DOI:
10.1039/C8RA04094J
(Paper)
RSC Adv., 2018,
8, 26407-26415
An efficient class of bis-NHC salts: applications in Pd-catalyzed reactions under mild reaction conditions†
Received
14th May 2018
, Accepted 26th June 2018
First published on 24th July 2018
Abstract
This study describes an efficient class of bis-N-heterocyclic carbene (bis-NHC) salts that can be easily made from commercially available and inexpensive starting materials. The application of these salts to Pd-catalyzed reactions is described. The palladium (Pd) catalyst generated in situ was highly effective under mild reaction conditions.
Introduction
Palladium-catalyzed carbon–carbon bond formation is one of the most versatile and useful tools for the synthesis of pharmaceutical compounds, advanced materials, and agricultural products.1 In the past decade, N-heterocyclic carbenes (NHCs) and their palladium complexes have demonstrated a high catalytic efficiency.2 In particular, bis-NHC-derived palladium complexes possess two strong metal–carbene bonds that are more stable than the corresponding complexes of similar monodentate carbene ligands. In addition, bis-NHC-derived palladium complexes offer many advantages, such as a superior control over the coordination sphere and tunable properties including their chirality. Recently, several bidentate bis-NHC–Pd complexes have been synthesized and efficient activity has been obtained in Pd-catalyzed reactions.3–5 However, Pd-catalyzed reactions are often carried out at a high reaction temperature in the presence of the bis-NHC–Pd catalytic system. Not only is the use of low boiling point compounds limited, but also the compounds are easily decomposed under a high temperature. It is very important to develop an efficient catalyst that allows the reaction to be carried out under mild reaction conditions.
Our group previously developed a series of bis-NHC salts that demonstrated good-to-excellent catalytic activity in Pd-catalyzed Suzuki–Miyaura coupling reactions of aryl bromides (Fig. 1).6 Generally, because it increases the electron-richness of bis-NHC, the bis-NHC–Pd catalytic system has a high activity in the oxidative addition step. In 2014, Strassner et al. discussed the mechanistic cycles of a Mizoroki–Heck reaction catalyzed by bis-NHC–Pd using a density functional theory (DFT) calculation.4e They revealed that the introduction of an electron-donating groups at the para position in the aryl substitution on imidazolium salts reduced the reaction barrier of the rate-determining step. To retain the advantage of an effective skeleton and further increase the electronic properties of bis-NHC, we used 1-(4-methoxyphenyl)benzimidazole as the main scaffold. This skeleton allows a high-potential modification through straightforward synthetic methods. In addition, the linker between the benzimidazole rings provides the flexibility to the bis-NHC–Pd complexes. Herein, we report the preparation of bis-benzimidazolium salts and the effects of different catalytic treatments in Pd-catalyzed coupling reactions.
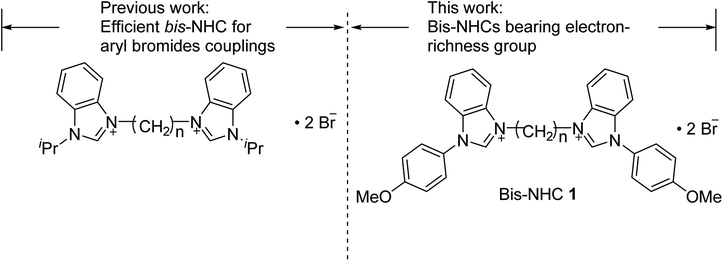 |
| | Fig. 1 Structure of bis-benzimidazolium salts 1. | |
Results and discussion
Synthesis of bis-NHC salts
The ligand syntheses started with the coupling of benzimidazole and 4-iodoanisole in the presence of copper(I) iodide (CuI) and 8-hydroxyquinalidine to form the desired product 2 with a quantitative yield.7 The bis-benzimidazolium salts 1 were obtained with an 89–96% yield through heating 2 with various dibromides in N,N-dimethylformamide (DMF) at 80 °C for 6 h and then 130 °C for 16 h in Scheme 1. To prevent the quick formation of a precipitate, DMF was used as the solvent and a two-stage heating system was needed in the preparation of the bis-benzimidazolium salts 1.
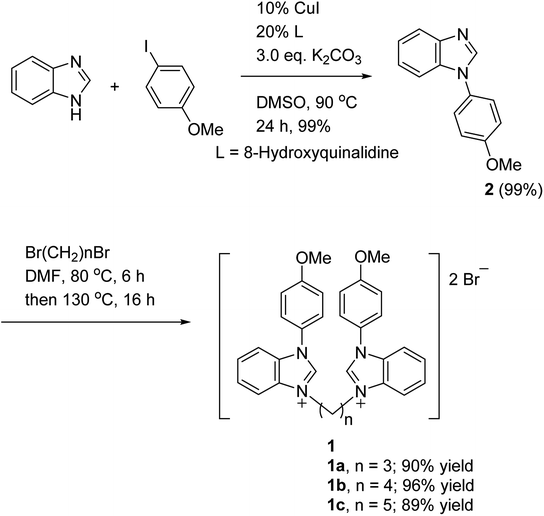 |
| | Scheme 1 The synthesis of bis-benzimidazolium salts 1. | |
Catalyst testing for the Suzuki–Miyaura reaction
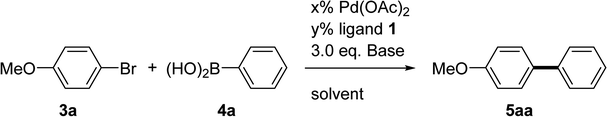
Biaryls formed via Suzuki–Miyaura cross-coupling are crucial scaffolds for pharmaceutical and natural products, advanced materials, and agrochemicals. The Suzuki–Miyaura cross-coupling reaction is also one of the most crucial methods for testing the efficiency of the catalyst. A general test protocol for the coupling reaction was developed by using the 4-bromoanisole 3a and phenylboronic acid 4a in a model study (Table 1). Initially, the reactions were performed using Pd(OAc)2, salts 1, and K3PO4·H2O in 1,4-dioxane at 60 °C for 24 h (entries 1–3). 1c was the optimal choice (entry 3), but a low conversion (40%) yield was obtained. Using our previous report,6 the conversion yield was increased after modifying the reaction conditions using a two-stage temperature process. Under the two-stage temperature conditions, the Pd/ligand ratio was varied because low conversions may be attributed to catalytically inactive bis-ligated species generated from an excess quantity of ligands.8 The results revealed an excellent conversion (91%) and yield (86%) for the coupling reaction conducted at room temperature with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Pd/1c ratio (entry 6). The reactions failed when acetonitrile or tetrahydrofuran (THF) were used as solvents (entries 7 and 8). The reaction could be completed in the presence of tert-butanol (t-BuOH) (entry 9). Then, different bases were screened under a standard two-stage heating condition (60 °C for 1 h followed by 30 °C for 24 h) (entries 10 and 11) which using t-BuOH as the solvent. Although both K2CO3 and K3PO4·H2O showed excellent conversion yields (entries 9 and 10), K3PO4·H2O was selected for further studies according to our previous works.6,9 Further studies demonstrated that the reaction could be completed even when only 0.5 mol% of Pd/1c was employed (entry 12), which was then used as our standard reaction condition for the substrate scope exploration.
1 Pd/1c ratio (entry 6). The reactions failed when acetonitrile or tetrahydrofuran (THF) were used as solvents (entries 7 and 8). The reaction could be completed in the presence of tert-butanol (t-BuOH) (entry 9). Then, different bases were screened under a standard two-stage heating condition (60 °C for 1 h followed by 30 °C for 24 h) (entries 10 and 11) which using t-BuOH as the solvent. Although both K2CO3 and K3PO4·H2O showed excellent conversion yields (entries 9 and 10), K3PO4·H2O was selected for further studies according to our previous works.6,9 Further studies demonstrated that the reaction could be completed even when only 0.5 mol% of Pd/1c was employed (entry 12), which was then used as our standard reaction condition for the substrate scope exploration.
Table 1 Optimization of the reaction conditions for Suzuki–Miyaura coupling catalyzed by 1/Pd
|
| Entry |
Ligand (mol%) |
Pd(OAc)2 (mol%) |
Conditiona |
Base |
Solvent |
Conv.b |
Yield (%)c |
| Condition A: 3a (1.0 mmol), 4a (1.5 mmol), Pd(OAc)2 (1.0 mol%), bis-NHC 1 (as indicated), K3PO4·H2O (3.0 mmol) and 1,4-dioxane (3.0 mL) were stirred under N2 for 24 h at the indicated temperature. Condition B: Pd(OAc)2 (1.0 mol%), bis-NHC 1 (as indicated), K3PO4·H2O (3.0 eq. to Pd), and 1,4-dioxane (3.0 mL) were stirred under N2 at 60 °C for 1 h, followed by 3a (1.0 mmol), 4a (1.5 mmol), and K3PO4·H2O (3.0 mmol) at 30 °C for 24 h. Determined by 400 MHz NMR. Isolated yield. |
| 1 |
1a (3.0) |
1.0 |
A |
K3PO4·H2O |
Dioxane |
0 |
— |
| 2 |
1b (3.0) |
1.0 |
A |
K3PO4·H2O |
Dioxane |
22 |
— |
| 3 |
1c (3.0) |
1.0 |
A |
K3PO4·H2O |
Dioxane |
40 |
— |
| 4 |
1c (3.0) |
1.0 |
B |
K3PO4·H2O |
Dioxane |
19 |
— |
| 5 |
1c (2.0) |
1.0 |
B |
K3PO4·H2O |
Dioxane |
54 |
— |
| 6 |
1c (1.0) |
1.0 |
B |
K3PO4·H2O |
Dioxane |
91 |
86 |
| 7 |
1c (1.0) |
1.0 |
B |
K3PO4·H2O |
MeCN |
<1 |
— |
| 8 |
1c (1.0) |
1.0 |
B |
K3PO4·H2O |
THF |
4 |
— |
| 9 |
1c (1.0) |
1.0 |
B |
K3PO4·H2O |
t-BuOH |
>99 |
99 |
| 10 |
1c (1.0) |
1.0 |
B |
K2CO3 |
t-BuOH |
>99 |
99 |
| 11 |
1c (1.0) |
1.0 |
B |
t-BuOK |
t-BuOH |
16 |
— |
| 12 |
1c (0.5) |
0.5 |
B |
K3PO4·H2O |
t-BuOH |
>99 |
99 |
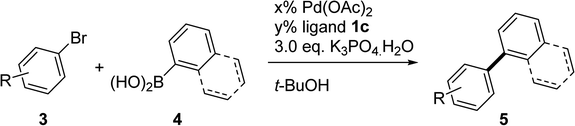
A wide range of aryl bromides were tested for the cross-coupling reaction in the presence of the Pd(OAc)2/1c (0.5 mol%) catalyst at room temperature. The results are summarized in Table 2. The coupling reaction of substrates with electron-donating or electron-withdrawing groups on the meta- or para-position of aryl bromide 3 provided excellent yields (entries 1–13), whereas the mono-ortho-substituted biaryls resulted in moderate-to-excellent yields (entries 14–16). Sterically hindered di-ortho-substituted products were also obtained in good-to-excellent yields (entries 17–20). Both the 1° and 3° amino groups remained intact after transformation, with 99% and 70% yields, respectively (entries 21 and 22). The catalytic system demonstrates high chemoselectivity at room temperature because that no Buchwald–Hartwig amination product was observed. The substrate scope could be further extended to benzyl halides with favorable yields (entries 23 and 24). The corresponding aryl bromides with naphthylboronic acid could generate various coupling products with excellent yields (entries 25–31). Furthermore, we demonstrated the high efficiency of this catalytic system. 4-Bromoanisole 3a could be coupled with phenylboronic acid 4a with an 87% yield with Pd loadings down to 0.005 mol% (entry 2). 4′-Bromoacetophenone 3d was a possible substrate, generating the corresponding coupling product with a 99% yield with Pd loadings down to 0.0005 mol% at room temperature (entry 7). The catalytic system was compatible with a wide range of functional groups, including methoxy, nitro, ketone, aldehyde and ester. Difficult Pd-catalyzed di-ortho-substituted biaryl syntheses were also achieved in the presence of Pd(OAc)2/1c with a particularly high chemoselectivity at room temperature.
Table 2 The coupling reaction of aryl bromides with arylboronic acids catalyzed by 1c/Pda
|
| Entry |
3 (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) ) |
4 |
Conv.b |
Yield (%)c |
| Reaction conditions: Pd(OAc)2 (0.5 mol%), bis-NHC 1c (0.5 mol%), K3PO4·H2O (3.0 mol%) and t-BuOH (3.0 mL) were stirred under N2 at 60 °C for 1 h, followed by 3 (1.0 mmol), 4 (1.5 mmol), and K3PO4·H2O (3.0 mmol) at 30 °C for 24 h. Determined by 400 MHz NMR. Isolated yield. Pd(OAc)2/1c (0.005 mol%) was used. Pd(OAc)2/1c (0.0005 mol%) was used. Pd(OAc)2/1c (0.00005 mol%) was used. |
| 1 |
4-OMe (3a) |
4a |
>99 |
5aa (99) |
| 2d |
4-OMe (3a) |
4a |
>99 |
5aa (87) |
| 3e |
4-OMe (3a) |
4a |
19 |
5aa (–) |
| 4 |
4-t-Bu (3b) |
4a |
>99 |
5ba (99) |
| 5 |
4-CO2Me (3c) |
4a |
>99 |
5ca (98) |
| 6 |
4-COMe (3d) |
4a |
>99 |
5da (99) |
| 7e |
4-COMe (3d) |
4a |
>99 |
5da (99) |
| 8f |
4-COMe (3d) |
4a |
10 |
5da (–) |
| 9 |
4-COEt (3e) |
4a |
>99 |
5ea (99) |
| 10d |
4-COEt (3e) |
4a |
>99 |
5ea (85) |
| 11e |
4-COEt (3e) |
4a |
81 |
5ea (72) |
| 12 |
3-COEt (3f) |
4a |
>99 |
5fa (99) |
| 13 |
4-CHO (3g) |
4a |
>99 |
5ga (99) |
| 14 |
2-CHO (3h) |
4a |
>99 |
5ha (84) |
| 15 |
2,4-DiNO2 (3i) |
4a |
>99 |
5ia (99) |
| 16 |
2-Me (3j) |
4a |
>99 |
5ja (70) |
| 17 |
2,6-DiMe (3k) |
4a |
98 |
5ka (85) |
| 18 |
1-Bromo-2-methylnaphthalene (3l) |
4a |
>99 |
5la (99) |
| 19 |
1-Bromo-2-methoxynaphthalene (3m) |
4a |
>99 |
5ma (99) |
| 20 |
1-Bromo-2,3-dimethoxynaphthalene (3n) |
4a |
>99 |
5na (99) |
| 21 |
4-NH2 (3o) |
4a |
>99 |
5oa (99) |
| 22 |
2-N,N-DiMe (3p) |
4a |
86 |
5pa (70) |
| 23 |
2-Methylbenzyl (3q) |
4a |
>99 |
5qa (89) |
| 24 |
4-Methlylbenzyl (3r) |
4a |
>99 |
5ra (77) |
| 25 |
4-OMe (3a) |
4b |
>99 |
5ab (99) |
| 26 |
4-t-Bu (3b) |
4b |
>99 |
5bb (96) |
| 27 |
4-CO2Me (3c) |
4b |
>99 |
5cb (97) |
| 28 |
4-COMe (3d) |
4b |
>99 |
5db (95) |
| 29 |
4-COEt (3e) |
4b |
>99 |
5eb (99) |
| 30 |
3-COEt (3f) |
4b |
>99 |
5fb (96) |
| 31 |
4-NH2 (3o) |
4b |
>99 |
5ob (99) |
Catalyst testing for the Mizoroki–Heck reaction
The Mizoroki–Heck reaction is a useful method to prepare alkylated olefins involving an sp2 carbon. In general, it is carried out in polar solvents such as N-methyl-2-pyrrolidone (NMP), N,N-dimethylacetamide (DMA) or N,N-dimethylformamide (DMF), in the presence of a base at a high temperature (100–150 °C). A more effective catalytic system for the Mizoroki–Heck reaction under milder conditions is needed to tolerate the coupling of low-boiling olefins with aryl halides. First, we studied the use of the bis-NHC–Pd catalytic system generated in situ from Pd(OAc)2 and bis-NHC 1c with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for the Mizoroki–Heck reaction of 4-bromoacetophenone 3d with methyl acrylate 6a with K3PO4·H2O as a base and DMF as the solvent at 60 °C. However, only a 4% conversion yield was achieved. Next, we increased the conversion yield to 75% by replacing Pd(OAc)2 with Pd(dba)2 (entry 1). The reaction was carried out at 60 °C in a variety of bases and solvents and the experimental results are summarized in Table 3. Using other bases such as triethylamine (Et3N), K2CO3, KOtBu and KF in the presence of the solvents, toluene, dioxane, acetonitrile or t-BuOH resulted in low conversion yields. When CsF in DMF was employed, the conversion yield of 7da was improved to 99% (entry 6).
1 ratio for the Mizoroki–Heck reaction of 4-bromoacetophenone 3d with methyl acrylate 6a with K3PO4·H2O as a base and DMF as the solvent at 60 °C. However, only a 4% conversion yield was achieved. Next, we increased the conversion yield to 75% by replacing Pd(OAc)2 with Pd(dba)2 (entry 1). The reaction was carried out at 60 °C in a variety of bases and solvents and the experimental results are summarized in Table 3. Using other bases such as triethylamine (Et3N), K2CO3, KOtBu and KF in the presence of the solvents, toluene, dioxane, acetonitrile or t-BuOH resulted in low conversion yields. When CsF in DMF was employed, the conversion yield of 7da was improved to 99% (entry 6).
Table 3 Optimizing the reaction conditions for Mizoroki–Heck reaction catalyzed by 1c/Pda
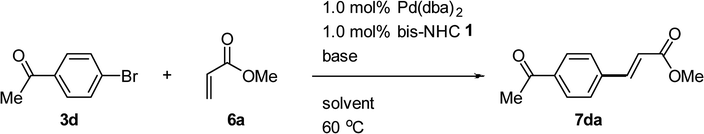
|
| Entry |
Base |
Solvent |
Conv.b |
Entry |
Base |
Solvent |
Conv.b |
| Reaction condition: 3d (1.0 mmol), 6a (1.5 mmol), Pd(dba)2 (1.0 mol%), bis-NHC 1c (1.0 mol%), base (3.0 mmol) and solvent (5.0 mL) were stirred under N2 for 24 h at 60 °C. Conversion was determined by 400 MHz NMR. |
| 1 |
K3PO4·H2O |
DMF |
75 |
6 |
CsF |
DMF |
99 |
| 2 |
K2CO3 |
DMF |
7 |
7 |
CsF |
Toluene |
6 |
| 3 |
KOtBu |
DMF |
8 |
8 |
CsF |
Dioxane |
9 |
| 4 |
KF |
DMF |
6 |
9 |
CsF |
tBuOH |
5 |
| 5 |
Et3N |
DMF |
18 |
10 |
CsF |
CH3CN |
14 |
After optimizing the reaction conditions, the substrate scope of the Mizoroki–Heck reaction was extended to a variety of substituted aryl bromides/iodides and substituted olefins (Table 4). Aryl bromides with electron-withdrawing groups, such as ketones and aldehydes, reacted with styrene to provide the corresponding products at high yields at 60 °C (entries 2 and 3). 4-Bromobenzaldehyde 3g can be coupled with 3-nitrostyrene 6c or methyl acrylate 6a with 96% and 98% yields (entries 4 and 5), respectively. Even a moderate yield (61%) was achieved by treating 4-bromobenzaldehyde 3g and deactivating 2-vinylnaphthelene 6d (entry 6). Moderate yields were achieved by treating 4-bromobenzaldehyde 3g or ethyl 4-bromobenzoate 3s with electron-donating groups on the olefin moiety 3a (entries 7 and 8). The bis-NHC–Pd catalytic system exhibited less reactivity toward aryl bromides with a strong electron-donating substituent. Aryl iodides with electron-donating groups, such as methoxy group 3a′, reacted with methyl acrylate 6a or acrylonitrile 6f to form the desired products with a 98% or 71% yield, respectively (entries 10 and 12). Iodobenzene 3t was coupled with olefins including methyl acrylate 6a, acrylonitrile 6f, and styrene 6b with yields between 72% and 96% (entries 9, 11 and 13). Indeed, the bis-NHC–Pd catalytic system exhibited a high catalytic reactivity under mild conditions that present the successful application to low boiling-point acrylonitrile and aryl halides.
Table 4 Mizoroki–Heck reactions with different substituents catalyzed by 1c/Pda
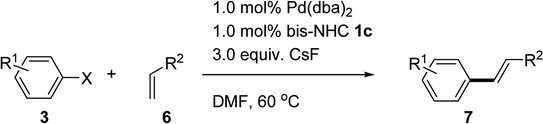
|
| Entry |
X |
R1 |
R2 |
Yield (%)b |
| Reaction condition: 2 (1.0 mmol), 3 (1.5 mmol), Pd(dba)2 (1.0 mol%), bis-NHC 1c (1.0 mol%), CsF (3.0 mmol) and DMF (5.0 mL) were stirred under N2 for 24 h at 60 °C. Isolated yield. |
| 1 |
Br |
4-COMe |
CO2Me |
7da (94) |
| 2 |
Br |
4-COEt |
C6H5 |
7eb (93) |
| 3 |
Br |
4-CHO |
C6H5 |
7gb (96) |
| 4 |
Br |
4-CHO |
3-NO2-C6H4 |
7gc (96) |
| 5 |
Br |
4-CHO |
CO2Me |
7ga (98) |
| 6 |
Br |
4-CHO |
2-Naphthyl |
7gd (61) |
| 7 |
Br |
4-CHO |
4-MeO-C6H4 |
7ge (67) |
| 8 |
Br |
4-CO2Et |
4-MeO-C6H4 |
7se (66) |
| 9 |
I |
H |
CO2Me |
7ta (96) |
| 10 |
I |
4-MeO |
CO2Me |
7aa (98) |
| 11 |
I |
H |
CN |
7tf (78) |
| 12 |
I |
4-MeO |
CN |
7af (71) |
| 13 |
I |
H |
C6H5 |
7tb (72) |
Catalyst testing for the Friedel–Crafts reaction
The Friedel–Crafts alkylation reaction of indoles with nitroalkenes is one of the most crucial methods for preparing tryptamine and its derivatives. There are very few reports on Friedel–Crafts alkylation catalyzed by bis-NHC–Pd. In fact, the Pd(II) complex can catalyze a Friedel–Crafts reaction as a Lewis acid.10 In 2008, Shi et al. have reported the synthesis of a bis-NHC–Pd complex derived from binaphthyl-2,2′-diamine as well as its application for the Friedel–Crafts alkylation reaction of indole with nitrostyrene.11 The Friedel–Crafts reaction was performed in the presence of 5 mol% bis-NHC–Pd complex and 5 equiv. of indole. Using low loadings of the bis-NHC–Pd complex and reducing the equivalents of indole are challenging for the Friedel–Crafts alkylation reaction catalyzed by bis-NHC–Pd.
In general, the acid-catalyzed conjugate addition of indoles requires careful control of the acidity to prevent polymerization.12 In 2011, Stephens et al. reported that sodium methoxide (NaOMe) led to the polymerization of the nitrostyrene.13 In fact, the use of a base was necessary for the in situ formation of the bis-NHC–Pd complex from bis-NHC and Pd(OAc)2. To avoid polymerization in basic conditions, the corresponding bis-NHC–Ag complex was employed as a carbene transfer agent to form the bis-NHC–Pd complex. The Friedel–Crafts alkylation reactions were tested in isopropyl alcohol (IPA) by the reaction of indole (3.0 equiv.) with nitrostyrene at 30 °C (Table 5). When varying the Pd(OAc)2/1c–Ag complex ratio from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we established that the ratio of 2
1, we established that the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provided the optimal yield at 96% (entry 4) (Fig. 2).
1 provided the optimal yield at 96% (entry 4) (Fig. 2).
Table 5 Screening optimized reaction condition for Friedel–Crafts alkylation reactiona
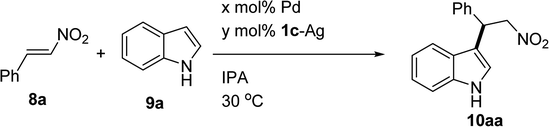
|
| Entry |
Pd salt (mol%) |
Pd/1c–Ag complex ratio |
Time (h) |
Yield (%)b |
| Reaction conditions: nitrostyrene 8a (0.5 mmol), indole 9a (1.5 mmol), Pd salt (as indicated), 1c–Ag complex (as indicated), and isopropanol (3.0 mL) are stirred for 24 h at 30 °C under N2. Isolated yield. |
| 1 |
Pd(OAc)2 (2.0) |
4/1 |
24 |
66 |
| 2 |
Pd(OAc)2 (1.0) |
2/1 |
24 |
61 |
| 3 |
Pd(OAc)2 (1.0) |
1/1 |
24 |
78 |
| 4 |
Pd(OAc)2 (2.0) |
2/1 |
24 |
96 |
| 5 |
Pd(OAc)2 (2.0) |
2/1 |
12 |
83 |
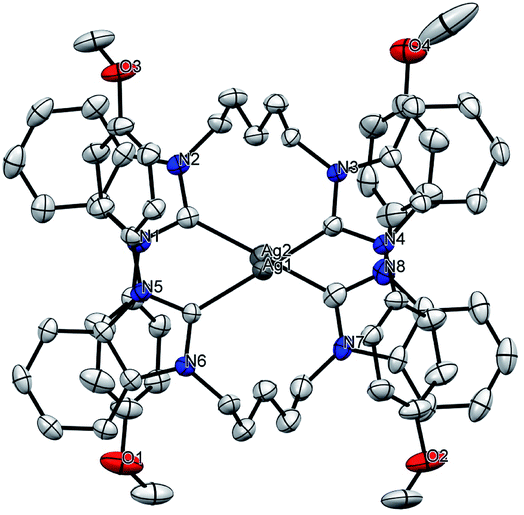 |
| | Fig. 2 Solid-state molecular structure of 1c–Ag. Hydrogen atoms, PF6− and solvent molecules are omitted for clarity. | |
A wide range of substituted nitrostyrene compounds were coupled with indole derivatives and examined in the presence of 2.0 mol% of the Pd(OAc)2/1c–Ag catalyst in IPA at room temperature and the results are summarized in Table 6. The results revealed that both electron-donating groups (8b–8d) and electron-withdrawing groups (8e–8g) on the nitroalkenes can form the corresponding products with favorable yields (70–86%) (entries 2–7). In addition, the catalytic system performed well with thiophene and naphthalene as substituents on the nitroalkenes with 87% and 90% yields, respectively (entries 8 and 9). The alkylation reaction using 2-methylindole 9b with nitroalkenes was also studied. Our experimental results demonstrated that when 9b was employed in the catalytic system, the yields were superior to those with 9a (entries 10–17). The electron-donating group on the C2 position of the indole increased the nucleophilicity of the indole. The yield using substrates with substituents in the ortho position were increased from 86% to 95% (entry 2 vs. entry 10) and from 80% to 94% (entry 5 vs. entry 11), respectively. Heterocyclic systems, such as thiophene and N-methylpyrrole, could also be used in the catalytic system with 92% and 91% yields, respectively (entries 14 and 15). Conjugated nitroalkene 8m could still undergo the 1,4-addition instead of the 1,6-addition with a high regioselectivity and a 93% yield (entry 16). The reactivity of indole with substituents on the nitrogen decreased drastically. A 56% yield was obtained at 80 °C for 24 h (entry 18). A fluorine atom on the 6-position of indole decreased its nucleophilicity and only provided a 61% yield for the Friedel–Crafts alkylation product (entry 19). Compound 9e, where a chlorine atom is present at the 5-position and a methyl group is attached on the 2-position of the indole, produced a 90% yield (entry 20). Electron-donating groups at the 2- and 5-positions of the indole also increase its nucleophilicity, which led to a 92% of yield after transformation (entry 21) (Fig. 3).
Table 6 The Friedel–Crafts alkylation reaction of alkenes and indoles catalyzed by 1c–Ag/Pda
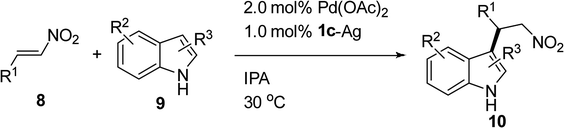
|
| Entry |
8 |
9 |
Yield (%)b |
| R1 |
R2 |
R3 |
| Reaction condition: Pd(OAc)2 (2.0 mol%), 1c–Ag (1.0 mol%), 8 (0.5 mmol), 9 (1.5 mmol), and IPA (3.0 mL) were stirred under N2 at 30 °C for 24 h. Isolated yield. The reaction was conducted at 80 °C for 24 h. |
| 1 |
C6H5 (8a) |
H |
H |
10aa (96) |
| 2 |
2-MeC6H4 (8b) |
H |
H |
10ba (86) |
| 3 |
3-MeC6H4 (8c) |
H |
H |
10ca (70) |
| 4 |
4-MeC6H4 (8d) |
H |
H |
10da (81) |
| 5 |
2-ClC6H4 (8e) |
H |
H |
10ea (80) |
| 6 |
3-ClC6H4 (8f) |
H |
H |
10fa (71) |
| 7 |
4-ClC6H4 (8g) |
H |
H |
10ga (70) |
| 8 |
2-Thiophen (8h) |
H |
H |
10ha (87) |
| 9 |
1-Naphthyl (8i) |
H |
H |
10ia (90) |
| 10 |
2-MeC6H4 (8b) |
H |
2-Me |
10bb (95) |
| 11 |
2-ClC6H4 (8e) |
H |
2-Me |
10eb (94) |
| 12 |
4-MeOC6H4 (8j) |
H |
2-Me |
10jb (85) |
| 13 |
2-Naphthyl (8k) |
H |
2-Me |
10kb (98) |
| 14 |
2-Thiophen (8h) |
H |
2-Me |
10hb (92) |
| 15 |
2-MeNC4H3 (8l) |
H |
2-Me |
10lb (91) |
| 16 |
C6H5CHCH (8m) |
H |
2-Me |
10mb (93) |
| 17 |
C6H5 (8a) |
H |
2-Me |
10ab (90) |
| 18c |
C6H5 (8a) |
H |
1-Me |
10ac (56) |
| 19 |
C6H5 (8a) |
6-F |
2-Me |
10ad (61) |
| 20 |
C6H5 (8a) |
5-Cl |
2-Me |
10ae (90) |
| 21 |
C6H5 (8a) |
5-Me |
2-Me |
10af (92) |
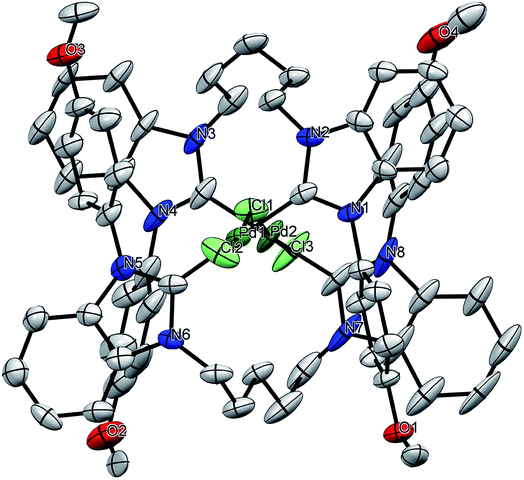 |
| | Fig. 3 Solid-state molecular structure of 1c–Pd. Hydrogen atoms, PF6− and solvent molecules are omitted for clarity. | |
Conclusion
In summary, the bis-NHC–Pd catalytic system exhibited a wide range of applications for the Pd-catalyzed reactions, such as the Suzuki–Miyaura reaction, the Mizoroki–Heck coupling reaction, and the Friedel–Crafts alkylation reaction. This was true whether it was generated from bis-NHC and Pd(OAc)2 in situ at 60 °C or using 1c–Ag with Pd(OAc)2 via transmetallation at 30 °C. The catalytic activity of the bis-NHC–Pd catalytic system underwent successful coupling reaction with high activities at 1.0 mol% catalyst loading. The catalytic system exhibited a high selectivity between Suzuki–Miyaura cross-coupling and Buchwald–Hartwig amination reactions at room temperature. The aryl–vinyl coupling reaction of low boiling-point acrylonitrile with aryl halides was possible under mild conditions. The catalytic system could form the corresponding adducts with favorable yields whether highly steric, electron-donating, or electron-withdrawing groups were attached on the nitroalkene. Moreover, the Friedel–Crafts adducts with indoles bearing different substituents were obtained, which overcome the issues encountered when the reaction is catalyzed by Lewis acids at ambient temperature.
Experimental section
Materials and methods
Reactions were analyzed using pre-coated silica gel 60 (F-254) plates (0.2 mm layer thickness). All products were purified by column chromatography (silica gel, 0.040–0.063 μm) using n-hexane/ethyl acetate as the eluent. Melting points were determined using a Mel-Temp 1001D (Barnstead/Thermo) digital melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Varian Mercury 400 spectrometer in a solution CDCl3 with chemical shifts (δ) given in ppm relative to TMS. J-values are given in Hz. Chloroform (δ = 7.26) was used as internal standard in the 1H NMR spectra. The central peak of CDCl3 (δ = 77.0) was used as internal standard in the 13C NMR spectra. High resolution mass spectra were recorded using a Finnigan/Thermo Quest MAT 95XL mass spectrometer. Mass spectra were obtained using either electron impact (EI) or electrospray ionization (ESI) method. The further details for catalysts synthesis and characterization can be found in the ESI.†
Typical reaction procedure
General procedures for Suzuki–Miyaura coupling reaction. All manipulations were carried out under nitrogen using dried solvent. The mixture of Pd(OAc)2 (0.5 mol%), 1c (0.5 mol%), K3PO4·H2O (1.5 mol%) and t-BuOH (3.0 mL) were stirred under N2 at 60 °C for 1 h, followed by addition of aryl halide 3 (1.0 mmol), arylboronic acid 4a or 4b (1.5 mmol), and K3PO4·H2O (3.0 mmol). The reaction mixture was stirred at 30 °C for 24 h. After completion of the reaction, monitored by TLC, the reaction was quenched by water (3.0 mL). The aqueous layer was extracted with EtOAc (3.0 mL × 3). The combined organic layers were dried over anhydrous MgSO4 and then filtered. The solvent was evaporated under reduced pressure and the corresponding crude product of the Suzuki–Miyaura coupling reaction was purified by chromatography.
General procedures for Mizoroki–Heck coupling reaction. All manipulations were carried out under nitrogen using dried solvent. Olefin 6 (1.50 mmol), aryl halide 3 (1.00 mmol), CsF (2.00 mmol), bis-NHC 1c (1.0 mol%) and Pd(dba)2 (1.0 mol%) were charged to the Schlenk tube. Further dry DMF (5.0 mL) was added. The mixture was stirred at 60 °C for 24 h. After completion of the reaction, monitored by TLC, the reaction was quenched by water (5.0 mL). The aqueous layer was extracted with EtOAc (5.0 mL × 3). The combined organic layers were dried over anhydrous MgSO4 and then filtered. The solvent was evaporated under reduced pressure and the corresponding crude product of the Mizoroki–Heck coupling reaction was purified by chromatography.
General procedures for Friedel–Crafts alkylation reaction. All manipulations were carried out under nitrogen using HPLC grade isopropanol. Indole 9 (1.50 mmol), nitroalkene 8 (0.50 mmol), 1c–Ag (0.50 mol%), and Pd(OAc)2 (1.0 mol%) were charged to the Schlenk tube. Isopropanol (3.0 mL) was added. The mixture was stirred at 30 °C for 24 h. After completion of the reaction, monitored by TLC, the reaction was quenched by water (3.0 mL). The aqueous layer was extracted with EtOAc (3.0 mL × 3). The combined organic layers were dried over anhydrous MgSO4 and then filtered. The solvent was evaporated under reduced pressure and the corresponding crude product of the Friedel−Crafts alkylation reaction was purified by chromatography.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We thank the Ministry of Science and Technology of the Republic of China (106WFA0550361) for financial support.
References
- Á. Molnár, in Palladium-Catalyzed Coupling Reactions, Wiley-VCH, Weinheim, 1st edn, 2013 Search PubMed.
-
(a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef PubMed;
(b) V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC.
- For selected samples for bis-NHC–Pd catalyzed Suzuki–Miyaura coupling reaction, see:
(a) L. Ngodwana, S. Bose, V. Smith, W. A. L. van Otterlo and G. E. Arnott, Eur. J. Inorg. Chem., 2017, 1923–1929 CrossRef;
(b) Ü. Yilmaz, N. Şireci, S. Deniz and H. Küçükbay, Appl. Organomet. Chem., 2010, 24, 414–420 Search PubMed;
(c) K. B. Avery, W. G. Devine, C. M. Kormos and N. E. Leadbeater, Tetrahedron Lett., 2009, 50, 2851–2853 CrossRef;
(d) Q. Xu, W.-L. Duan, Z.-Y. Lei, Z.-B. Zhua and M. Shi, Tetrahedron, 2005, 61, 11225–11229 CrossRef;
(e) G. Nan, B. Rao and M. Luo, ARKIVOC, 2011, 29–40 Search PubMed;
(f) M. Shi and H.-X. Qian, Tetrahedron, 2005, 61, 4949–4955 CrossRef;
(g) L. Seva, W.-S. Hwang and S. Sabiah, J. Mol. Catal. A: Chem., 2016, 418, 125–131 CrossRef;
(h) Q.-X. Liu, L.-X. Zhao, X.-J. Zhao, Z.-X. Wang, A.-H. Chen and X.-G. Wang, J. Organomet. Chem., 2013, 731, 35–48 CrossRef;
(i) S. Demir, I. Özdemir and B. Çetinkaya, Appl. Organomet. Chem., 2006, 20, 254–259 CrossRef;
(j) M. Charbonneau, G. Addoumich, P. Oguadinma and A. R. Schmitzer, Organometallics, 2014, 33, 6544–6549 CrossRef.
- For selected samples for bis-NHC–Pd catalyzed Mizoroki–Heck coupling reaction, see:
(a) S. Demir, I. Özdemir and B. Cetinkaya, Appl. Organomet. Chem., 2009, 23, 520–523 CrossRef;
(b) J. D. Blakemore, M. J. Chalkley, J. H. Farnaby, L. M. Guard, N. Hazari, C. D. Incarvito, E. D. Luzik, Jr. and H. W. Suh, Organometallics, 2011, 30, 1818–1829 CrossRef;
(c) D. S. Clyne, J. Jin, E. Genest, J. C. Gallucci and T. V. RajanBabu, Org. Lett., 2000, 2, 1125–1128 CrossRef PubMed;
(d) H. M. Lee, C. Y. Lu, C. Y. Chen, W. L. Chen, H. C. Lin, P. L. Chiu and P. Y. Cheng, Tetrahedron, 2004, 60, 5807–5825 CrossRef;
(e) C. Allolio and T. Strassner, J. Org. Chem., 2014, 79, 12096–12105 CrossRef PubMed.
- For selected samples for bis-NHC–Pd catalyzed reaction, see:
(a) J. M. Asensio, P. Gomez-Sal, R. Andres and E. de Jesus, Dalton Trans., 2017, 46, 6785–6797 RSC;
(b) Y. J. Cho, Y. N. Lim, W. Yoon, H. Yun and H. Y. Jang, Eur. J. Org. Chem., 2017, 1139–1142 CrossRef;
(c) S. Schick, T. Pape and F. E. Hahn, Organometallics, 2014, 33, 4035–4041 CrossRef;
(d) C. Marshall, M. F. Ward and W. T. A. Harrison, Tetrahedron Lett., 2004, 45, 5703–5706 CrossRef;
(e) M. C. Perry, X. Cui and K. Burgess, Tetrahedron: Asymmetry, 2002, 13, 1969–1972 CrossRef;
(f) C. Zhang and M. L. Trudell, Tetrahedron, 2000, 41, 595–598 CrossRef;
(g) A. B. Mullick, M. S. Jeletic, A. R. Powers, I. Ghiviriga, K. A. Abboud and A. S. Veige, Polyhedron, 2013, 52, 810–819 CrossRef;
(h) Z. Liu and M. Shi, Tetrahedron: Asymmetry, 2009, 20, 119–123 CrossRef.
- Y.-R. Lin, C.-C. Chiu, H.-T. Chiu, D.-S. Lee and T.-J. Lu, Appl. Organomet. Chem., 2018, 32, e3896 CrossRef.
- Z. H. Zhang, T. S. Li and J. J. Li, Monatsh. Chem., 2007, 138, 89–94 CrossRef.
- S. Shekhar, P. Ryberg, J. F. Hartwig, J. S. Mathew, D. G. Blackmond, E. R. Strieter and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 3584–3591 CrossRef PubMed.
- M.-P. Wang, C.-C. Chiu, D.-S. Lee and T.-J. Lu, Appl. Organomet. Chem., 2018, 32, e4348 CrossRef.
- K. Mikami, M. Hatano and K. Akiyama, Top. Organomet. Chem., 2005, 14, 279–321 CrossRef.
- Z. Liu, T. Zhang and M. Shi, Organometallics, 2008, 27, 2668–2671 CrossRef.
-
(a) Z.-P. Zhan, R.-F. Yang and K. Lang, Tetrahedron Lett., 2005, 46, 3859–3862 CrossRef;
(b) H. Firouzabadi, N. Iranpoor and F. Nowrouzi, Chem. Commun., 2005, 789–791 RSC;
(c) M. M. Alam, R. Varala and S. R. Adapa, Tetrahedron Lett., 2003, 44, 5115–5119 CrossRef.
- D. P. Gavin and J. C. Stephens, ARKIVOC, 2011, 407–421 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1551115 and 1536791. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra04094j |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article * and
Ta-Jung Lu
* and
Ta-Jung Lu
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Pd/1c ratio (entry 6). The reactions failed when acetonitrile or tetrahydrofuran (THF) were used as solvents (entries 7 and 8). The reaction could be completed in the presence of tert-butanol (t-BuOH) (entry 9). Then, different bases were screened under a standard two-stage heating condition (60 °C for 1 h followed by 30 °C for 24 h) (entries 10 and 11) which using t-BuOH as the solvent. Although both K2CO3 and K3PO4·H2O showed excellent conversion yields (entries 9 and 10), K3PO4·H2O was selected for further studies according to our previous works.6,9 Further studies demonstrated that the reaction could be completed even when only 0.5 mol% of Pd/1c was employed (entry 12), which was then used as our standard reaction condition for the substrate scope exploration.
1 Pd/1c ratio (entry 6). The reactions failed when acetonitrile or tetrahydrofuran (THF) were used as solvents (entries 7 and 8). The reaction could be completed in the presence of tert-butanol (t-BuOH) (entry 9). Then, different bases were screened under a standard two-stage heating condition (60 °C for 1 h followed by 30 °C for 24 h) (entries 10 and 11) which using t-BuOH as the solvent. Although both K2CO3 and K3PO4·H2O showed excellent conversion yields (entries 9 and 10), K3PO4·H2O was selected for further studies according to our previous works.6,9 Further studies demonstrated that the reaction could be completed even when only 0.5 mol% of Pd/1c was employed (entry 12), which was then used as our standard reaction condition for the substrate scope exploration.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )
)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for the Mizoroki–Heck reaction of 4-bromoacetophenone 3d with methyl acrylate 6a with K3PO4·H2O as a base and DMF as the solvent at 60 °C. However, only a 4% conversion yield was achieved. Next, we increased the conversion yield to 75% by replacing Pd(OAc)2 with Pd(dba)2 (entry 1). The reaction was carried out at 60 °C in a variety of bases and solvents and the experimental results are summarized in Table 3. Using other bases such as triethylamine (Et3N), K2CO3, KOtBu and KF in the presence of the solvents, toluene, dioxane, acetonitrile or t-BuOH resulted in low conversion yields. When CsF in DMF was employed, the conversion yield of 7da was improved to 99% (entry 6).
1 ratio for the Mizoroki–Heck reaction of 4-bromoacetophenone 3d with methyl acrylate 6a with K3PO4·H2O as a base and DMF as the solvent at 60 °C. However, only a 4% conversion yield was achieved. Next, we increased the conversion yield to 75% by replacing Pd(OAc)2 with Pd(dba)2 (entry 1). The reaction was carried out at 60 °C in a variety of bases and solvents and the experimental results are summarized in Table 3. Using other bases such as triethylamine (Et3N), K2CO3, KOtBu and KF in the presence of the solvents, toluene, dioxane, acetonitrile or t-BuOH resulted in low conversion yields. When CsF in DMF was employed, the conversion yield of 7da was improved to 99% (entry 6).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we established that the ratio of 2
1, we established that the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provided the optimal yield at 96% (entry 4) (Fig. 2).
1 provided the optimal yield at 96% (entry 4) (Fig. 2).









