonal and spatial differences in these two different watersheds; (2) if the water quality and isotopic data could indicate the NO3− sources, i.e., if NO3− originates from fertilizer or manure in the JYN watershed or from organism decomposition or sewage in the JYA watershed; and (3) is there different occurrence of NO3− transformation with the microbial nitrification or denitrification in these two rivers. The possible source of NO3− from fertilizer, manure, septic system, or sewage explanation could be identified due to different land use in these two watersheds. The expected results could provide important base for the risk assessment and management of the nitrate contaminant from the comparable watersheds with different land use practices.
2. Materials and methods
2.1 Study area description
The study area (237.1 km2) is within the Jinyun and Jinyang watershed (117°01′–117°14′ E, 36°19′–36°28′ N; elevation range of 150–175 m, with respective 55.2 km2 and 181.9 km2 basin areas). These two watersheds have the Cambrian gneisses and Ordovician limestone landscape. The research region has a warm temperate continental monsoon climate with four distinct seasons. The annual mean precipitation is 710 mm (∼65% between June and September). The annual mean temperature is 14.7 °C (−0.4 °C in January and +27.5 °C in July) (Chinese Meteorological Administration Public Service Center, website: http://www.weather.com.cn). The soil types are primary the calcareous cinnamon soil and brown soil, which promote water loss and soil erosion.19Jinyun river (JYN) and Jinyang river (JYA), locating in southern Jinan city, Shandong, China, are typical watersheds, with a total 55.2 km2 and 181.9 km2 basin areas, respectively. Both rivers together contribute about half of water to the Wohushan reservoir, which supplies the primary drinking water source for 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 residents in the south central Jinan city and a variety of industrial and agricultural activities of Jinan city (Fig. S1,†).20 The rivers' source originates from the northern slope of Mountain Tai at >1500 m above sea level, and the rivers traverse the southern part of Jinan city before flowing into the Wohushan reservoir (Fig. S1†). Both the JYN and JYA are fed by both precipitation and ground water throughout the year. The input of these two rivers is mainly dominated by surface runoff from local precipitation during the summer rainy season (June to September), whilst, the rivers are sustained by precipitation and seepage of ground water during the remainder of the year. In general, the daily discharge shows a distinct seasonal variation, ranging from 0.67 to 1.26 m3 s−1 in January (winter, lowest) and 4.15 to 7.82 m3 s−1 in August (summer, highest) for both the JYN and JYA (the office of Wohushan reservoir Administration, see website: http://www.whssk.com). The water contribution to the Wohushan reservoir from the JYN watershed is similar to the JYA watershed, whilst their land use and anthropogenic activities are very contrasting. For instance, high N input from chemical fertilizer and manure applications for intensive agricultural activities and orchard plantations are common in the JYN watershed. The NO3− contribution is thus from runoff of precipitation in this watershed. In contrast, more natural tour sites and residential properties are along the JYA watershed. This JYA watershed is thus associated with septic systems and potential point sources of contamination.21
000 residents in the south central Jinan city and a variety of industrial and agricultural activities of Jinan city (Fig. S1,†).20 The rivers' source originates from the northern slope of Mountain Tai at >1500 m above sea level, and the rivers traverse the southern part of Jinan city before flowing into the Wohushan reservoir (Fig. S1†). Both the JYN and JYA are fed by both precipitation and ground water throughout the year. The input of these two rivers is mainly dominated by surface runoff from local precipitation during the summer rainy season (June to September), whilst, the rivers are sustained by precipitation and seepage of ground water during the remainder of the year. In general, the daily discharge shows a distinct seasonal variation, ranging from 0.67 to 1.26 m3 s−1 in January (winter, lowest) and 4.15 to 7.82 m3 s−1 in August (summer, highest) for both the JYN and JYA (the office of Wohushan reservoir Administration, see website: http://www.whssk.com). The water contribution to the Wohushan reservoir from the JYN watershed is similar to the JYA watershed, whilst their land use and anthropogenic activities are very contrasting. For instance, high N input from chemical fertilizer and manure applications for intensive agricultural activities and orchard plantations are common in the JYN watershed. The NO3− contribution is thus from runoff of precipitation in this watershed. In contrast, more natural tour sites and residential properties are along the JYA watershed. This JYA watershed is thus associated with septic systems and potential point sources of contamination.21
2.2 Water sampling
To investigate seasonal and spatial distribution pattern of the nitrate concentration and isotopic composition in these two watersheds, water samples from 22 sites were collected four times in January (winter, low-water stage), May (spring, rising-water stage), August (summer, high-water stage) and October (fall, falling-water stage) 2011 and 2014 (Fig. 1S†). Four sites were distributed at the upstream Jinyang river (JYA-U), the middle reach of Jinyang river (JYA-M), and the downstream Jinyang river (JYA-D), respectively. Three samples were collected and the samples were combined into one sample at each site. The total samples were four at upstream, middle stream, and downstream Jinyang river, respectively. Four of the sites were selected at the upstream and downstream Jinyun river (JYN-U and JYN-D), respectively. Two sites were from the integrated channel because they are shorter than the Jinyang river. The total number of samples is 8 for Jinyun river. Three samples were collected from integrated channel. The samples were from combined two sites and each of two. In 2014, the samples were only collected from the sites where the data were not obtained well. These sampling sites (Fig. S1†) were selected to avoid fixed point-source contamination. At each site, a depth-integrated (0.5–1.5 m) mixed sample was taken from the center of the river and its temperature, pH and electrical conductivity (EC) were immediately measured. Samples for chemical and isotopic analyses were passed through a 0.45 μm membrane filter and kept at 4 °C until analyses within 2 weeks. In order to reduce the interference of Cl1−, the pretreatment was implemented with AG/H SPE column. Anions were analyzed by ion chromatography (Dionex ICS-1500, USA) at Shandong Analysis and Test Center, Jinan, China. Ammonium N (NH4+–N) concentrations were concluded by the indophenol blue method (Ministry of Environment Protection of PRC, 1989).2.3 Analytical methods
Three collected water samples from upstream, middle stream and downstream river were selected for isotope analysis. The water was filtered with 0.45 μm cellulose acetate membrane. Before measurement, the water samples were stored in refrigerator at 4 °C. In order to prevent biological activity, the samples were preserved with HCl for isotope analysis within one month. The refrigerated samples were measured for δ15N–NO3− and δ18O–NO3− using the denitrifier method at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, Guizhou, China. Briefly, the denitrifying bacterium Pseudomonas auroeofaciens converts NO3− to gaseous nitrous oxide (N2O) for isotopic analysis (Casciotti et al., 2002; Kaushal et al., 2011). With a minimum of 50 nmol NO3− requirement to a continuous flow Trace Gas Pre-concentrator-IsoPrime isotope ratio mass spectrometer (Isoprime 100, UK), the δ15N and δ18O values (‰) in the NO3− samples were corrected using international reference standards USGS32, USGS34, IAEA-N3 and values are reported in parts per thousand (‰) relative to atmospheric N2 and Vienna Standard Mean Ocean Water (V-MOW) for δ15N and δ18O, respectively.22 The oxygen-18 (18O) isotope of water was analyzed through equilibrating with carbon dioxide gas at 25.0 ± 0.1 °C (Lee et al., 2008).8 The carbon dioxide gas was then extracted and cryogenically purified. The δ18O values in the water were determined using a Sercon Integra stable isotope ratio mass spectrometer (Sercon 20, UK) at Nanjing Wanso Testing Services, China.The following equation was used to calculate the δ15N and δ18O values:
| δ (‰) = [(Rsample/Rstandard) − 1] × 1000 | (1) |
In order to quantify the measurement, data presentation, calculation and statistical analysis were performed using Microsoft office Excel and SPSS 16.0 (SPSS Inc., USA). The paired t-test and one-way ANOVA were performed at a significant level of P = 0.05.
3. Results and discussion
3.1 Seasonal and spatial distribution pattern of nitrate
The averaged NO3− concentrations were not significantly different between the upstream and downstream site (P > 0.05) in the whole hydrological year of the JYN and JYA (Table 1). However, the average NO3− was higher in the JYN than in the JYA, especially in spring and winter (Table 1), which probably resulted from more extensive distributions of agricultural farmlands and orchards in the JYN drainage basin compared with the JYA drainage basin. The NO3− concentrations varied with seasons in both the JYN and JYA and were lower in spring (rising-water stage) and summer (high-water stage) both in the JYN and JYA (Table 1). Although the NO3− concentrations of integrated channel showed a mixed effect, the same temporal patterns were found (Table 1). The possible reasons were the hydrological change, runoff of precipitation, and the transformation between organic N and inorganic N.23 Both NO3− and Cl− concentrations in the JYN and JYA showed little spatial variation (Table 2 and Fig. 1). All of the Cl− values in the sites were out of the compositional range of precipitation.24 No rock salts or evaporation sediments were found in the two watersheds.20 The elevated Cl− concentration in the rivers of south Jinan indicated that a potential large contribution of anthropogenic sources such as fertilizer, manure and sewage may dominate the Cl− concentrations of these two rivers. This also suggested that the NO3− contamination in these two rivers may be from anthropogenic sources rather than from atmospheric deposition.| Seasons | Winter | Spring | Summer | Fall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample sites | Upstream | Middle stream | Downstream | Upstream | Middle stream | Downstream | Upstream | Middle stream | Downstream | Upstream | Middle stream | Downstream | |
| a Values reported are means (n = 4) and standard errors in the brackets. The different lowercase letters of a and b in each column of upstream and downstream indicate significant differences between rivers in the same sampling position. | |||||||||||||
| T (°C) | JYN | 0.6(0.3)aa | 0.5(0.1)a | 22.5(1.9)a | 22.5(1.2)a | 28.5(2.0)a | 27.2(1.7)a | 16.6(1.5)a | 13.9(2.1)a | ||||
| JYA | 0.5(0.1)a | 0.8(0.2) | 0.5(0.1)a | 22.8(0.4)a | 23.6(0.4) | 24.0(0.1)a | 26.5(0.2)a | 26.8(0.3) | 27.0(0.1)a | 13.5(0.1)a | 13.7(0.3) | 13.8(0.2)a | |
| IntC | 0.6(0.1)a | 0.5(0.1)a | 23.1(0.3)a | 22.5(0.1)a | 26.8(1.3)a | 27.4(0.1)a | 14.5(0.2)a | 15.2(0.1)a | |||||
| pH | JYN | 7.7(0.1)a | 8.1(0.3)a | 8.1(0.1)a | 8.0(0.1)a | 8.0(0.1)a | 7.7(0.2)a | 7.7(0.1)a | 8.1(0.1)a | ||||
| JYA | 8.0(0.01)a | 8.1(0.04) | 8.0 (0.02)a | 8.2(0.08)a | 8.0(0.05) | 8.2(0.02)a | 8.0(0.03)a | 7.9(0.02) | 8.0(0.04)a | 7.9(0.03)a | 8.0(0.05) | 8.0(0.02)a | |
| IntC | 8.0(0.01)a | 8.0(0.01)a | 8.1(0.03)a | 8.1(0.02)a | 7.9(0.02)a | 8.0(0.01)a | 8.1(0.01)a | 7.9(0.02)a | |||||
| DO (mg L−1) | JYN | 14.6(1.5)a | 12.3(1.1)a | 9.7(1.5)a | 8.9(1.2)a | 11.2(1.5)a | 7.0(1.0)a | 10.2(0.9)a | 9.8(1.0)a | ||||
| JYA | 12.8(1.2)b | 11.5(1.1) | 15.7(1.4)b | 10.2(1.4)b | 8.8(1.3) | 13.4(1.2)b | 9.4(0.4)b | 8.7(0.8) | 8.2(0.4)a | 9.8(0.3)b | 10.7(1.0) | 10.2(0.2)a | |
| IntC | 12.7(1.4)ab | 13.2(1.2)a | 9.6(0.8)a | 8.8(1.1)a | 8.0(1.3)b | 9.2(1.0)a | 9.1(2.2)b | 9.3(1.9)a | |||||
| Cl− (mg L−1) | JYN | 27.6(3.2)a | 23.4(2.8)a | 23.9(3.0)a | 24.1(3.4)a | 19.1(1.7)a | 21.0(3.5)a | 19.4(3.2)a | 21.7(2.5)a | ||||
| JYA | 25.4(9.2)a | 26.2(0.1) | 27.6(0.03)a | 23.6(0.03)a | 23.0(0.1) | 21.8(0.03)a | 18.1(0.4)a | 16.4(0.3) | 13.6(0.1)a | 20.7(0.1)a | 19.9(0.03) | 20.2(0.5)a | |
| IntC | 26.4(5.1)a | 25.8(3.5)a | 23.8(2.8)a | 23.7(4.2)a | 19.2(5.0)a | 18.8(2.7)a | 20.9(3.5)a | 21.2(4.4)a | |||||
| NH4+ (mg L−1) | JYN | 0.19(0.02)a | 0.13(0.01)a | 0.14(0.01)a | 0.15(0.03)a | 0.25(0.02)a | 0.65(0.04)a | 0.37(0.02)a | 0.22(0.01)a | ||||
| JYA | 0.09(0.01)b | 0.12(0.03) | 0.15(0.01)a | 0.25(0.01)b | 0.12(0.01) | 0.23(0.02)a | 0.13(0.01)b | 0.13 (0.01) | 0.18 (0.01)b | 0.15 (0.01)b | 0.44(0.01) | 0.53(0.03)b | |
| IntC | 0.98(0.01)c | 0.12(0.02)a | 0.15(0.09)b | 0.15(0.01)a | 0.37(0.03)a | 0.46(0.03)a | 0.38(0.01)a | 0.39(0.11)a | |||||
| NO3− (mg L−1) | JYN | 11.47(0.07)a | 11.00(0.09)a | 6.27(0.05)a | 7.08(0.05)a | 5.56(0.13)a | 4.47(0.15)a | 8.31(0.02)a | 5.76(0.01)a | ||||
| JYA | 8.49(0.43)b | 8.92(0.04) | 9.98(0.02)b | 4.73(0.01)b | 3.92(0.01) | 2.48(0.01)b | 6.32(0.16)b | 5.43(0.16) | 5.36(0.09)b | 7.25(0.22)b | 6.05(0.01) | 7.70(0.01)a | |
| IntC | 9.87(0.33)b | 10.05(0.29)b | 5.46(0.41)a | 5.21(0.38)b | 5.70(0.22)a | 5.59(0.25)b | 7.21(1.29)b | 7.19(1.03)b | |||||
| δ18O–H2O (‰) | JYN | −7.33(1.42)a | −7.92(1.38)a | −9.31(0.38)a | −8.45(0.36)a | −8.34(0.35)a | −7.68(0.33)a | −11.07(2.27)a | −9.22(1.90)a | ||||
| JYA | −9.60(0.42)a | −8.15(0.39) | −8.72(0.73)a | −8.73(0.25)a | −9.24(0.43) | −9.08(0.37)a | −9.20(0.23)a | −10.51(0.33) | −8.73(0.51)a | −8.74(0.22)b | −9.45(0.15) | −7.94(0.26)b | |
| IntC | −9.20(0.65)a | −7.98(1.14)a | −9.52(0.57)a | −8.19(0.95)a | −8.85(0.48)a | −9.52(0.36)b | −8.76(2.60)b | −9.13(1.58)a | |||||
| δ15N–NO3− (‰) | JYN | 6.68(0.09)a | 6.81(0.07)a | 9.39(0.05)a | 9.32(0.04)a | 8.55(0.13)a | 8.73(0.19)a | 7.41(0.01)a | 8.41(0.23) | 4.61(0.02)a | |||
| JYA | 8.26(0.29)b | 8.07(0.32) | 8.24(0.27)b | 9.68(0.24)b | 9.35(0.19) | 9.47(0.26)b | 8.25(0.13)b | 8.07(0.17) | 8.15(0.20)b | 8.72(0.22)a | 8.52(0.20)b | ||
| IntC | 8.16(0.07)b | 7.86(0.19)a | 9.65(0.08)b | 9.54(0.22)a | 8.24(0.08)b | 8.76(0.17)a | 8.21(0.11)a | 8.35(0.30)b | |||||
| δ18O–NO3− (‰) | JYN | 7.31(1.42)a | 7.30(1.40)a | 6.14(0.36)a | 6.65(0.39)a | 8.22(0.33)a | 8.68(0.29)a | 7.43(0.35)a | 5.71(0.28) | 5.11(0.28)a | |||
| JYA | 2.89(0.40)b | 3.46(0.42) | 3.25(0.38)b | 6.41(0.54)a | 5.65(0.57) | 6.18(0.52)b | 6.29(0.32)b | 5.84(0.30) | 6.13(0.34)b | 6.13(0.30)b | 6.09(0.31)a | ||
| IntC | 5.46(0.52)a | 6.13(0.44)a | 6.52(0.37)a | 7.03(0.55)c | 7.84(0.62)a | 9.21(0.49)b | 6.54(0.35)b | 5.98(0.43)a | |||||
| Spatial site | Cl− (mg L−1) | NH4+ (μg L−1) | NO3− (mg L−1) | δ18O–H2O (‰) | δ15N–NO3− (‰) | δ18O–NO3− (‰) |
|---|---|---|---|---|---|---|
| AVR ± SD | AVR ± SD | AVR ± SD | AVR ± SD | AVR ± SD | AVR ± SD | |
| a JYU-U, the upstream Jiyun river; JYU-D, the downstream Jiyun river; JYA-U, the upstream Jiyang river; JYA-M, the middle reach of Jiyang river; JYA-D, the downstream Jiyang river. Values reported are means (n ≥ 12) ± standard errors. The different lowercase letters of a and b in each column indicate significant differences among the sampling positions. | ||||||
| JYN-Ua | 22.49 ± 4.08a | 94.84 ± 9.00a | 7.90 ± 2.65a | −9.01 ± 1.59a | 8.01 ± 1.20a | 6.57 ± 1.56a |
| JYN-D | 22.58 ± 1.44a | 84.13 ± 9.32b | 7.07 ± 2.82b | −8.32 ± 0.68a | 8.07 ± 1.15a | 6.94 ± 1.48a |
| JYA-U | 21.94 ± 3.24a | 85.84 ± 5.33a | 6.70 ± 1.58b | −9.07 ± 0.42a | 8.73 ± 0.67a | 5.43 ± 1.70a |
| JYA-M | 21.38 ± 4.21a | 88.08 ± 11.56b | 6.08 ± 2.09b | −9.34 ± 0.97a | 8.46 ± 0.61a | 5.17 ± 1.14b |
| JYA-D | 20.78 ± 5.75a | 84.41 ± 16.50b | 6.38 ± 3.21a | −8.62 ± 0.48a | 8.60 ± 0.60a | 5.41 ± 1.44a |
| Integrated channel | 22.46 ± 2.90a | 90.36 ± 9.31b | 7.24 ± 1.96c | −8.89 ± 0.57a | 8.60 ± 0.67 ab | 6.84 ± 1.20a |
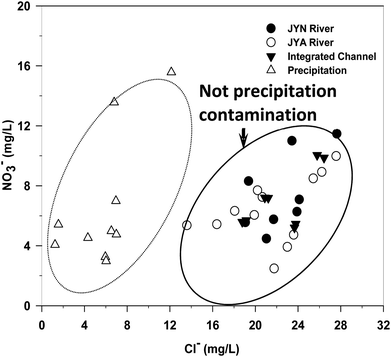 | ||
| Fig. 1 Plots of NO3− and Cl− in the Jinyun river and Jinyang river and integrated channel of Jinan, China. | ||
The nitrate concentration in JYA is lower than JYN during winter, spring and fall, but higher than JYN during summer. This nitrate seasonal variation corresponds to the seasonal variation of nitrogen isotope and oxygen isotope, which displayed high nitrate concentration with high nitrogen isotopes and low oxygen isotope. The results showed that the possible nitrate sources is from manure or sewage waste. The possible reasons are that there are more agricultural lands distributed in the subwatershed of JYN. The application of manures and runoff in the summer may result in the drastic disturbance.
3.2 Nitrate sources
Both δ15N–NO3− and δ18O–NO3− can be a good indicator to quantify the different contribution of nitrate sources.2,16 Values of δ15N–NO3− and δ18O–NO3− were not significantly different between the JYN and JYA across the whole hydrological years (Table 2). The δ15N–NO3− values were slightly higher from JYA than from JYN (Table 2). The δ18O–NO3− values showed contrary pattern compared with the δ15N–NO3− in these two rivers. Both the δ15N–NO3− and δ18O–NO3− presented the different seasonal patterns in the two rivers.The Cl− concentrations in JYN and JYA were distinctly higher than in the precipitation (Fig. 1). It was primarily concluded that NO3− were not only from precipitation. δ15N–NO3− values varied between +4.61 and +9.39‰ (averaged 8.54 ± 0.92‰, n = 56, Tables 1 and 2) and were lower in the upstream (JYN-U, 8.01 ± 1.20‰) than in the downstream (JYN-D, 8.07 ± 1.15‰) of the Jinyun river (Fig. 2A). δ15N–NO3− values were not significantly different among the upstream of the Jinyang river (JYA-U, averaged 7.33‰), the middle reach of the Jinyang river (JYA-M, averaged 7.08‰), and the downstream of the Jinyang river (JYA-D, averaged 8.02‰); they varied between +8.07–9.68‰ (Fig. 2A). Across these spatial river sites, δ15N–NO3− values were significantly higher in spring than other seasons (Fig. 2B). The addition of NO3− from manure or sewage might produce elevations of both the NO3− concentration and the δ15N–NO3− signature.7 In this study, although the runoff of precipitation is more drastic in summer than other season, δ15N–NO3− was not higher in the summer. This indicated that NO3− from discharged sewage may be the main sources.
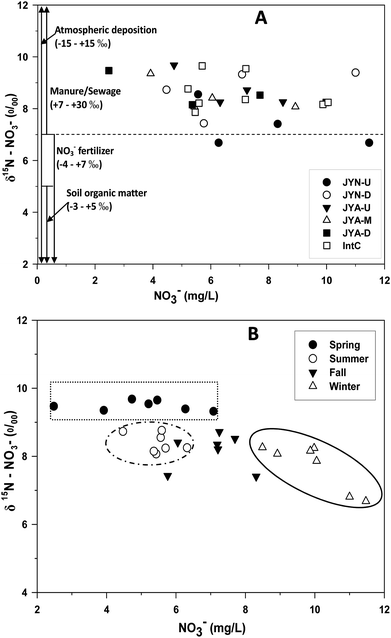 | ||
| Fig. 2 NO3− versus δ15N–NO3− in spatial sites of these two watershed rivers (A) and in different seasons across the watersheds (B). The ranges in A are from Isotope Hydrology (Joel R Gat, 2010). | ||
The combination of analyzing the concentration and the isotopic composition of NO3− is a useful tool to identify the NO3− source and the denitrification process in a watershed.17,18,25 Values of NO3− and δ15N–NO3− were widely scattered in both the JYN and JYA (Fig. 2A). Values of δ15N–NO3− for these two rivers were not significantly different by paired t-test (P = 0.22). The integrated channel showed similar patterns and the values were between the JYN and JYA rivers, reflecting the mixing of these two rivers (Table 1).
The ranges of δ15N–NO3− indicated the NO3− originated primarily from the manure or sewage, with only a little from NO3− fertilizer in the JYN. However, the NO3− concentrations did not increase with δ15N–NO3− (Fig. 2A and B). Therefore, it was likely that the state of NO3− from manure or sewage changed with nitrification and denitrification controlled by microbes.
3.3 Nitrification and denitrification
δ18O–NO3− values in the two watershed rivers ranged from +2.89 to +9.21‰, indicating that neither atmospheric NO3− deposition (+25–75‰) nor NO3−-containing fertilizer (+17–25‰) was a dominant source of riverine NO3− in these two rivers (Fig. 3). Values of δ15N–NO3− (+6.68 to +9.68‰) and δ18O–NO3− (+2.89 to +9.21‰) were distributed within the ranges of manure/sewage and the microbial nitrification (Fig. 3). Instead, the data indicated that most of the NO3− were derived from manure/sewage and microbial nitrification (Fig. 3)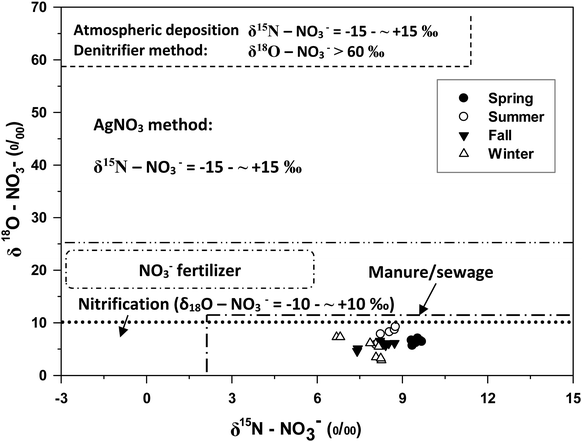 | ||
| Fig. 3 Patterns of δ18O–H2O and δ18O–NO3− for the Jinyun river and Jinyang river in different seasons. | ||
Although all δ18O–NO3− values fell in the range typically cited as representing ammonium fertilizer sources (−8‰ < δ18O–NO3− < +14‰), whilst, δ15N–NO3− values were out of the range for ammonium fertilizer sources (−7‰ < δ15N–NO3− < +5‰).26,27 The δ18O–NO3− values produced by microbial nitrification in watersheds range from −10 to +10‰ (Kendall et al., 2007).16 All of these sample values had some overlap range of typical soil nitrate (+3‰ < δ15N–NO3− < +8‰, −8‰ < δ18O–NO3−< +14‰). Soil NO3− is a product of bacterial decomposition of organic N that results from the decomposition of plants and animals and their products (i.e. organic waste). Inorganic or organic N based fertilizers are a primary source of nitrate in agricultural areas, while septic waste plays a significant role in residential areas.18,28 The results indicated that the NO3− for these two rivers could be derived from the nitrification of manure/sewage other than ammonium fertilizers.26–28 Furthermore, it did support our hypothesis that different land uses could result in different NO3− sources in these two rivers. However, there was no information available on the rates of various fractionation processes, the usage of manure in these two watersheds, or the amount of sewage discharging to the JYN and JYA, and therefore soil NO3− could not be separated from other NO3− sources derived from based on δ15N–NO3− values alone.
δ18O–NO3− values in these two rivers were within −10 to +10‰ (Fig. 2B), possibly suggesting that most of NO3− contributed by soil nitrate could be from microbial nitrification before flowing into the JYN and JYA. Nitrogen-fixing bacteria account for two-thirds of the oxygen atoms in NO3− from soil water and one-third from atmospheric oxygen.29,30 As shown in Fig. 4, the δ18O–NO3− and δ18O–H2O values in these two rivers were highly and positively correlated (r2 = 0.393, P = 0.04), further suggesting that the δ18O–NO3− values were largely controlled by the δ18O–H2O in the river during the microbial nitrification. However, there was no regular seasonal distribution of δ18O–H2O in these two rivers compared with the oxygen isotopic composition of dissolved NO3−, indicating that the nitrification capacity from microbial activities might not be always consistent with the seasons change (Fig. 4). The correlations between the δ18O–NO3− and δ18O–H2O in the spring and autumn are better than the summer and winter. The possible reasons are the temperatures and water quantities in these two seasons are apt to the microbial nitrification in the river.
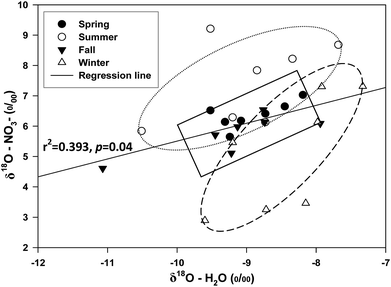 | ||
| Fig. 4 Patterns of δ18O–H2O and δ18O–NO3− for the Jinyun river and Jinyang river in different seasons. | ||
Denitrification can occur in anaerobic pockets within a water body and can cause a characteristic increase of both the δ15N–NO3− and δ18O–NO3− values in the remaining NO3−.16,31 The process of ammonia evaporation causes an enrichment of heavier N isotope and hence increases the δ15N values.7,16 Indeed, these changes of isotopic compositions would correlate with decreasing NO3− concentrations as nitrate is being transformed to nitrogen gas and then out of the system. It is important to validate whether the observed changes in the isotopic composition of the remaining NO3− correspond to a decrease of the total dissolved NO3− in the water body. This can be done by plotting δ15N against a concentration scale.32 Although the ratio of changes in δ15N–NO3− and δ18O–NO3− is not typically close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the δ15N–NO3− values across these two river water negatively correlated with NO3− in the winter in this study (Fig. 2B). The variation in the negative correlation between δ15N–NO3− and NO3− concentrations showed that the denitrification had happened although the dissolved oxygen is higher in the winter river (Kendall, 1998).26
2, the δ15N–NO3− values across these two river water negatively correlated with NO3− in the winter in this study (Fig. 2B). The variation in the negative correlation between δ15N–NO3− and NO3− concentrations showed that the denitrification had happened although the dissolved oxygen is higher in the winter river (Kendall, 1998).26
4. Conclusion
Results in this study showed that NO3− concentrations had distinct seasonal patterns. They were lower in spring and summer either in the Jinyun river or Jinyang river and were higher in the Jinyun river than the Jinyang river, especially in spring and winter. Both river waters indicated that NO3− concentration deviated from manure/sewage and anthropogenic contamination might be a dominant NO3− origination over the watersheds. The sources of NO3− could be from microbial nitrification and manure/sewage in the Jinyun river and Jinyang river. The nitrification would have taken place in manure/sewage and soil, but not in the ammonium fertilizer. This study provides a reference in identifying the sources of nitrate in the similar multipurpose watershed around the world. It is necessary to control or reduce nitrate contamination in the waters.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was jointly supported by Natural Science Foundation of Shandong Province, China (ZR2017MD022 and ZR2018MD002) and Natural Science Foundation of China (31270586). We thank Bonnie Chapman Beers, working in the SUNY Upstate Medical University, for polishing the language. We are appreciated with Qian Li and Fujun Yue for chemical analyses.References
- K. S. Lee, Y. S. Bong, D. Lee, Y. Kim and K. Kim, Sci. Total Environ., 2008, 395, 117–124 CrossRef PubMed.
- E. Pastén-Zapata, R. Ledesma-Ruiz, T. Harter, A. Ramírez and J. Mahlknecht, Total Environ., 2014, 470–471, 855–864 CrossRef PubMed.
- C. Liu, S. Li, Y. Lang and H. Xiao, Environ. Sci. Technol., 2006, 40, 6928–6933 CrossRef PubMed.
- S. S. Kaushal, P. M. Groffman, L. E. Band, E. M. Elliott, C. A. Shields and C. Kendall, Environ. Sci. Technol., 2011, 45, 8225–8232 CrossRef PubMed.
- A. Menció, M. Boy and J. Mas-Pla, Sci. Total Environ., 2011, 409, 3049–3058 CrossRef PubMed.
- M. Itoh, Y. Takemon, A. Makabe, C. Yoshimizu, A. Kohzu, N. Ohte, D. Tumurskh, I. ayasu, N. Yoshida and T. Nagata, Sci. Total Environ., 2011, 409, 1530–1538 CrossRef PubMed.
- M. Zhang, Y. Zhi, J. Shi and L. Wu, Sci. Total Environ., 2018, 639, 1175–1187 CrossRef PubMed.
- K. S. Lee, Y. S. Bong, D. Lee, Y. Kim and K. Kim, Sci. Total Environ., 2008, 395, 117–124 CrossRef PubMed.
- M. Alvarez-Cobelas, D. G. Angeler and S. Sanchez-Carrillo, Export of nitrogen from catchments: a worldwide analysis, Environ. Pollut., 2008, 156, 261–269 CrossRef PubMed.
- S. Li, C. Liu, J. Li, X. Liu, B. Chetelat, B. Wang and F. Wang, Environ. Sci. Technol., 2010, 44, 1753–1758 Search PubMed.
- N. Chen and H. Hong, Biogeochemistry, 2011, 106, 311–321 CrossRef.
- L. M. Kellman and C. Hillaire-Marcel, Agric., Ecosyst. Environ., 2003, 95, 87–102 CrossRef.
- M. L. Cole, K. D. Kroeger, J. W. McClelland and I. Valiela, Biogeochemistry, 2006, 77, 199–215 CrossRef.
- C. C. Y. Chang, C. Kendall, S. R. Silva, W. A. Battaglin and D. H. Campbell, J. Fish. Aquat. Sci., 2002, 59, 1874–1885 CrossRef.
- M. M. Savard, A. Smirnoff, D. Paradis, E. Van Bochove and S. Liao, J. Hydrol., 2010, 381, 134–141 CrossRef.
- C. Kendall, E. M. Elliott and S. D. Wankel, Stable isotopes in ecology and environmental science, 2007, pp. 375–449 Search PubMed.
- D. A. Burns and C. Kendall, Water Resour. Res., 2002, 38, 1051–1061 CrossRef.
- S. Danielescu and K. T. MacQuarrie, Biogeochemistry, 2013, 115, 111–127 CrossRef.
- L. Wang and Y. Qin, J. Soil Water Conserv., 2005, 25, 87–91 Search PubMed.
- M. Liu, Q. Wang, M. Li and D. Liu, J. Shandong Norm. Univ., Nat. Sci., 2000, 15, 238–240 Search PubMed.
- M. Niu, K. Kong and Z. Xu, J. Jinan Univ., Sci. Technol., 2013, 27, 418–422 Search PubMed.
- F. Yue, S. Li, C. Liu, N. An and H. Cai, Chin. J. Ecol., 2012, 31, 1–6 Search PubMed.
- S. Zhang, H. Wang, Q. Chen, B. Yang and L. Yang, J. Soil Water Conserv., 2012, 26, 169–200 Search PubMed.
- W. Wang, S. Qu, Q. Ye and X. Sun, J. Hydraul. Eng., 2011, 42, 477–489 Search PubMed.
- Y. Xia, Y. Li, X. Zhang and X. Yan, J. Geophys. Res.: Biogeosci., 2017, 122(1), 2–14 CrossRef.
- E. Minet, R. Goodhue, W. Meier-Augenstein, R. Kalin, O. Fenton, K. Richards and C. Coxon, Water Res., 2017, 124, 85–96 CrossRef PubMed.
- C. Kendall and R. Aravena, Environmental tracers in subsurface hydrology, 2000, pp. 261–297 Search PubMed.
- Z. Jin, Q. Zheng, C. Zhu, Y. Wang, J. Cen and F. Li, Appl. Geochem., 2018, 93, 10–19 CrossRef.
- D. Marconi, PhD thesis, ProQuest Dissertations Publishing, 2017.
- L. Bristow, M. Altabet, I. Gregory-Eaves and R. Maranger, Biogeochemistry, 2017, 135(3), 221–237 CrossRef.
- G. Anornu and D. Adomako, Sci. Total Environ., 2017, 603–604, 687–698 CrossRef PubMed.
- C. Leibundgut, P. Maloszewski and C. Külls, Tracers in Hydrology, 2009, 50–65 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04364g |
| This journal is © The Royal Society of Chemistry 2018 |
