Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antidepressant activity of an aqueous extract from okra seeds†
Fangbo Xia,
Chenchen Li,
Mengqiu Li,
Yonghong Liao,
Xinmin Liu,
Jianyong Si ,
Qi Chang* and
Ruile Pan
,
Qi Chang* and
Ruile Pan *
*
Institute of Medicinal Plant Development, Peking Union Medical College, Chinese Academy of Medical Sciences, No. 151, North Road Malianwa, Haidian District, Beijing 100193, PR China. E-mail: rlpan@implad.ac.cn
First published on 21st September 2018
Abstract
Faced with the increasing incidence of major depression disorder (MDD) and the unsatisfactory effect of current drugs, there has been growing attention on the relation between dietary supplements and MDD prevention. In this research, the antidepressant activity of okra seed extract (OSE) was evaluated with behavioral tests including an open field test, tail suspension test (TST), forced-swimming test (FST) and novelty suppressed feeding test (NSFT) for sub-chronic treatment and chronic sleep-interruption (CSI) animal models. The chemical constituents of OSE were identified by using UPLC-DAD/Q-TOF MS. To investigate the mechanism, the prefrontal cortex and hippocampus were collected to determine neurotransmitters, total antioxidant capacity (T-AOC), superoxide dismutase (SOD) and malondialdehyde (MDA). Blood serum was prepared for the determination of corticosterone (CORT) and adrenocorticotropic hormone (ACTH). Results demonstrated that OSE possessed an antidepressant effect in both sub-chronic treatment and CSI animal models through suppressing the hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis, alleviating oxidative stress and regulating neurotransmitter levels in the hippocampus and frontal cortex. Besides, chemical analysis based on the UPLC-DAD/ESI-Q-TOF MS approach showed that OSE mainly contained catechin and quercetin derivatives. The present study provided a scientific basis for developing okra seeds to be a dietary supplement for MDD prevention.
1. Introduction
Major depression disorder (MDD) is a common brain disorder, which has been considered a leading cause of disease burden worldwide.1 MDD is not only characterized by profound dysregulation of emotion or mood, interest loss and low self-worth, but also associated with cognitive dysfunction, disturbed sleep and appetite, fatigue, and other endocrine or metabolic alterations.2 Due to the poor understanding of the etiology, the available antidepressants in clinical practice still focus on the monoamine disorder hypothesis based on the theory that the monoamine neuro-transmitters are depleted in major depression.Most of current antidepressants are not completely effective (only 33% of depressed patients are sensitive to their first antidepressant medication).3 What's worse, they are associated with many serious adverse effects, such as cardiac toxicity, sexual dysfunction, body weight gain and sleep disorder.4 Recently, there has been growing attention on the relation between dietary supplement (and potential nutritional deficiencies) and mental health. It has been presented that diet and nutrition offer key modifiable targets for the prevention of mental disorders, which play a fundamental role in the promotion of mental health.5 From this point of view, it is important to find a safer, better-tolerated antidepressant from natural plants.
Plant polyphenols are widespread in the natural green plants, and are important secondary metabolites. Plant polyphenols involve various biological activities, such as antioxidant,6 neuroprotection,7,8 anti-inflammation9,10 and curing neurodegenerative and cardiovascular diseases.11 Recently, several phenolic phytochemicals, such as resveratrol,12 curcumin,13 tea polyphenols,14 ferulic acid,15 have been found to exhibit antidepressant-like effects by protecting nerve cells from oxidative damage, and resulting in the remission and functional recovery from oxidative damage.
Okra (Abelmoschus esculentus (L.) Moench) is one of the members of family Malvaceae. It is known by many names, such as lady's finger, bamyah and bhindi, and its pods are eaten raw as well as cooked as a vegetable. Okra is of African origin where it has been cultivated for more than 4000 years, now is also grown in different tropical and warm temperature regions of the world, like Greece, Iran, Egypt, India, Japan, southern United States and Turkey, Philippines and China. The previous studies has proved that okra pods contained high contents of polyphenols and flavonoids16 and possessed antioxidant, neuroprotective, antidiabetic, antihyperlipidemic effects.17–20 Besides, our previous study demonstrated that the contents of polyphenols in okra seeds is about 24 times as much as that in okra skin with Folin–Ciocalteu method, and also proved that the okra seed water extract (OSE) possessed the strong antioxidant and anti-fatigue effects.21 Thus, it is speculated that OSE may have the promising antidepressant function. As for the quality control of okra seeds, it has been proved that the contents of total polyphenols played important roles. To clarify the chemical composition of polyphenols, UPLC-DAD/Q-TOF MS approach was applied to study the chemical constituents of OSE in this research.
The present study aims to illuminate the antidepressant activity of OSE with sub-chronic and chronical sleep interruption (CSI) models. Besides, the phenolic compounds of OSE are identified with UPLC-DAD/Q-TOF MS.
2. Experimental
2.1. Plant material
Fresh okra pods, authenticated by Professor Ruile Pan (the Institute of Medicinal Plant, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China), were gathered from Zhengzhou, Henan Province, China in July, 2015 and the voucher specimens (no. 20150809) have been deposited in Herbarium of the institute.2.2. Sample preparation
Five kilograms of fresh okra pods were divided into okra seeds (1 kg) and okra skins (4 kg). The okra seeds were extracted with 3000 mL boiling water for 3 times (each time for 1 h). Each filtered liquid was combined and concentrated under vacuum, to yield okra seed extract (OSE, 40.5 g). The content of total polyphenols in OSE was 29.5%, which was determined using our previous method.21 Sample was stored at −20 °C for subsequent chemical analysis and animal experiments.2.3. Chemicals and reagents
Paroxetine (PAR) was purchased from the National institutes for Food and Drug Control (Beijing, China). The standard substances of dopamine (DA), 5-hydroxytryptamine (5-HT), norepinephrine (NE), epinephrine (E) and acetylcholine (Ach) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Corticosterone (CORT) and adrenocorticotrophic hormone (ACTH), total antioxidant capacity (T-AOC) assay kit (FRAP method), malondialdehyde (MDA) and superoxide dismutase (SOD) were purchased from Nanjing Jiancheng Biotechnology Institute (Nanjing, China). Acetonitrile and ammonium formate of HPLC grade were obtained from Thermo Fisher Scientific (MA, USA). Ultrapure water was obtained from a Milli-Q water purification system (Millipore, USA). All other chemicals used for analysis were analytical grade, obtained from Beijing Chemical Works (Beijing, China).Fourteen reference compounds for UPLC-DAD/Q-TOF MS analysis were isolated from okra seeds by our research group. Their structures were identified by 13C NMR, 1H NMR and HR-MS, and the purities determined with HPLC method were all over 95%. Their structures were shown in ESI 1.†
2.4. UPLC-DAD/Q-TOF MS analysis
OSE (100 mg) was dissolved in chromatographic methanol (10 mL), and the solution was filtered through 0.22 μm polytetrafluoroethylene filters before analysis.A Waters Acquity Ultra Performance LC system (Waters Corporation, Milford, MA, USA) equipped with a photo diode array detector (DAD, 190–400 nm) was used for analysis, and the system was controlled by the MassLynx V4.1 software (Waters Co., USA). Separations were performed using a Waters HSS T3 column (2.1 mm × 100 mm, 1.7 μm). The mobile phase was composed of methyl alcohol (A) and 0.1% formic acid–water (B). The following solvent gradient system: 3−10% A from 0 to 10 min, 10–65% A from 10 to 35 min, 90% A from 35 to 37 min. The flow rate was 0.3 mL min−1, and column temperature was set at 35 °C. The injection volume was 3 μL.
The mass spectrometric data were collected using a Q-TOF analyzer in a SYNAPT HDMS system (Waters Corporation, Milford, MA, USA) in negative ion modes. The optimization parameters were set as following: the source temperature was set at 100 °C with a cone gas flow of 100 L h−1, a desolvation gas temperature at 350 °C and a desolvation gas flow of 800 L h−1. For the negative ion modes, the capillary voltage was set to 2.5 kV, and the cone voltage was set to 40 V. Centroid data were collected from m/z 100 to 1500 with a scan time of 0.1 s and an interscan delay of 0.02 s over analysis time. Leucine-enkephalin was used as the lock mass (m/z 554.2615 in negative ion mode) at a concentration of 0.5 mg mL−1 with a flow rate of 80 mL min−1.
2.5. Animals
Male ICR mice (17–19 g, 8 weeks) were purchased from the Vital River Laboratories (Qualified no. SCXK 2012-0001, Beijing, China). The mice were housed in groups of 6 animals per cage under a constant temperature (23 ± 2 °C) and humidity (50% ± 10%) on a 12/12 h light/dark cycle (lights on from 08:00 to 20:00). Mice had free access to standard chow diet and sterilized drinking water in the SPF animal house. All experimental procedures were conducted under the supervision and approval of the Academy of Experimental Animal Center of the Institute of Medicinal Plant Development and in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals (Eighth edition).2.6. Experimental design
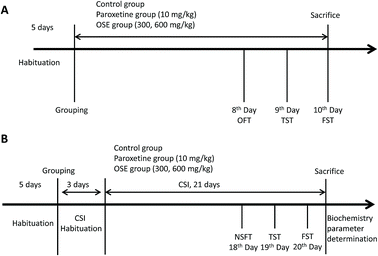
Behavioral tests were carried as follows: open field test (8th day); tail suspension test (9th day) and forced-swimming test (10th day). After finishing the behavioral tests, the mice were sacrificed with ether anesthesia. Detail experimental scheme is displayed in Fig. 1(A).
All groups except for the normal group were subjected to CSI using the Sleep Interruption Apparatus (SIA, a computer controlled rotating drum, Chinese no. 201210356645.X) from 8 am to 11 am each day. The parameters of SIA were set as follows: rotation speed, 60 s per revolution; rotation frequency, rotating 1 min after 2 min pause.
Mice in the control group were housed in a static SIA copy apparatus, and the other groups were taken back to the animal room for 3 days for acclimatization. After 3 days of CSI habituation, the 21 day CSI period began. The mice were provided with water and food in the SIA during the CSI acclimatization period, the 21 day CSI period and the behavioral test periods. Behavioral tests were carried as follows: novelty suppressed feeding test (18th day), tail suspension test (19th day) and forced-swimming test (20th day). After finishing the behavioral tests, the mice were sacrificed for biochemistry parameter analysis. Detail experimental scheme is displayed in Fig. 1(B).
2.7. Behavioral tests
2.8. Tissue collection and biochemical parameter measurement
After the forced swim test, mice were sacrificed under ether anesthesia. The prefrontal cortexes and hippocampals were collected, and immediately put into liquid nitrogen, and then stored at −80 °C until the determination of neurotransmitter, T-AOC, SOD and MDA. Blood serum was prepared for the determination of CORT and ACTH.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. An aliquot of supernatant (100 μL) was added to 10 μL of internal standard solution (300 μg mL−1, 3,4-dihydroxybenzylamine, DHBA), 50 μL of the prepared sample was injected into a LC-MS/MS instrument for assay.
000 rpm for 10 min at 4 °C. An aliquot of supernatant (100 μL) was added to 10 μL of internal standard solution (300 μg mL−1, 3,4-dihydroxybenzylamine, DHBA), 50 μL of the prepared sample was injected into a LC-MS/MS instrument for assay.LC-MS/MS analyses were carried out with an Shimadzu Prominence ultrafast liquid chromatography (UFLC) connected with an Applied Biosystem 5500 Q-Trap mass detector equipped with electrospray ionization source (ESI). Samples were separated on a Phenomenex C18 column (50 × 2.0 mm, 5 μm) kept at 35 °C. The mobile phases consisted of 0.05% formic acid in water (solvent A) and acetonitrile (solvent B), and the flow was set at 0.4 mL min−1. The linear gradient elution program was as follows: 20% B (0–1 min), 20–80% B (1–2 min), 80–20% B (2–3 min), and 20% B (3–5 min). The parameters of mass spectrometer with ESI under positive electrospray ionization mode were set as follows: source temperature, 550 °C; the needle voltage, 4.5 kV; the curtain gas, the ion source gas 1 and the ion source gas 2 were set at 50 psi, 50 psi and 10 psi. MS data were acquired by multiple reaction monitoring (MRM) with m/z 154.2 → 137.1 (DA), m/z 184.4 → 166.2 (E), m/z 170.3 → 152.2 (NE), m/z 177.2 → 160.0 (5-HT), m/z 146.1 → 87.1 (Ach), m/z 140.1 → 123.1 (DHBA, IS).
2.9. Statistical analysis
Data were analyzed by SPSS statistical software (SPSS 19.0 Inc., Chicago, IL, USA). A one-way analysis of variance (One-way ANOVA) with least-significant difference (LCSI) test was used for inter-group comparison. The p value less than 0.05 was considered statistically significant. The results were expressed as mean ± standard error of mean (SEM).3. Results
3.1. Identification of phenolic compounds from OSE by UPLC-DAD/Q-TOF MS analysis
After investigating the influence of mobile phase, kind of chromatographic column, column temperature, flow rate and the intra- and inter-day precisions of UPLC method, typical UPLC-MS profiles in the negative-ion mode of OSE was shown in ESI Fig. 2.† It could be seen that 17 peaks were detected, which were identified by matching with the molecular weight, MS/MS data, UV-visible spectral characteristics, and retention time. The information of 17 tentative identification were shown in Table 1.| No. | tr (min) | MS− (m/z) | MS/MS (m/z) | Formula | Identification |
|---|---|---|---|---|---|
| a Identification by comparing with reference substances. | |||||
| 1 | 5.07 | 1218.2329 | 913.1798 | C60H50O24 | Epigallocat tetramer |
| 609.1238 | |||||
| 305.0684 | |||||
| 2 | 7.71 | 913.1882 | 305.0663 | C45H38O18 | Epigallocat trimmer |
| 609.1227 | |||||
| 3 | 14.30 | 289.0682 | C15H14O6 | Epicatechin | |
| 4 | 14.50 | 305.0654 | C15H14O7 | Gallocatechin | |
| 5 | 18.11 | 289.0735 | C15H14O6 | Catechin (stda) | |
| 6 | 18.85 | 477.1194 | 387.1658 | C22H22O12 | Isorhamnetin-3-O-β-D-glucoside |
| 299.0641 | |||||
| 163.0414 | |||||
| 7 | 20.38 | 595.4586 | 300.0255 | C26H28O16 | Quercetin-3-O-β-D-xyl(1→2)-β-D-glucoside (stda) |
| 8 | 21.98 | 609.4293 | 300.0311 | C27H30O16 | Quercetin-7-O-rutinoside |
| 9 | 22.42 | 479.0829 | 317.0225 | C21H20O13 | Myricetin-3-O-β-D-glucoside |
| 163.0011 | |||||
| 10 | 22.66 | 595.1301 | 300.0277 | C26H28O16 | Quercetin-3-O-β-D-xyl(1→2)-β-D-galactoside |
| 11 | 23.14 | 625.1394 | 300.027 | C27H30O17 | Quercetin-3-O-gentiobioside (stda) |
| 12 | 24.48 | 463.0878 | 300.026 | C21H20O12 | Isoquercitrin (stda) |
| 13 | 24.67 | 609.1459 | 463.0863 | C27H30O16 | Rutin (stda) |
| 301.0346 | |||||
| 14 | 25.32 | 505.1017 | 446.0776 | C23H22O13 | Quercetin-3-O-β-D-6′′-(acetyl)glucopyranoside |
| 301.0360 | |||||
| 15 | 28.86 | 301.0363 | C15H10O7 | Quercetin (stda) | |
| 16 | 31.30 | 299.0226 | 285.0389 | C16H12O6 | Kaempferide (stda) |
| 17 | 31.94 | 315.0535 | C16H12O7 | Isorhamnetin (stda) | |
In conclusion, as shown in the Table 1, 17 compounds, including 5 phenolic acids and 12 flavonoids, were identified or tentatively characterized by analyzing the MS/MS data and comparing with literature data and reference substances.
3.2. Antidepressant effects of OSE on sub-chronic treatment in mice
3.3. Antidepressant effects of OSE on chronical sleep interruption (CSI) mice
4. Discussion
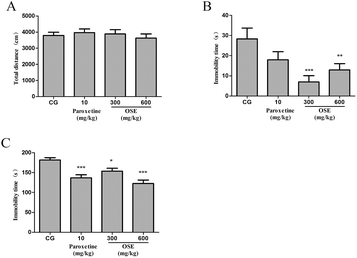
To the best of our current knowledge, it is the first time to demonstrate the antidepressant effects of OSE using both sub-chronic and CSI animal models. From the results of present research, it could be concluded that supplement of OSE could inhibit depression-like behavior by suppressing the hyperactivation of hypothalamic–pituitary–adrenal (HPA) axis, alleviating oxidative stress and normalizing the levels of neurotransmitters in the hippocampus and frontal cortex.TST and FST are two validated models used to assess putative antidepressant compounds, which are based on the fact that animals subjected to short-term, inescapable stress can develop an immobile posture. The immobility, referred to as behavioral despair in animals, is claimed to reproduce a condition similar to human depression.26 In the present study, oral treatment with OSE for 10 days significantly reduced the immobility time in TST and FST, suggesting that OSE has antidepressant effects on mice. To exclude the possibility that the antidepressant-like effects of OSE were attributable to stimulatory effects on locomotor function, OSE-induced spontaneous activity was evaluated in the open field test. Results revealed OSE-treated mice exhibited no alterations in spontaneous activity, indicating that the decreases in immobility time in the FST and TST were not caused by motor stimulation but rather to increase in active movements, such as struggling and swimming.
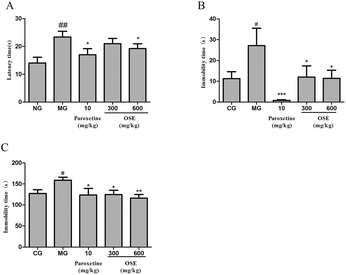
Based on the sub-chronic experiment, the effect of OSE on CSI mice was evaluated. CSI model can induce depression-like behavioral alterations, and is usually used for evaluating the efficacy of antidepressants through behavioral tests, such as FST, TST and NSFT. Both FST and TST are mentioned above. The NSFT is usually employed to define anhedonia (a loss of responsiveness to pleasant events), which is a core symptom in the diagnosis of depression and can be modeled by inducing a reduction of sucrose consumption in depression-like animal models.27 In the present study, as can be seen from Fig. 3, CSI induced significant increase of immobility in the FST and TST, and the latency in NSFT compared with control group. However, treatment with OSE (300 and 600 mg kg−1) and paroxetine (10 mg kg−1) were found to prevent changes induced by CSI. These results demonstrated OSE possessed antidepressant effects in the CSI model.
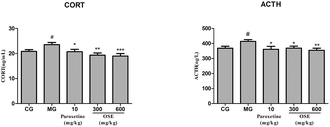
Currently, the pathogenesis of depression remains poorly understood. Among several putative hypothesis, stress is one of the most important factors responsible for depressive disorders, and the hyperactivation of hypothalamic–pituitary–adrenal (HPA) axis has attracted much more appreciation. Corticotrophin releasing factor (CRF) is produced from the paraventricular nucleus (PVN) to induce adrenocorticotropic hormone (ACTH) release from the pituitary, which in turn stimulates glucocorticoids (GCs) from the adrenal.28 Under normal conditions, glucocorticoid level in blood is sensitively regulated by HPA axis via negative feedback; when chronically stressful situation, such as CSI or chronic unpredictable mild stress (CUMS) models, occurs, HPA axis will be activated via stimulation of adrenocorticotropic hormone (ACTH) release and the subsequent peripheral release of steroids/cortisol from the adrenal grand. The persistently high concentration of blood glucocorticoids causes the dysfunction of HPA axis, exacerbates the lesion in the nervous system, and even aggravates the depression.29,30 In present research, it was observed that CSI mice displayed an obvious elevation of GC and ACTH levels in serum. However, OSE treatment could significantly reversed the elevated trend of GC and ACTH levels in CSI mice, indicating the alleviation of depression severity.
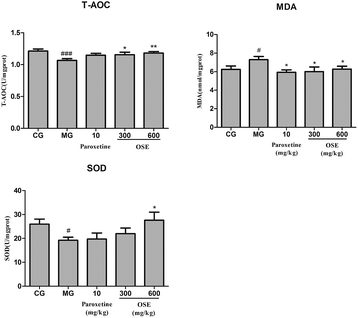
Growing evidence suggests that oxidative stress plays an important role in the development of chronic diseases such as cardiovascular disease and some psychiatric disorders such as depression. The brain is particularly vulnerable to oxidative stress due to a high consumption of oxygen, the generation of reactive oxygen species (ROS), high long chain polyunsaturated fatty acid content (which, in turn, are vulnerable to ROS attacks) and relatively poor antioxidant capacity. When living cells are threatened by certain physical and psychological events, it will lead to oxidative damage. The oxidative damage could be indicated by level of MDA, a product of lipid peroxidation, and the activity of antioxidant enzymes such as SOD and T-AOC. In present study, administration of OSE reversed the increase of MDA in the hippocampus of mice induced by CSI significantly, while obviously increased the activities of SOD and T-AOC as compared with the model mice. The data suggested the antioxidant activity of OSE have a potential relationship with its antidepressant effects.
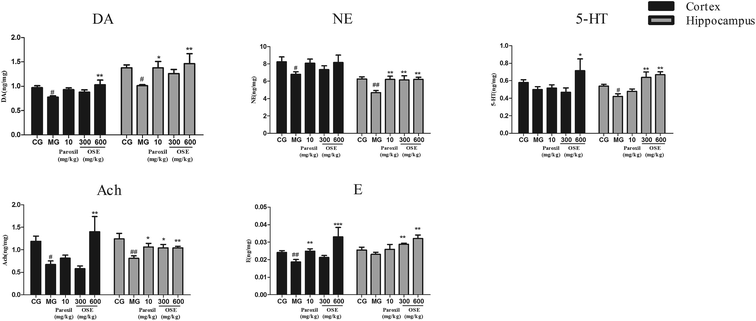
It is well known that currently treatment of MDD is focused on the monoamine hypothesis, which prop that depression is related to the low-level of brain monoamines including serotonin (5-HT), dopamine (DA) and norepinephrine (NE).31 5-HT is involved in many neurological and psychiatric diseases related to mood, sleep, learning, memory and sexual behavior;32 NE has long been known to play prominent roles in aggression, anxiety, reward and other stress-related behaviors;33 dopaminergic pathways are more likely altered in psychomotor retarded depressive patients.34 Additionally, increasing number of evidences indicate that MDD is associated with altered function of the major excitatory and inhibitory neurotransmitters. Peripheral administration of the acetylcholinesterase (AChE) antagonist physostigmine induces symptoms of anxiety and depression in human subjects by decreasing the breakdown of Ach and increasing level of Ach in the brain.35 To detect the brain monoamine and Ach levels in CSI mice, the hippocampus and the frontal cortex, which are critically involved in the regulation of emotion, motivation, learning and memory, were investigated in present study. Our results revealed that OSE (600 mg kg−1) led to a marked increase of DA, NE, 5-HT, Ach and E levels in hippocampus, and also promoted the levels of DA, 5-HT, Ach and E levels in frontal cortex. These results demonstrated that OSE could promote levels of hippocampus and frontal cortex monoamines to display antidepressant activity.
When it comes to the chemical compositions of OSE, various chromatographic methods were used to isolate 14 compounds from OSE, and their structures were identified based on spectrum data of 1H NMR, 13C NMR and MS as shown in ESI Fig. 1.† Based on these isolated substances, the chemical compositions of OSE were further analyzed with UPLC-DAD/Q-TOF MS. UPLC is a novel approach to chromatographic separation, which is based on the use of columns with smaller packing (1.7 μm particle) and operated at higher pressures (up to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi). Compared with traditional HPLC, UPLC provides a higher peak capacity, greater resolution, increased sensitivity and higher speed of analysis. When UPLC is coupled to mass spectrometry, it has been widely used as a powerful tool in the analysis of characterization and quantification of different complicated samples. In present study, 17 phenolic compounds, including epigallocat tetramer, epigallocat trimmer, epicatechin, gallocatechin, catechin, isorhamnetin-3-O-β-D-glucoside, quercetin-3-O-β-D-xyl(1→2)-β-D-glucoside, isoquercitrin, rutin, quercetin-7-O-rutinoside, myricetin-3-O-β-D-glucoside, quercetin-3-O-β-D-xyl(1→2)-β-D-galactoside, quercetin-3-O-gentiobioside, quercetin-3-O-β-D-6′′-(acetyl)glucopyranoside, quercetin, kaempferide and isorhamnetin from okra seed extract were identified using UPLC-DAD/ESI-Q-TOF MS. Our results revealed that OSE mainly contained catechin and quercetin derivatives, which is consistant with the previous report.16 As for the relationship between the active ingredients and curative effects of OSE, it can be concluded that phenolic compounds play the important roles for its antidepressant activity. Numerous previous literatures have proved that phenolic acids and quercetin-related flavonoids showed antidepressant activity due to their antioxidant effects.36,37 Meanwhile, the results of chemical analysis in present study showed that the catechin and quercetin derivatives are the major compositions of OSE.
000 psi). Compared with traditional HPLC, UPLC provides a higher peak capacity, greater resolution, increased sensitivity and higher speed of analysis. When UPLC is coupled to mass spectrometry, it has been widely used as a powerful tool in the analysis of characterization and quantification of different complicated samples. In present study, 17 phenolic compounds, including epigallocat tetramer, epigallocat trimmer, epicatechin, gallocatechin, catechin, isorhamnetin-3-O-β-D-glucoside, quercetin-3-O-β-D-xyl(1→2)-β-D-glucoside, isoquercitrin, rutin, quercetin-7-O-rutinoside, myricetin-3-O-β-D-glucoside, quercetin-3-O-β-D-xyl(1→2)-β-D-galactoside, quercetin-3-O-gentiobioside, quercetin-3-O-β-D-6′′-(acetyl)glucopyranoside, quercetin, kaempferide and isorhamnetin from okra seed extract were identified using UPLC-DAD/ESI-Q-TOF MS. Our results revealed that OSE mainly contained catechin and quercetin derivatives, which is consistant with the previous report.16 As for the relationship between the active ingredients and curative effects of OSE, it can be concluded that phenolic compounds play the important roles for its antidepressant activity. Numerous previous literatures have proved that phenolic acids and quercetin-related flavonoids showed antidepressant activity due to their antioxidant effects.36,37 Meanwhile, the results of chemical analysis in present study showed that the catechin and quercetin derivatives are the major compositions of OSE.
5. Conclusions
The present study firstly demonstrated OSE possessed antidepressant effects on sub-chronic treatment mice and CSI animal model through behavior tests, which. And the potential mechanisms may be via dampening oxidative stress, supressing HPA axis, as well as regulating the neurotransmitter levels. UPLC-DAD/Q-TOF MS analysis showed OSE mainly contained catechin and quercetin derivative. From the present data, the phenolic compounds should be the antidepressant constituents of OSE.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Mega-project for Innovative Drugs (2017ZX09305008); National Science and Technology major projects (2012ZX09301002-001).Notes and references
- S. Moussavi, S. Chatterji, E. Verdes, A. Tandon, V. Patel and B. Ustun, Lancet, 2007, 370, 851–858 CrossRef.
- B. Haenisch and H. Bönisch, Pharmacol. Ther., 2011, 129, 352 CrossRef PubMed.
- M. H. Trivedi, A. J. Rush, S. R. Wisniewski, A. A. Nierenberg, D. Warden, L. Ritz, G. Norquist, R. H. Howland, B. Lebowitz and P. J. Mcgrath, Am. J. Psychiatry, 2006, 163, 28 CrossRef PubMed.
- J. M. Bostwick, Mayo Clinic Proceedings, Mayo Clinic, 2010, vol. 85, pp. 538–550 Search PubMed.
- J. Sarris, A. C. Logan, T. N. Akbaraly, G. P. Amminger, V. Balanzá-Martínez, M. P. Freeman, J. Hibbeln, Y. Matsuoka, D. Mischoulon and T. Mizoue, Lancet Psychiatry, 2015, 2, 271–274 CrossRef PubMed.
- M. J. Rahman, A. Costa de Camargo and F. Shahidi, Food Chem., 2018, 240, 917–925 CrossRef PubMed.
- L. Letenneur, C. Proustlima, G. A. Le, J. F. Dartigues and P. Barbergergateau, Am. J. Epidemiol., 2007, 165, 1364 CrossRef PubMed.
- M. Singh, M. Arseneault, T. Sanderson, V. Murthy and C. Ramassamy, J. Agric. Food Chem., 2008, 56, 4855 CrossRef PubMed.
- G. L. Tipoe, T. M. Leung, M. W. Hung and M. L. Fung, Cardiovasc. Hematol. Disord.: Drug Targets, 2007, 7, 135 CrossRef.
- J. A. Nichols and S. K. Katiyar, Arch. Dermatol. Res., 2010, 302, 71–83 CrossRef PubMed.
- A. Garcíalafuente, E. Guillamón, A. Villares, M. A. Rostagno and J. A. Martínez, Inflammation Res., 2009, 58, 537–552 CrossRef PubMed.
- Y. Xu, Z. C. Wang, W. T. You, X. H. Zhang, S. Li, P. A. Barish, M. M. Vernon, X. Du, G. W. Li and J. C. Pan, Eur. Neuropsychopharmacol., 2010, 20, 405–413 CrossRef PubMed.
- L. L. Hurley, L. Akinfiresoye, O. Kalejaiye and Y. Tizabi, Behav. Brain Res., 2014, 268, 1–7 CrossRef PubMed.
- W. L. Zhu, H. S. Shi, Y. M. Wei, S. J. Wang, C. Y. Sun, Z. B. Ding and L. Lu, Pharmacol. Res., 2012, 65, 74–80 CrossRef PubMed.
- Y. J. Zhang, X. Huang, Y. Wang, Y. Xie, X. J. Qiu, P. Ren, L. C. Gao, H. H. Zhou, H. Y. Zhang and M. Q. Qiao, Brain Res. Bull., 2011, 86, 222 CrossRef PubMed.
- P. Arapitsas, Food Chem., 2008, 110, 1041–1045 CrossRef PubMed.
- W. Tongjaroenbuangam, N. Ruksee, P. Chantiratikul, N. Pakdeenarong, W. Kongbuntad and P. Govitrapong, Neurochem. Int., 2011, 59, 677–685 CrossRef PubMed.
- S. Fan, Y. Zhang, Q. Sun, L. Yu, M. Li, B. Zheng, X. Wu, B. Yang, Y. Li and C. Huang, J. Nutr. Biochem., 2014, 25, 702–709 CrossRef PubMed.
- H. Wang, G. Chen, D. Ren and S. T. Yang, Phytother. Res., 2014, 28, 268–273 CrossRef PubMed.
- L. Hu, W. Yu, L. Ying, N. Prasad and Z. Tang, BioMed research international, 2015, 2014, 341291 Search PubMed.
- F. Xia, Y. Zhong, M. Li, Q. Chang, Y. Liao, X. Liu and R. Pan, Nutrients, 2015, 7, 8846–8858 CrossRef PubMed.
- X.-P. Sun, S.-D. Li, Z. Shi, T.-F. Li, R.-L. Pan, Q. Chang, C. Qin and X.-M. Liu, Neurosci. Bull., 2013, 29, 737–744 CrossRef PubMed.
- Y. Liu, N. Zhao, C. Li, Q. Chang, X. Liu, Y. Liao and R. Pan, PLoS One, 2017, 12, e0183702 CrossRef PubMed.
- H. Dang, Y. Chen, X. Liu, Q. Wang, L. Wang, W. Jia and Y. Wang, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2009, 33, 1417–1424 CrossRef PubMed.
- M.-Z. Yan, Q. Chang, Y. Zhong, B.-X. Xiao, L. Feng, F.-R. Cao, R.-L. Pan, Z.-S. Zhang, Y.-H. Liao and X.-M. Liu, J. Agric. Food Chem., 2015, 63, 9277–9285 CrossRef PubMed.
- J. F. Cryan and A. Holmes, Nat. Rev. Drug Discovery, 2005, 4, 775–790 CrossRef PubMed.
- A. Mathews, K. Mogg, J. Kentish and M. Eysenck, Behav. Res. Ther., 1995, 33, 293–303 CrossRef PubMed.
- P. J. Lucassen, J. Pruessner, N. Sousa, O. F. Almeida, A. M. Van Dam, G. Rajkowska, D. F. Swaab and B. Czeh, Acta Neuropathol., 2014, 127, 109–135 CrossRef PubMed.
- B. E. Murphy, Psychoneuroendocrinology, 1997, 22(suppl 1), S125–S132 Search PubMed.
- R. M. Sapolsky, Arch. Gen. Psychiatry, 2000, 57, 925–935 CrossRef PubMed.
- M. Maan het Rot, S. J. Mathew and D. S. Charney, Can. Med. Assoc. J., 2009, 180, 305–313 CrossRef PubMed.
- M. Naughton, J. B. Mulrooney and B. E. Leonard, Hum. Psychopharmacol., 2000, 15, 397–415 CrossRef.
- S. A. Montgomery, J. Psychopharmacol., 1997, 11, S9–S15 Search PubMed.
- P. S. D'Aquila, M. Collu, G. L. Gessa and G. Serra, Eur. J. Pharmacol., 2000, 405, 365–373 CrossRef.
- S. C. Risch, R. M. Cohen, D. S. Janowsky, N. H. Kalin and D. L. Murphy, Science, 1980, 209, 1545–1546 CrossRef PubMed.
- L. Pathak, Y. Agrawal and A. Dhir, Expert Opin. Invest. Drugs, 2013, 22, 863–880 CrossRef PubMed.
- Y. Liu, G. Jia, L. Gou, L. Sun, X. Fu, N. Lan, S. Li and X. Yin, Pharmacol., Biochem. Behav., 2013, 104, 27–32 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03201g |
| This journal is © The Royal Society of Chemistry 2018 |