Open Access Article
Open Access ArticleOne-pot preparation of MnOx impregnated cotton fibers for methylene blue dye removal
Jing Wang *,
Lidong Kou,
Zuohua Huang and
Liang Zhao
*,
Lidong Kou,
Zuohua Huang and
Liang Zhao
Institute of Chemistry, Henan Academy of Sciences, 56 Hongzhuan Rd., Zhengzhou, 450002, Henan Prov., P. R. China. E-mail: wangj12@163.com
First published on 13th June 2018
Abstract
Using natural cotton fibers (CF) as a matrix, a series of novel MnOx impregnated cotton fibers (designated as Mn-X@BCF) were prepared in this study through a one-pot sono-assisted KMnO4 reduction process with no additional reducers. The as-prepared Mn-X@BCF was covered by a uniform and dense layer of MnOx nanospheres (10–30 nm), the amount of which was significantly improved by CF pretreatment and linearly correlated with KMnO4 concentration. Specifically, when KMnO4 concentrations increased from 5 mmol L−1 to 100 mmol L−1, the impregnation ratios of MnOx on BCF increased from 0.34% to 14.98% accordingly. Mn-X@BCF showed substantial removal for the typical dye MB. Under the studied conditions, MB removal equilibrium was reached within 10 minutes and solution pH showed no significant influence over a wide pH range (2–11). MB adsorption by Mn-25@BCF obeyed the pseudo-second-order kinetic model and Langmuir model well. The calculated maximum adsorption capacity (Qm) of Mn-25@BCF was 46.3 mg g−1, close to the cumulative adsorption capacity (Qc 45 mg g−1) of MB determined during the eight cycles of adsorption. It was proposed that adsorption followed by partial oxidation was the main mechanism of MB removal by Mn-X@BCF. The as-prepared Mn-25@BCF has great potential as an efficient and low-cost material for dye wastewater treatment because of its inexpensive and renewable source of raw materials, fast MB removal kinetics, wide working pH range and easy solid–liquid separation.
1. Introduction
Manganese oxides (MnOx) have wide applications in environmental remediation because of their excellent adsorption, oxidation and catalytic degradation properties. It was reported that MnOx could be used solely as the adsorbent or oxidant or coupled with other oxidants like hydrogen peroxide (H2O2), potassium peroxymonosulfate (PMS), and ozone (O3) in the catalytic oxidation system for removal of organometallic complexes and organic pollutants (e.g. substituted phenols, dyes, aromatic amines, pesticides) (Table 1).1–3 For example, Cao et al.1 prepared α/β-MnO2 nanorods by a hydrothermal method and found that their removal of rhodamine B (RB) and methylene blue (MB) was extremely high in the presence of H2O2 (i.e. 95%) as compared with that in the absence of H2O2 (i.e. 10%). This indicated that α/β-MnO2 functioned mainly as the catalyst for degradation of dyes using H2O2 as the oxidant. However, the widespread application of MnOx in the form of powders or nanoparticles is limited due to their easy self-aggregation, difficult solid/liquid separation, serious dust contamination, etc. Therefore, direct synthesis of nanomaterials on solid matrixes is attracting increasing interest in terms of engineering applications and synthetic challenges.| Material | Synthetic method | Pollutant | Ref. |
|---|---|---|---|
| a MB-methylene blue.b RB-rhodamine B.c IC-indigo carmine.d CR-Congo red (CR). | |||
| MnO2 nanorods | Hydrothermal | Dyes MBa, RBb | 1 |
| MnO2 nanocorals | KMnO4–MnSO4 reaction | Dye MB | 2 |
| MnOx | Calcination | Phenol | 3 |
| δ-MnO2@clay | KMnO4–MnSO4 reaction | Dye MB | 4 |
| MnO2@cellulose fiber | KMnO4 reduction at RT (by ethanol) | Pb2+ | 5 |
| MnO2@cellulose fiber | KMnO4 reduction at RT (by oleic acid) | HCHO | 6 |
| MnO2@fique fiber | KMnO4 reduction under sonication | Dye ICc | 7 |
| MnO2@cellulose nanofiber | KMnO4 reduction under sonication | Dye MB | 8 |
| MnO2@cotton fiber | KMnO4–MnSO4 reaction | Cu2+, Pb2+, CRd, MB | 9 |
| MnO2@PET fiber | KMnO4 reduction at 90 °C (by oxalate) | HCHO | 10 |
| MnO2/PDA/PAN fiber | KMnO4 reduction at 80 °C | Pb2+ | 11 |
| MnO2@MWCNTs | Co-precipitation | HCHO | 12 |
| MnO2@chitin | KMnO4 reduction at RT | Dye MB | 13 |
| MnO2@Al2O3 | MnAc2 impregnation and calcination | NO | 14 |
Several matrixes have been studied for immobilization of MnOx powders including clays, chitin, MWCNTs, Al2O3, natural and synthetic fibers, etc. (Table 1).4–14 Among them, fibers seem to be one of the most promising matrixes in the practical use due to their various application forms (e.g. filaments, nonwoven fabrics, threads, and cloths), small mass transfer distance, fast reaction kinetic and extremely low pressure drop as compared with traditional materials.15 For example, using a natural fique fiber as the matrix, Chacón-Patiño et al.7 prepared a novel MnO2@fique fiber by the sono-assisted KMnO4 reduction method. The bionanocomposite was able to remove up to 98%, in less than 5 minutes, of the colour present in indigo carmine (IC) contaminated water samples and it can be reused with no substantial drop in dye degradation efficiency. Using a commercial cotton fiber as the matrix, Jiao et al.9 prepared a novel cotton fiber-MnO2 hierarchical composite (C-MHCs) by a two-step strategy “ion exchange-KMnO4–MnSO4 redox reaction” method. The composite showed efficient removal for various pollutants like Cu2+, Pb2+, MB and Congo red (CR), probably through the adsorption mechanism. Using an electrospun polyacrylonitrile (PAN) fiber as the matrix, Li et al.11 prepared a novel MnO2/PDA/PAN fiber by the KMnO4 reduction method at 80 °C. Results showed that PDA coating on PAN fibers was necessary for loading uniform MnO2, and MnO2 and PDA both contributed to the extremely high Pb2+ adsorption capacity (i.e. 185 mg g−1).
Our interest in natural fibers arises from the fact that they offer a sustainable, resourceful and environment friendly venue to create novel functional materials. Cotton, the most widely used natural fiber in the world, is low-cost and clean to produce, and has wide applications in textile cloth, daily care, and medicine.16,17 The main component of cotton fiber is cellulose, made up of repeating units of α-glucose. Previous studies showed that various reagents like organic acids (e.g. oleic acid, oxalate), alcohols (e.g. ethanol), polysaccharides (e.g. glucose, chitin) and low-valence Mn (e.g. MnSO4) could be used as the reducing agents for KMnO4 for preparation of MnOx and its composites.4–11,13 Therefore, it's reasonable to propose that cotton fiber, composed primarily of α-glucose, can be used to reduce KMnO4 itself, without any additional reducing agents. Besides, the abundant OH groups in cotton structure are apt to interact and stabilize MnOx nanostructures, which is beneficial for MnOx uniform deposition.
Methylene Blue (MB), as a cationic phenothiazine dye, has a wide application in industries like textile, paper, rubber, plastics, cosmetics and food, etc.13 Besides, it can also be used as chemical indicators, biological stains and drugs. However, due to its potential hazards to humans and high resistance to biodegradation, MB has become one of the most attracting environmental pollutants that should be removed by various methods, among which adsorption and catalytic degradation are deemed as the most efficient methods.1,2,4,8,9,13,18–22 As summarized in Table 2, all of the studied materials, either MnOx-based materials or other materials showed significant removal for MB. However, there is still a controversy about whether or not oxidants, microwave, UV-Vis radiation or other additives should be applied as assistants for the MnOx-based materials. Moreover, it is still imperative to develop more efficient and cost-effective materials that are more appropriate to practical applications.
| Material | MB removal property | Ref. |
|---|---|---|
| MnO2 nanocorals | 98.6% in 20 min (C0 5 mg L−1) Qm 41 mg g−1 | 2 |
| δ-MnO2@clay | 92% in 100 min (C0 80 mg L−1) | 4 |
| MnO2@cellulose nanofiber | 99.8% in 2 min (C0 80 mg L−1) | 8 |
| MnO2@cotton fiber | Qm 247 mg g−1 | 9 |
| MnO2@chitin | 99.9% in 2.5 min (C0 20 mg L−1) | 13 |
| MnOx | Qm 230 mg g−1 (microwave) or 27 mg g−1 (MnOx alone) | 18 |
| Co–Mn–Fe complex oxide | 92% in 60 min (C0 50 mg L−1) | 19 |
| Fe3O4 nanoparticles | 99.9% in 2 min (C0 10 mg L−1) | 20 |
| ZnO/Ag/CdO | 98.3% in 90 min | 21 |
| Guar gum-cerium(IV) tungstate | 96% in 120 min (C0 0.32 mg L−1) | 22 |
In this study, we used medical absorbent cotton fiber as the matrix and developed a one-pot sono-assisted KMnO4 reduction approach to deposit MnOx uniformly onto cotton fiber. The as-synthesized composites were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and scanning electron microscope (SEM). Their removal performance and the related mechanism for the typical MB dye were studied by both static adsorption tests and instrumental analyses. These composites can not only avoid the disadvantages of MnOx powders encountered in water treatment but also improve the MnOx performance, providing promising potentials in a variety of practical applications.
2. Experimental
2.1 Materials
Commercial medical absorbent cotton fibers (CF, filament length 3–7 cm, tensile strength 0.02–0.06 N) were used as the matrix for preparation of the MnOx composites. Spectroscopic grade KBr (purity above 99.0%) was purchased from Sinopharm Chemical Reagent Co. Ltd. (China) for FTIR analyses. All other chemicals like methylene blue (MB, purity above 96.0%), HCl, NaOH, KMnO4 were of analytical grade and used as received.2.2 Preparation of Mn-X@BCF
Prior to use, the fibers were washed thoroughly by deionized (DI) water, then pre-treated sequentially by 5% HCl-DI water–6% NaOH-DI water, and finally oven-dried at 60 °C until constant weight (the product was designated as BCF). Then, certain amount of raw CF or pre-treated BCF were submerged into a series of KMnO4 solutions (5–100 mmol L−1), and the mixtures were irradiated under sonication (28 kHz, 140 W) for 1 hour. MnOx nanoparticles were deposited in situ on the surface of CF or BCF. After standing overnight, the final products were separated, washed with DI water for 3 times to remove any possible residuals, dried at 60 °C until constant weight and then stored in a desiccator prior to use. The products were labelled as Mn-X@CF and Mn-X@BCF (X equals to 5–100, indicating KMnO4 concentrations). Amounts of MnOx on the composites were determined by the mass gain (G, %) calculated using the following equation
 | (1) |
2.3 Characterization of Mn-X@BCF
Deposition of MnOx on BCF surfaces were investigated by SEM, FTIR and XPS. Surface morphologies of the samples were observed by SEM (S-4800, Hitachi, Japan) with 1000-, 5000-, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000- and 100
000- and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold magnification. FTIR spectra were collected in 400–4000 cm−1 using a Thermo Nicolet IR 200 instrument (Thermo Electron Corp., USA). Chemical compositions of the sample surface were analysed by XPS on an Axis Ultra spectrometer (Kratos Analytical, UK). XPS peak 4.1 software was used to peak-fit the high resolution spectra of C1s, Mn2p, S2p and N1s after being calibrated to the binding energy of C1s at 284.6 eV.23
000-fold magnification. FTIR spectra were collected in 400–4000 cm−1 using a Thermo Nicolet IR 200 instrument (Thermo Electron Corp., USA). Chemical compositions of the sample surface were analysed by XPS on an Axis Ultra spectrometer (Kratos Analytical, UK). XPS peak 4.1 software was used to peak-fit the high resolution spectra of C1s, Mn2p, S2p and N1s after being calibrated to the binding energy of C1s at 284.6 eV.23
2.4 Batch adsorption tests
Batch tests were conducted to assess MB removal properties by the as-synthesized Mn-X@BCF samples. In a typical test, 0.1 g of fiber was mixed with 50 mL of MB solution (pH0 2–11, C0 20 mg L−1) on a shaking table (THZ-98C, Shanghai, China) at 25 °C and 150 rpm. After reaching equilibrium, the fibers were taken out and analysed by FTIR, SEM and XPS, while the filtrate was analysed for MB concentration by spectrophotometer (UV1800, Shimadzu, Japan) at 664 nm according to previously reported procedure.24 Every sample was analysed in duplicate to guarantee accuracy of the data. MB removal rate (R, %) and adsorption capacity (Qe, mg g−1) were obtained using the equations
 | (2) |
 | (3) |
3. Results and discussion
3.1 Preparation of Mn-X@BCF
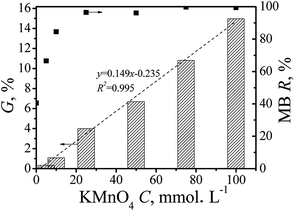
Deposition of MnOx was evaluated as a function of matrix properties and KMnO4 concentrations. Preliminary tests showed pre-treatment of CF affected MnOx deposition substantially. For the raw CF, MnOx was hardly deposited as indicated from the nearly zero mass gain and the resulting very pale brown colour. However, for the pre-treated BCF, significant amounts of MnOx were deposited as a function of KMnO4 concentrations. As shown in Fig. 1, when KMnO4 concentration was 5 mmol L−1, the mass gain of BCF was 0.34%. Increasing KMnO4 concentrations up to 10–100 mmol L−1 resulted in a steady increase of mass gain of BCF to 1.13–14.98%. Under the studied conditions, the mass gain of BCF could be fitted linearly with KMnO4 concentrations, and the fitting correlation coefficient R2 was 0.995. Depending on MnOx amount, colour of Mn-X@BCF turned brown or dark brown. As compared with the study of Jiao et al.,9 a more convenient one-pot method was used in this study to deposit MnOx directly on cotton fiber.3.2 Characterization of Mn-X@BCF
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000- and 100
000- and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold magnification. As shown in Fig. 2, it was obvious that BCF used in this study was flat rather than round, with a diameter of 10–20 μm and numerous lines parallel to the length of the fiber. After MnOx deposition, a dense and uniform layer of particles were formed on the BCF surface. By close observation, these particles were nanospheres mostly in the diameter of 10–30 nm. Further analyses by Nano Measurer software of randomly selected 100 particles showed that the average particle size of MnOx nanospheres on Mn-25@BCF, Mn-100@BCF and Mn-25@BCF with MB were 19.5 ± 5.3 nm, 16.7 ± 4.6 nm, 20.0 ± 6.8 nm, respectively (Fig. 2), much smaller than the previously reported MnO2 granules (i.e. 200–350 nm).9 It was obvious that even at an extremely high KMnO4 concentration, the fibers retained their morphology and shape well during the modification reactions. Besides, particle size of MnOx nanoparticles, distributed uniformly on BCF surface, did not show a substantial increase as compared with that prepared at low KMnO4 concentration. This indicated that nanoparticle aggregation, reported in previous literature,7 did not occur in this study, probably because of the dual function of sonication and cotton fiber stabilization. Furthermore, MB adsorption showed no significant influence on morphology and particle size of the nanostructured MnOx.
000-fold magnification. As shown in Fig. 2, it was obvious that BCF used in this study was flat rather than round, with a diameter of 10–20 μm and numerous lines parallel to the length of the fiber. After MnOx deposition, a dense and uniform layer of particles were formed on the BCF surface. By close observation, these particles were nanospheres mostly in the diameter of 10–30 nm. Further analyses by Nano Measurer software of randomly selected 100 particles showed that the average particle size of MnOx nanospheres on Mn-25@BCF, Mn-100@BCF and Mn-25@BCF with MB were 19.5 ± 5.3 nm, 16.7 ± 4.6 nm, 20.0 ± 6.8 nm, respectively (Fig. 2), much smaller than the previously reported MnO2 granules (i.e. 200–350 nm).9 It was obvious that even at an extremely high KMnO4 concentration, the fibers retained their morphology and shape well during the modification reactions. Besides, particle size of MnOx nanoparticles, distributed uniformly on BCF surface, did not show a substantial increase as compared with that prepared at low KMnO4 concentration. This indicated that nanoparticle aggregation, reported in previous literature,7 did not occur in this study, probably because of the dual function of sonication and cotton fiber stabilization. Furthermore, MB adsorption showed no significant influence on morphology and particle size of the nanostructured MnOx.
| Samples | Atomic concentration, % | |||||
|---|---|---|---|---|---|---|
| C | O | N | S | Mn | O/C | |
| BCF | 60.83 | 38.73 | 0.44 | — | — | 0.64 |
| Mn-25@BCF | 57.72 | 40.99 | 0.37 | — | 0.92 | 0.71 |
| Mn-100@BCF | 55.41 | 42.95 | 0.27 | — | 1.38 | 0.78 |
| Mn-25@BCF, with MB | 62.24 | 36.04 | 0.83 | 0.27 | 0.62 | 0.58 |
Detailed surface information of the samples was further deduced by the high-resolution scan of C1s, Mn2p, S2p and N1s (Fig. 4). From the C1s scan, it can be seen that C1s spectra of BCF could be grouped into three peaks with binding energy (BE) being centred at 284.6, 286.2 and 287.6 eV, corresponding to skeleton carbon (C–C/C–H, C1), single oxygen bonded carbon (C–O, C2), and glucoside bonded carbon or single oxygen double-bonded carbon (O–C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C3), respectively.23 After MnOx loading, especially at a high KMnO4 concentration (e.g. 100 mmol L−1), a small amount of carboxyl carbon (O
O, C3), respectively.23 After MnOx loading, especially at a high KMnO4 concentration (e.g. 100 mmol L−1), a small amount of carboxyl carbon (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, C4, 292.6 eV) appeared and the ratio of C3 increased obviously. This was probably due to the redox reaction between KMnO4 and BCF. In accordance with the reduction of KMnO4 to MnOx, some amounts of carbon in low oxidation states (e.g. C1, C2) were oxidized to their high oxidation states (e.g. C3, C4). This indicated that BCF used in this study functioned not only as the stabilizer for MnOx, but also as the reducer for MnOx formation. Peak fitting of the Mn2p scan illustrated that Mn2p could be fitted with one doublet-peak (ΔBE = 11.6 eV) with Mn2p3/2 being situated at 641.7 eV, which could be ascribed to Mn2O3 in agreement with the earlier reports.5,14 It should be noted that KMnO4 concentration and MB adsorption showed no significant influence on MnOx existing forms. After contact with MB, characteristic peaks of S2p (164.5 eV) and N1s (398.5 eV) appeared on XPS spectra of Mn-25@BCF.24,27,28 The symmetric shape of N1s peak demonstrated that there was only one state of nitrogen because a positive charge in MB+ was evenly distributed in the molecule due to the p-conjugated effect.27,28 While the S2p3/2 BE was similar to that of MB as reported previously, which was very close to S2p3/2 of thiophene (164.3 eV).28 These results illustrated that the chemical environment of N and S heterocycle was relatively stable and MB was removed by Mn-25@BCF mainly through the adsorption mechanism.
C–O, C4, 292.6 eV) appeared and the ratio of C3 increased obviously. This was probably due to the redox reaction between KMnO4 and BCF. In accordance with the reduction of KMnO4 to MnOx, some amounts of carbon in low oxidation states (e.g. C1, C2) were oxidized to their high oxidation states (e.g. C3, C4). This indicated that BCF used in this study functioned not only as the stabilizer for MnOx, but also as the reducer for MnOx formation. Peak fitting of the Mn2p scan illustrated that Mn2p could be fitted with one doublet-peak (ΔBE = 11.6 eV) with Mn2p3/2 being situated at 641.7 eV, which could be ascribed to Mn2O3 in agreement with the earlier reports.5,14 It should be noted that KMnO4 concentration and MB adsorption showed no significant influence on MnOx existing forms. After contact with MB, characteristic peaks of S2p (164.5 eV) and N1s (398.5 eV) appeared on XPS spectra of Mn-25@BCF.24,27,28 The symmetric shape of N1s peak demonstrated that there was only one state of nitrogen because a positive charge in MB+ was evenly distributed in the molecule due to the p-conjugated effect.27,28 While the S2p3/2 BE was similar to that of MB as reported previously, which was very close to S2p3/2 of thiophene (164.3 eV).28 These results illustrated that the chemical environment of N and S heterocycle was relatively stable and MB was removed by Mn-25@BCF mainly through the adsorption mechanism.
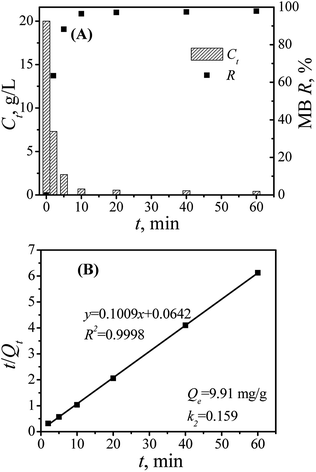
3.3 MB removal by Mn-X@BCF
Pseudo-second-order kinetic model:
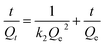 | (4) |
Langmuir:
 | (5) |
4. Conclusions
A series of novel MnOx impregnated cotton fibers were prepared in this study through a one-pot sono-assisted KMnO4 reduction process. Both of cotton fiber pre-treatment and KMnO4 concentrations impacted the impregnation ratio of MnOx a lot, which ranged from 0.34% to 14.98%. Analyses of FTIR, SEM and XPS showed that MnOx existed primarily as Mn2O3 nanospheres (10–30 nm) and distributed uniformly on Mn-X@BCF surface with no obvious particle aggregation even at a high KMnO4 concentration (i.e. 100 mmol L−1). The optimal Mn-25@BCF showed an extremely fast removal for MB, with ignorable impact by solution pH in a wide pH range (i.e. pH 2–11). Moreover, Mn-X@BCF can be easily separated from the solution by simply taking the fiber out, making it a competitive material for dye removal in practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding for this research was provided by the Major Science and Technology Special Projects of Henan Province (grant 181100310100). The anonymous reviewers are gratefully acknowledged for their valuable comments and suggestions that help to substantially improve this manuscript.Notes and references
- G. Cao, L. Su, X. Zhang and H. Li, Mater. Res. Bull., 2010, 45, 425 CrossRef.
- M. A. Salam, Chem. Eng. J., 2015, 270, 50 CrossRef.
- E. Saputra, S. Muhammad, H. Sun, H. M. Ang, M. O. Tadé and S. Wang, Appl. Catal., B, 2013, 142, 729 CrossRef.
- M. Zhu, Z. Wang, S. Xu and T. J. Li, J. Hazard. Mater., 2010, 181, 57 CrossRef PubMed.
- S. M. Maliyekkal, K. P. Lisha and T. Pradeep, J. Hazard. Mater., 2010, 181, 986 CrossRef PubMed.
- L. Zhou, J. He, J. Zhang, Z. He, Y. Hu, C. Zhang and H. He, J. Phys. Chem. C, 2011, 115, 16873 Search PubMed.
- M. L. Chacón-Patiño, C. Blanco-Tirado and J. P. Hinestroza, Green Chem., 2013, 15, 2920 RSC.
- Y. Wang, X. Zhang, X. He, W. Zhang, X. Zhang and C. Lu, Carbohydr. Polym., 2014, 110, 302 CrossRef PubMed.
- C. Jiao, J. Tao, S. Xu, D. Zhang, Y. Chen and H. Lin, RSC Adv., 2017, 7, 31475 RSC.
- J. Wang, R. Yunus, J. Li, P. Li, P. Zhang and J. Kim, Appl. Surf. Sci., 2015, 357, 787 CrossRef.
- Y. Li, R. Zhao, S. Chao, B. Sun, C. Wang and X. Li, Chem. Eng. J., 2018, 344, 277 CrossRef.
- J. Miao, C. Li, H. Liu and X. Zhang, J. Nanosci. Nanotechnol., 2018, 18, 3982 CrossRef PubMed.
- R. S. Dassanayake, E. Rajakaruna, H. Moussa and N. Abidi, Int. J. Biol. Macromol., 2016, 93(Pt A), 350 CrossRef PubMed.
- F. Lin, Z. Wang, Q. Ma, Y. Yang and R. Whiddon, Appl. Catal., B, 2016, 198, 100 CrossRef.
- J. Wang, L. Zhao, W. Duan, L. Han and Y. Chen, Ind. Eng. Chem. Res., 2012, 51, 13655 CrossRef.
- J. Wang, B. Zhao, L. Zhao, X. Zhang and D. Zhao, Synth. Met., 2015, 204, 10 CrossRef.
- C. Liu, X. Lei, X. Liang, J. Jia and L. Wang, RSC Adv., 2017, 7, 9744 RSC.
- X. Wang, L. Mei, X. Xing, L. Liao, G. Lv, Z. Li and L. Wu, Appl. Catal., B, 2014, 160–161, 211 CrossRef.
- D. Huang, J. Ma, C. Fan, K. Wang, W. Zhao, M. Peng and S. Komarneni, Appl. Clay Sci., 2018, 152, 230 CrossRef.
- M. Ghaedi, S. Hajjati, Z. Mahmudi, I. Tyagi, S. Agarwal, A. Maity and V. K. Gupta, Chem. Eng. J., 2015, 268, 28 CrossRef.
- R. Saravanan, K. M. Mansoob, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, J. Colloid Interface Sci., 2015, 452, 126 CrossRef PubMed.
- V. K. Gupta, D. Pathania, P. Singh, A. Kumar and B. S. Rathore, Carbohydr. Polym., 2014, 101, 684 CrossRef PubMed.
- J. Wang, B. Deng, H. Chen, X. Wang and J. Zheng, Environ. Sci. Technol., 2009, 43, 5223 CrossRef PubMed.
- K. Zhang, J. Wang, Y. Wang, L. Zhao and Q. Xu, Chem. Eng. J., 2014, 247, 50 CrossRef.
- N. Abidi, L. Cabrales and C. H. Haigler, Carbohydr. Polym., 2014, 100, 9 CrossRef PubMed.
- S. Mopoung and T. Bunterm, Am. J. Appl. Sci., 2016, 13, 814 CrossRef.
- X. Yang, L. Xiong, X. Hu, B. He and G. Chu, Res. Chem. Intermed., 2012, 38, 67 CrossRef.
- L. Zhao, X. Wang, Y. Guo, N. Wu and Y. Xie, Acta Phys.-Chim. Sin., 2003, 19, 896 Search PubMed.
- Y. Li, J. Qu, F. Gao, S. Lv, L. Shi, C. He and J. Sun, Appl. Catal., B, 2015, 162, 268 CrossRef.
- A. Asfaram, M. Ghaedi, A. Goudarzi and S. Hajati, RSC Adv., 2015, 5, 72300 RSC.
- N. Wang, X. Li, J. Yang, Y. Shen, J. Qu, S. Hong and Z. Yu, RSC Adv., 2016, 6, 88897 RSC.
| This journal is © The Royal Society of Chemistry 2018 |