Open Access Article
Open Access Articlecis-1 Isomers of tethered bismethano[70]fullerene as electron acceptors in organic photovoltaics†
Tomokazu Umeyama *a,
Shogo Takaharaa,
Sho Shibataa,
Kensho Igarashia,
Tomohiro Higashino
*a,
Shogo Takaharaa,
Sho Shibataa,
Kensho Igarashia,
Tomohiro Higashino a,
Kenji Mishimab,
Koichi Yamashitab and
Hiroshi Imahori
a,
Kenji Mishimab,
Koichi Yamashitab and
Hiroshi Imahori *ac
*ac
aDepartment of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
bDepartment of Chemical System Engineering, School of Engineering, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
cInstitute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: umeyama@scl.kyoto-u.ac.jp; imahori@scl.kyoto-u.ac.jp; Fax: +81-75-383-2571; Tel: +81-75-383-2568 Tel: +81-75-383-2566
First published on 18th May 2018
Abstract
Isomer-controlled [70]fullerene bis-adducts can achieve high performance as electron-acceptors in organic photovoltaics (OPVs) because of their stronger absorption intensities than [60]fullerene derivatives, higher LUMO energy levels than mono-adducts, and less structural and energetic disorder than random isomer mixtures. Especially, attractive are cis-1 isomers that have the closest proximity of addends owing to their plausible more regular close packed structure. In this study, propylene-tethered cis-1 bismethano[70]fullerene with two methyl, ethyl, phenyl, or thienyl groups were rationally designed and prepared for the first time to investigate the OPV performances with an amorphous conjugated polymer donor (PCDTBT). The cis-1 products were found to be a mixture of two regioisomers, α-1-α and α-1-β as major and minor components, respectively. Among them, the cis-1 product with two ethyl groups (Et2-cis-1-[70]PBC) showed the highest OPV performance, encouraging us to isolate its α-1-α isomer (Et2-α-1-α-[70]PBC) by high-performance liquid chromatography. OPV devices based on Et2-cis-1-[70]PBC and Et2-α-1-α-[70]PBC with PCDTBT showed open-circuit voltages of 0.844 V and 0.864 V, respectively, which were higher than that of a device with typical [70]fullerene mono-adduct, [70]PCBM (0.831 V) with a lower LUMO level. However, the short-circuit current densities and resultant power conversion efficiencies of the devices with Et2-cis-1-[70]PBC (9.24 mA cm−2, 4.60%) and Et2-α-1-α-[70]PBC (6.35 mA cm−2, 3.25%) were lower than those of the device with [70]PCBM (10.8 mA cm−2, 5.8%) due to their inferior charge collection efficiencies. The results obtained here reveal that cis-1 [70]fullerene bis-adducts do not guarantee better OPV performance and that further optimization of the substituent structures is necessary.
Introduction
Organic photovoltaic (OPV) devices have attracted scientific and industrial attention due to their advantages of low cost, lightweight, flexibility, and solution processing.1–5 The photoactive layer generally comprises a blend film with a bulk heterojunction (BHJ) structure of electron-donating conjugated polymers and electron-accepting organic semiconductors. Fullerene derivatives have been widely utilized as electron-acceptors5 because of their reversible reduction behaviors, outstanding electron affinities, and excellent electron-transporting properties originating from their small reorganization energies of electron transfer,6 whereas non-fullerene electron acceptors, exhibiting high power conversion efficiencies (PCEs), have recently emerged as alternatives.7–10PCEs of OPV devices are determined by the product of short-circuit current density (JSC), open-circuit voltage (VOC), and fill factor (FF); therefore, all three factors should be increased to achieve a high PCE.1,2 [70]Fullerene derivatives such as [6,6]-phenyl-C71-butyric acid methyl ester ([70]PCBM) are preferentially employed in high-performance OPVs instead of [60]fullerene derivatives owing to the former's better light-harvesting ability in the visible region, which improves JSC.11,12 The higher solubility of [70]fullerene derivatives than those of [60]fullerene derivatives is beneficial for device fabrication processes. Another strategy for developing high-performance acceptors is the use of fullerene bis-adducts.1,13 By increasing the number of addends from one to two, the degree of π-conjugation is reduced and the energy level of the lowest unoccupied molecular orbital (LUMO) is raised. Since VOC is generally proportional to the energy difference between the LUMO of the electron-acceptor and the highest occupied molecular orbital (HOMO) of the electron-donor,1,2 the rise in the LUMO energy of electron-acceptors improves VOC. Bis-[6,6]-phenyl-C61-butyric acid methyl ester (bis-[60]PCBM),14 indene-C60 bis-adduct ([60]ICBA),15 and indene-C70 bis-adduct ([70]ICBA)12 are successful examples of fullerene bis-adduct acceptors in OPVs. However, even if both the addends are identical and symmetric and additions are limited to occur at [6,6]-bonds, there are 8 possible regioisomers of [60]fullerene bis-adducts. Furthermore, [70]fullerene bis-adducts have 38 possible regioisomers16 because a [70]fullerene cage has four different types of non-equivalent [6,6]-bonds, i.e., α-, β-, γ-, and δ-type bonds (Fig. 1a),17–20 although the differences in reactivity (α > β > γ > δ) and steric hindrance reduce the number of bis-adduct regioisomers actually obtained.21,22 Due to different structural and electronic properties of each regioisomer, disorders in molecular packing and energy levels are found in BHJ films while using the regioisomer mixture, which may deteriorate JSC and FF of BHJ OPV devices.12,14,15 Therefore, the development of regioisomer-controlled acceptors is effective for improving OPV device performance, which has been proven by various examples of regioisomer-free [60]fullerene bis-adducts.23–35
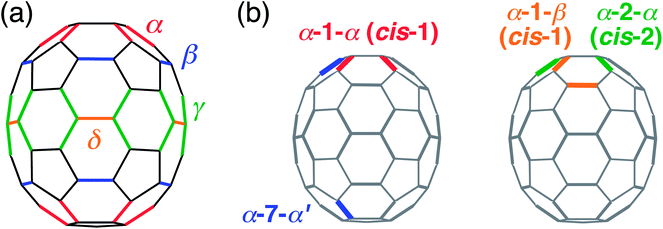 | ||
| Fig. 1 (a) Structure of [70]fullerene. α-, β-, γ-, and δ-type [6,6]-bonds are represented by red, blue, green and orange lines. (b) Examples of names for [70]fullerene bis-adduct patterns. | ||
Regioisomer-controlled [70]fullerene bis-adducts are expected to be high-performance acceptors for BHJ OPVs, as is evident from the above argument. However, the number of reports that demonstrate this is limited36–39 compared to the case of regioisomer-free [60]fullerene bis-adducts.23–35 This is due to the synthetic difficulty originating from the formation of a complicated mixture containing multiple regioisomers of [70]fullerene bis-adducts. In a pioneering work by Wong and coworkers,37 a single regioisomer of [70]ICBA, i.e., α-7-α′ isomer (Fig. 1b),40 in which two indene units were attached at α-bonds belonging to different hemispheres with an angle of “2 o'clock”, was carefully isolated from the regioisomer mixture by high-performance liquid chromatography (HPLC). The regioisomer pure α-7-α′-[70]ICBA exhibited better OPV device performance than the [70]ICBA regioisomer mixture. We devised tether-directed bis-functionalization of [70]fullerene with an ethylene-bridged indene dimer (1,2-bis(3-indenyl)ethane, BIE) as reactant to selectively obtain a relatively close substituent pattern, i.e., cis-2 (α-2-α) type isomer (Fig. 1b),40 in which two indene units were attached at α-bonds of hexagons next to each other.38 Note here that steric hindrance between the addends would inhibit the formation of such a close substitution pattern if the tether-directed effect was absent.23,27 The OPV based on BIE–[70]fullerene bis-adduct isomer with cis-2 configuration, cis-2-[70]BIEC and poly(3-hexylthiophene) showed a remarkable PCE of 4.2%, which was higher than those with the regioisomer mixture of BIE–[70]fullerene bis-adduct (2.2%), BIE–[70]fullerene mono-adduct (2.2%), BIE–[60]fullerene bis-adduct isomer with cis-2 configuration (2.8%), and even [70]PCBM (3.8%). This result has encouraged us to use cis-1 isomers of [70]fullerene bis-adducts having the closest proximity of two addends on a [70]fullerene cage because of their plausible regular close packing in the BHJ structure with conjugated polymers.
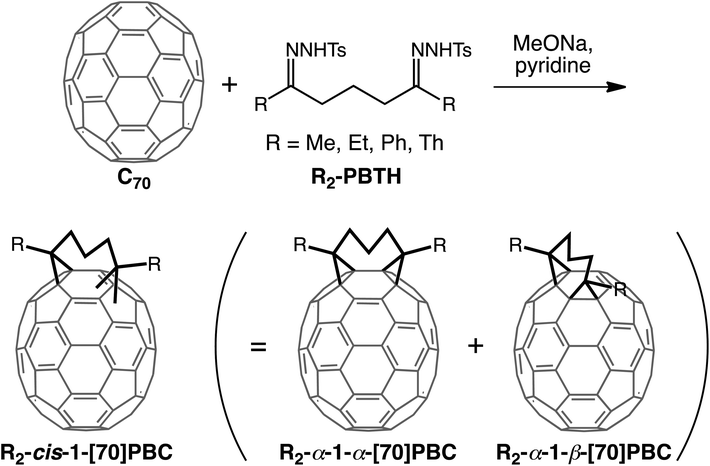
Recently, Echegoyen et al. succeeded in synthesizing a bismethano[70]fullerene derivative with cis-1 configuration, i.e., attached to [6,6]-bonds in the same hexagon, through a tether-directed remote functionalization method.16,41 They conducted a reaction between [70]fullerene and 1,3-dibenzoylpropane bis-p-toluenesulfonyl hydrazone (Ph2-PBTH) as addend precursor, yielding propylene-tethered cis-1 bismethano[70]fullerene with two phenyl groups (Ph2-cis-1-[70]PBC, Scheme 1). Ph2-cis-1-PBC was further separated to α-1-α and α-1-β isomers (Fig. 1b) by preparative thin layer chromatography and their structures were fully characterized. However, the photovoltaic properties of these tethered bis-adducts of [70]fullerene were not evaluated. In this study, for the first time, we utilized propylene-tethered cis-1 bismethano[70]fullerene as an electron-accepting material in OPV devices. Bis-adducts with methyl, ethyl, phenyl and thienyl groups (Scheme 1) were designed as substituents based on theoretical calculations on the degree of molecular packing (i.e., packing density) and prepared for investigating the substituent effects on film structures and photovoltaic properties of blend films with an amorphous conjugated polymer donor, poly[N-9′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT).42,43
Results and discussion
Densely packed fullerenes in blend films are advantageous over loosely packed ones because the former provides films with higher electron mobilities than the latter.17 This helps achieve higher performance for fullerene acceptor-based OPV devices. In addition, well-packed fullerenes at the donor–acceptor interface facilitate charge dissociation.44,45 Therefore, optimized structures for solid systems of propylene-tethered bismethano[70]fullerenes with various substituents, i.e., R2-α-1-α-[70]PBC (R = methyl (Me), ethyl (Et), i-propyl (iPr), cyano (CN), phenyl (Ph), thienyl (Th), and pyridyl (Py)) (Fig. S1†), were calculated using periodic boundary conditions on MOPAC to sort the suitable substituents. Volumes of one molecule (V0) and of a unit cell (V) were obtained based on the optimized structures (Table 1 and Fig. S2†). The unit cells included four molecules and the degree of molecular packing was defined as 4V0/V.46 Among alkyl-substituted bis-adducts (R = Me, Et, iPr), the ethyl-substituted one showed the smallest V (5170 Å3) and highest degree of packing (0.712), suggesting high potential as an electron-accepting material in OPV devices. The packing degree of Et2-α-1-α-[70]PBC was higher than that of [70]PCBM (0.683, Table 1). The packing degree of iPr2-α-1-α-[70]PBC (0.667) was slightly higher than that of Me2-α-1-α-[70]PBC (0.643). However, the insulating alkyl chain of iPr2-α-1-α-[70]PBC was longer than that of Me2-α-1-α-[70]PBC. Overall, Et2-α-1-α-[70]PBC and Me2-α-1-α-[70]PBC were chosen as synthetic targets. In propylene-tethered bismethano[70]fullerenes with aromatic substituent groups (R = Ph, Th, Py), the phenyl- and thienyl-substituted ones possessed higher packing degrees (0.660 and 0.670, respectively) than the pyridyl one (0.639). Thus, Ph2-α-1-α-[70]PBC and Th2-α-1-α-[70]PBC were selected. Although CN2-α-1-α-[70]PBC exhibited a higher packing degree (0.672), synthetic difficulty impeded the attempt.| Fullerene | V0 (bohr3 per molecule)a | V (Å3)b | Packing degreec |
|---|---|---|---|
| a Estimated by DFT at RB3LYP/6-31G(d) level.b Calculated after optimizations of fullerene solid systems under periodic boundary conditions at PM6-D3 based on MOPAC. All unit cells contain 4 molecules.c Defined as 4V0/V. | |||
| Me2-α-1-α-[70]PBC | 5660 | 5220 | 0.643 |
| Et2-α-1-α-[70]PBC | 6210 | 5170 | 0.712 |
| iPr2-α-1-α-[70]PBC | 6240 | 5550 | 0.667 |
| CN2-α-1-α-[70]PBC | 5890 | 5200 | 0.672 |
| Ph2-α-1-α-[70]PBC | 6870 | 6170 | 0.660 |
| Th2-α-1-α-[70]PBC | 6230 | 5520 | 0.670 |
| Py2-α-1-α-[70]PBC | 7400 | 6860 | 0.639 |
| [70]PCBM | 6190 | 5380 | 0.683 |
Propylene-tethered cis-1 bismethano[70]fullerene with methyl, ethyl, or thienyl groups (R2-cis-1-[70]PBC (R = Me, Et, Th)) was synthesized using the method used for Ph2-cis-1-[70]PBC (Scheme 1).16 The reaction between [70]fullerene and addend precursor, 1,3-dicarbonylpropane bis-p-toluenesulfonyl hydrazone (R2-PBTH) yielded a crude product, which was purified by silica gel chromatography using CS2 as eluent and then preparative HPLC with a Buckyprep column using toluene as eluent. Only three [70]fullerene-based fractions, i.e., unreacted pristine [70]fullerene, R2-cis-1-[70]PBC, and multiple adducts, were eluted during HPLC. The overall yields of R2-cis-1-[70]PBC with Me, Et, and Th groups were 5.2%, 28%, and 17%, respectively. Analyses of the HPLC traces revealed that the cis-1 products consisted of two regioisomers, α-1-α as the major component (60–78%) and α-1-β as the minor one (Table 2 and Fig. S3, ESI†). This was consistent with the previous result for Ph2-cis-1-[70]PBC (α-1-α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-1-β = 78
α-1-β = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22).16,47 Owing to the difference in the reactivities of [6,6]-bonds in the [70]fullerene cage (α > β > γ > δ), the first reaction occurred mainly at an α-bond. In addition, the short propylene chain limited the second reaction site to the α- or β-bond on the same hexagon. The structures of R2-cis-1-[70]PBC (R = Me, Et, Th) were characterized by 1H-NMR and high-resolution mass spectrometry (Experimental section). UV-vis absorption spectra of R2-cis-1-[70]PBC (R = Me, Et, Ph, Th) showed similar absorption shapes and intensities as those of [70]PCBM (Fig. S4, ESI†).
22).16,47 Owing to the difference in the reactivities of [6,6]-bonds in the [70]fullerene cage (α > β > γ > δ), the first reaction occurred mainly at an α-bond. In addition, the short propylene chain limited the second reaction site to the α- or β-bond on the same hexagon. The structures of R2-cis-1-[70]PBC (R = Me, Et, Th) were characterized by 1H-NMR and high-resolution mass spectrometry (Experimental section). UV-vis absorption spectra of R2-cis-1-[70]PBC (R = Me, Et, Ph, Th) showed similar absorption shapes and intensities as those of [70]PCBM (Fig. S4, ESI†).
| Fullerene | Ratio of α-1-α isomera (%) | Solubilityb (g mL−1) | LUMOc (eV) |
|---|---|---|---|
| a Estimated by the peak area ratio in HPLC traces.b Measured in ODCB at room temperature.c LUMO = −e(4.80 + E1) eV; E1 is the first reduction potential of fullerenes vs. ferrocene/ferrocenium (Fc/Fc+) couple.d Not determined due to the low solubility. | |||
| Me2-cis-1-[70]PBC | 67 | 3.6 | —d |
| Et2-cis-1-[70]PBC | 78 | 11.6 | −3.67 |
| Ph2-cis-1-[70]PBC | 68 | 13.8 | −3.67 |
| Th2-cis-1-[70]PBC | 60 | 5.8 | −3.66 |
| Et2-α-1-α-[70]PBC | >99 | 6.2 | −3.65 |
| [70]PCBM | — | 62.9 | −3.70 |
Solubility tests of R2-cis-1-[70]PBC (R = Me, Et, Ph, Th) in a standard organic solvent for OPV device fabrication, i.e., o-dichlorobenzene (ODCB), were carried out at room temperature (Table 2). The solubility of Me2-cis-1-[70]PBC (3.6 g mL−1) was found to be unsatisfactory for film formation by the spin-coating technique. Considering the low solubility and theoretically-predicted undesirable loose packing (vide supra), the properties of Me2-cis-1-[70]PBC were not examined subsequently. The bis-adduct with thienyl groups, Th2-cis-1-[70]PBC, exhibited solubility (5.8 mg mL−1) somewhat being higher than that of Me2-cis-1-[70]PBC and was used for OPV device fabrication (vide infra). Other bis-adducts, Et2-cis-1-[70]PBC and Ph2-cis-1-[70]PBC, possessed solubilities (11.6 and 13.8 mg mL−1, respectively) sufficient for solution processes, whereas they were much lower than that of a widely used [70]fullerene mono-adduct acceptor, [70]PCBM (62.9 mg mL−1).
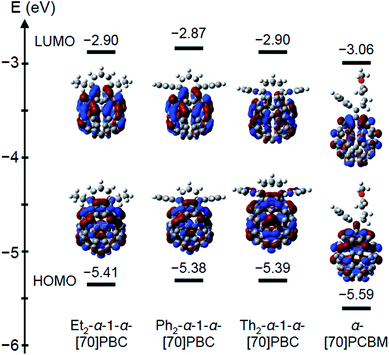
Electrochemical properties of R2-cis-1-[70]PBC (R = Et, Ph, Th) containing α-1-α (60–78%) and α-1-β isomers were evaluated by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements (Fig. S5, ESI†). The LUMO energy levels determined from the electrochemical data are listed in Table 2 together with that of [70]PCBM. The LUMO levels of R2-cis-1-[70]PBC were 30–40 mV higher than that of [70]PCBM, which was desirable for photovoltaic application, improving VOC.1,2 To gain insight into the electronic structures of R2-cis-1-[70]PBC, we performed DFT calculations of α-1-α isomers using the RB3LYP/6-31G(d) model. LUMO levels were obtained after geometry optimization, as illustrated in Fig. 2. The theoretical LUMO levels of R2-α-1-α-[70]PBC (R = Et, Ph, Th) were similar (−2.90, −2.87, and −2.90 V, respectively) and significantly higher than that of [70]PCBM (−3.06 V), which agreed with those obtained from electrochemical measurements (Table 2). The orbital distributions of the HOMOs and LUMOs of the fullerene bis-adducts were primarily found only in the [70]fullerene cage and delocalized throughout the entire cage (Fig. 2). Theoretical calculations of R2-α-1-β-[70]PBC contained in R2-cis-1-[70]PBC as minor component (22–40%) showed similar LUMO energy levels and orbital distributions as those of R2-α-1-α-[70]PBC (Fig. S6, ESI†).
To investigate the photovoltaic properties of R2-cis-1-[70]PBC (R = Et, Ph, Th) containing α-1-α and α-1-β isomers as major and minor components, OPV devices with the ITO/PEDOT:PSS/PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene/TiOx/Al configuration were fabricated. The detailed device fabrication process is described in the Experimental section. The average current density–voltage characteristics and photovoltaic parameters are shown in Fig. 3a and Table 3, respectively. Since the solubility of Th2-cis-1-[70]PBC in ODCB was lower than those of Et2-cis-1-[70]PBC and Ph2-cis-1-[70]PBC (Table 2), the optimized weight ratio of fullerene derivative in a mixed solution with PCDTBT was lower in PCDTBT
fullerene/TiOx/Al configuration were fabricated. The detailed device fabrication process is described in the Experimental section. The average current density–voltage characteristics and photovoltaic parameters are shown in Fig. 3a and Table 3, respectively. Since the solubility of Th2-cis-1-[70]PBC in ODCB was lower than those of Et2-cis-1-[70]PBC and Ph2-cis-1-[70]PBC (Table 2), the optimized weight ratio of fullerene derivative in a mixed solution with PCDTBT was lower in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC ([PCDTBT]
Th2-cis-1-[70]PBC ([PCDTBT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [fullerene] = 1
[fullerene] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w)) than in PCDTBT
1 (w/w)) than in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC and PCDTBT
Et2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC (1
Ph2-cis-1-[70]PBC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w)) (Table 3).
3 (w/w)) (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene films
fullerene films
| Fullerene | [PCDTBT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [fullerene] (w/w) [fullerene] (w/w) |
JSC (mA cm−2) | VOC (V) | FF | PCE (%) | Thickness (nm) | μe (10−4 cm2 V−1 s−1) | rms (nm) |
|---|---|---|---|---|---|---|---|---|
| a The data represent the average values with standard deviations from ten independent devices.b Measured by SCLC.c Measured by AFM.d Film thicknesses at sites without fullerene aggregates.e Not determined because the inhomogeneous film thickness due to the large aggregate formation hampered the accurate mobility estimations. | ||||||||
| Et2-cis-1-[70]PBC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
9.24 ± 0.09 | 0.844 ± 0.005 | 0.590 ± 0.006 | 4.60 ± 0.05 | 75 | 0.59 | 0.59 |
| Ph2-cis-1-[70]PBC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
3.81 ± 0.07 | 0.866 ± 0.006 | 0.364 ± 0.004 | 1.20 ± 0.03 | 57d | —e | 2.3 |
| Th2-cis-1-[70]PBC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.13 ± 0.08 | 0.787 ± 0.002 | 0.327 ± 0.003 | 0.59 ± 0.03 | 50d | —e | 1.9 |
| Et2-α-1-α-[70]PBC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6.35 ± 0.04 | 0.864 ± 0.005 | 0.593 ± 0.005 | 3.25 ± 0.06 | 52d | 0.51 | 0.78 |
| [70]PCBM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
10.8 ± 0.06 | 0.831 ± 0.004 | 0.618 ± 0.003 | 5.55 ± 0.05 | 91 | 6.9 | 0.35 |
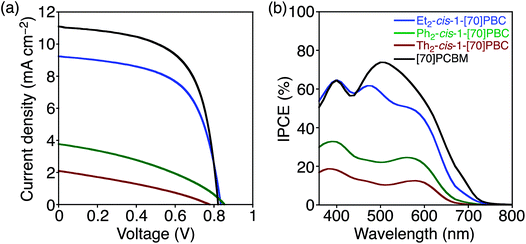
The OPV device with Et2-cis-1-[70]PBC revealed a significantly higher PCE of 4.60% than those with Ph2-cis-1-[70]PBC (1.20%) and Th2-cis-1-[70]PBC (0.59%) (Table 3). JSC (3.81 and 2.13 mA cm−2) and FF (0.364 and 0.327) of the devices with Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC were remarkably lower than those of the Et2-cis-1-[70]PBC-based device (9.24 mA cm−2 and 0.590). Reflecting the inferior JSC values, the incident photon-to-current efficiencies (IPCEs) of devices with Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC were significantly lower than those of the Et2-cis-1-[70]PBC-based device over the entire visible region (Fig. 3b). Although PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC film exhibited higher absorption than PCDTBT
Et2-cis-1-[70]PBC film exhibited higher absorption than PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC and PCDTBT
Ph2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC in the region 430–530 nm (Fig. S7, ESI†), the difference in absorption intensity could not explain the significant difference in the IPCE values. Additionally, X-ray diffraction (XRD) measurements of all blend films displayed no significant signals, reflecting the amorphous nature of PCDTBT.
Th2-cis-1-[70]PBC in the region 430–530 nm (Fig. S7, ESI†), the difference in absorption intensity could not explain the significant difference in the IPCE values. Additionally, X-ray diffraction (XRD) measurements of all blend films displayed no significant signals, reflecting the amorphous nature of PCDTBT.
To search for a reason regarding poor OPV performances of the devices with Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC, the blend film structures of photoactive layers were examined with an optical microscope. As shown in Fig. 4, aggregates with sizes of tens of micrometers were formed in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC and PCDTBT
Ph2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC. Similar large aggregates were absent in PCDTBT
Th2-cis-1-[70]PBC. Similar large aggregates were absent in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC, suggesting that the aggregates did not consist of PCDTBT but fullerenes in the blend films. Atomic force microscopy (AFM) visualized that the film surface roughnesses of PCDTBT
Et2-cis-1-[70]PBC, suggesting that the aggregates did not consist of PCDTBT but fullerenes in the blend films. Atomic force microscopy (AFM) visualized that the film surface roughnesses of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC and PCDTBT
Ph2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC (rms = 2.3 and 1.9 nm, respectively) at sites other than the aggregate were much larger than that of PCDTBT
Th2-cis-1-[70]PBC (rms = 2.3 and 1.9 nm, respectively) at sites other than the aggregate were much larger than that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (rms = 0.59 nm) (Fig. S8, ESI†). These results suggested that Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC had strong cohesive natures even in the blend film with amorphous PCDTBT. Bicontinuous network structures of donor and acceptor domains were poorly formed in PCDTBT
Et2-cis-1-[70]PBC (rms = 0.59 nm) (Fig. S8, ESI†). These results suggested that Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC had strong cohesive natures even in the blend film with amorphous PCDTBT. Bicontinuous network structures of donor and acceptor domains were poorly formed in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC and PCDTBT
Ph2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC, resulting in inferior JSC and FF values.
Th2-cis-1-[70]PBC, resulting in inferior JSC and FF values.
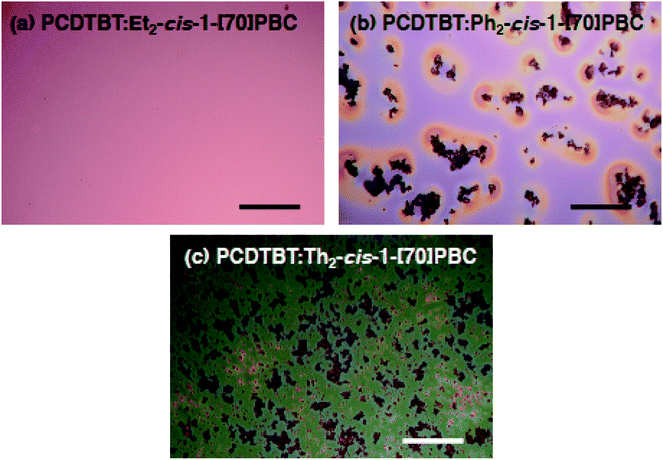 | ||
Fig. 4 Optical microscopy images of (a) PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC, (b) PCDTBT Et2-cis-1-[70]PBC, (b) PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC, and (c) PCDTBT Ph2-cis-1-[70]PBC, and (c) PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC on ITO/PEDOT:PSS substrates. Scale bars represent 50 μm. Th2-cis-1-[70]PBC on ITO/PEDOT:PSS substrates. Scale bars represent 50 μm. | ||
To investigate the diffusion efficiency of excitons generated in the polymer domain to the polymer–fullerene interface, we measured the steady-state photoluminescences of the blend films (Fig. S9, ESI†). The emission from PCDTBT was quenched efficiently (>98%) in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC, PCDTBT
Et2-cis-1-[70]PBC, PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC, and PCDTBT
Ph2-cis-1-[70]PBC, and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC. This result suggested that Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC molecules also existed outside the large aggregates and that most of the excitons generated by polymer absorption were quenched by fullerene molecules scattered across the polymer domain. However, such quenching did not lead to efficient photocurrent generation because of poor formation of an effective electron-transporting pathway to the electrode.
Th2-cis-1-[70]PBC. This result suggested that Ph2-cis-1-[70]PBC and Th2-cis-1-[70]PBC molecules also existed outside the large aggregates and that most of the excitons generated by polymer absorption were quenched by fullerene molecules scattered across the polymer domain. However, such quenching did not lead to efficient photocurrent generation because of poor formation of an effective electron-transporting pathway to the electrode.
As expected, VOC of the OPV with Et2-cis-1-[70]PBC (0.844 V) was slightly higher than that with a prevalent high-performance [70]fullerene acceptor, [70]PCBM (0.831 V) (Table 3), as the result of the elevated LUMO level of Et2-cis-1-[70]PBC by bis-functionalization. However, JSC and FF of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC-based device (9.24 mA cm−2 and 0.590) were slightly lower than those of the PCDTBT
Et2-cis-1-[70]PBC-based device (9.24 mA cm−2 and 0.590) were slightly lower than those of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM-based one (10.8 mA cm−2 and 0.618), irrespective of the comparable light-harvesting efficiency (Fig. S7†). The lower JSC and FF led to the inferior PCE of PCDTBT
[70]PCBM-based one (10.8 mA cm−2 and 0.618), irrespective of the comparable light-harvesting efficiency (Fig. S7†). The lower JSC and FF led to the inferior PCE of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (4.60%) relative to that of PCDTBT
Et2-cis-1-[70]PBC (4.60%) relative to that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (5.55%). The smoother film surface of PCDTBT
[70]PCBM (5.55%). The smoother film surface of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (rms = 0.35 nm) than that of PCDTBT
[70]PCBM (rms = 0.35 nm) than that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (rms = 0.59 nm) indicated the formation of a more suitable bicontinuous donor–acceptor network structure in PCDTBT
Et2-cis-1-[70]PBC (rms = 0.59 nm) indicated the formation of a more suitable bicontinuous donor–acceptor network structure in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (Fig. S10, ESI†). More importantly, the electron mobility of PCDTBT
[70]PCBM (Fig. S10, ESI†). More importantly, the electron mobility of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (5.9 × 10−5 cm2 V−1 s−1) estimated by space-charge-limited current (SCLC) measurement was much lower than that of PCDTBT
Et2-cis-1-[70]PBC (5.9 × 10−5 cm2 V−1 s−1) estimated by space-charge-limited current (SCLC) measurement was much lower than that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (6.9 × 10−4 cm2 V−1 s−1) (Table 3). The plausible lower charge collection efficiency of PCDTBT
[70]PCBM (6.9 × 10−4 cm2 V−1 s−1) (Table 3). The plausible lower charge collection efficiency of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC than PCDTBT
Et2-cis-1-[70]PBC than PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM as a consequence of the difference in the electron mobilities resulted in the inferior OPV device performance.
[70]PCBM as a consequence of the difference in the electron mobilities resulted in the inferior OPV device performance.
The lower OPV performances of devices with R2-cis-1-[70]PBC (R = Et, Ph, Th) than that with [70]PCBM might be due to inhomogeneity of R2-cis-1-[70]PBC containing two regioisomers, i.e., α-1-α and α-1-β. Disorders of molecular packing structures and electronic properties caused by regioisomer inhomogeneity may decrease electron mobility in fullerene domains. To shed light into the regioisomer effect on film structure and OPV performance, the major α-1-α isomer was further isolated from the Et2-cis-1-[70]PBC isomer mixture, which had showed the highest PCE among the R2-cis-1-[70]PBC isomer mixtures (R = Et, Ph, Th), using preparative HPLC technique with a 5PBB column (Fig. S11(a), ESI†). Collection of the minor α-1-β isomer could not be conducted because of the low composition ratio (α-1-α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-1-β = 78
α-1-β = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22, Table 2) in Et2-cis-1-[70]PBC that made it difficult to obtain sufficient amount of the α-1-β isomer for OPV device fabrication. The purity of the isolated α-1-α isomer sample (denoted as Et2-α-1-α-[70]PBC) was estimated by HPLC trace and found to be >99% (Fig. S11(b), ESI†).48 The solubility test in ODCB at room temperature revealed that Et2-α-1-α-[70]PBC possessed lower solubility (6.2 g mL−1) than Et2-cis-1-[70]PBC (11.6 g mL−1, Table 2). The highly symmetrical substitution structure of Et2-α-1-α-[70]PBC (Scheme 1) may have enhanced its tendency to form insoluble aggregates. The optical and electrochemical properties of Et2-α-1-α-[70]PBC were almost identical to those of Et2-cis-1-[70]PBC, reflecting the high content of Et2-α-1-α-[70]PBC in Et2-cis-1-[70]PBC (Fig. S12, ESI†).
22, Table 2) in Et2-cis-1-[70]PBC that made it difficult to obtain sufficient amount of the α-1-β isomer for OPV device fabrication. The purity of the isolated α-1-α isomer sample (denoted as Et2-α-1-α-[70]PBC) was estimated by HPLC trace and found to be >99% (Fig. S11(b), ESI†).48 The solubility test in ODCB at room temperature revealed that Et2-α-1-α-[70]PBC possessed lower solubility (6.2 g mL−1) than Et2-cis-1-[70]PBC (11.6 g mL−1, Table 2). The highly symmetrical substitution structure of Et2-α-1-α-[70]PBC (Scheme 1) may have enhanced its tendency to form insoluble aggregates. The optical and electrochemical properties of Et2-α-1-α-[70]PBC were almost identical to those of Et2-cis-1-[70]PBC, reflecting the high content of Et2-α-1-α-[70]PBC in Et2-cis-1-[70]PBC (Fig. S12, ESI†).
The OPV device based on PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC blend film was fabricated by the same procedure as the device with PCDTBT
Et2-α-1-α-[70]PBC blend film was fabricated by the same procedure as the device with PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC. The optimized weight ratio of fullerene in mixed solution with PCDTBT ([PCDTBT]
Et2-cis-1-[70]PBC. The optimized weight ratio of fullerene in mixed solution with PCDTBT ([PCDTBT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Et2-α-1-α-[70]PBC] = 1
[Et2-α-1-α-[70]PBC] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w)) was lower than PCDTBT
2 (w/w)) was lower than PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC ([PCDTBT]
Et2-cis-1-[70]PBC ([PCDTBT]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Et2-cis-1-[70]PBC] = 1
[Et2-cis-1-[70]PBC] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
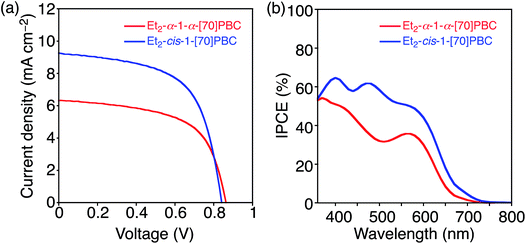
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w)) (Table 3) on account of the lower solubility of Et2-α-1-α-[70]PBC. PCE of the OPV device with Et2-α-1-α-[70]PBC was lower (3.25%) than that with Et2-cis-1-[70]PBC (4.60%) (Fig. 5a and Table 3). Although VOC and FF of the PCDTBT
3 (w/w)) (Table 3) on account of the lower solubility of Et2-α-1-α-[70]PBC. PCE of the OPV device with Et2-α-1-α-[70]PBC was lower (3.25%) than that with Et2-cis-1-[70]PBC (4.60%) (Fig. 5a and Table 3). Although VOC and FF of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC-based device (0.864 V and 0.593) were rather comparable to those of the PCDTBT
Et2-α-1-α-[70]PBC-based device (0.864 V and 0.593) were rather comparable to those of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC-based one (0.844 V and 0.590), JSC of the former (6.35 mA cm−2) was significantly lower than that of the latter (9.24 mA cm−2). Consistently, IPCE of the former device was lower than that of the latter over the entire visible region (Fig. 5b). The slightly decreased absorption intensity of PCDTBT
Et2-cis-1-[70]PBC-based one (0.844 V and 0.590), JSC of the former (6.35 mA cm−2) was significantly lower than that of the latter (9.24 mA cm−2). Consistently, IPCE of the former device was lower than that of the latter over the entire visible region (Fig. 5b). The slightly decreased absorption intensity of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC relative to that of PCDTBT
Et2-α-1-α-[70]PBC relative to that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (Fig. S13(a), ESI†) was one of the reasons for inferior JSC. The decrease in film thickness (Table 3) owing to the lower solubility of Et2-α-1-α-[70]PBC was compatible with the decrease in the absorption intensity of PCDTBT
Et2-cis-1-[70]PBC (Fig. S13(a), ESI†) was one of the reasons for inferior JSC. The decrease in film thickness (Table 3) owing to the lower solubility of Et2-α-1-α-[70]PBC was compatible with the decrease in the absorption intensity of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC. Although the efficient fluorescence quenching of PCDTBT in the blend film with Et2-α-1-α-[70]PBC (Fig. S13(b)†) indicated high efficiency of exciton diffusion to the donor–acceptor interface, the optical microscopy image visualized the existence of micrometer-sized aggregates in PCDTBT
Et2-α-1-α-[70]PBC. Although the efficient fluorescence quenching of PCDTBT in the blend film with Et2-α-1-α-[70]PBC (Fig. S13(b)†) indicated high efficiency of exciton diffusion to the donor–acceptor interface, the optical microscopy image visualized the existence of micrometer-sized aggregates in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC (Fig. 6). The film surface roughness of PCDTBT
Et2-α-1-α-[70]PBC (Fig. 6). The film surface roughness of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC (rms = 0.78 nm) at sites other than the aggregate (Fig. S14, ESI†) was larger than that of PCDTBT
Et2-α-1-α-[70]PBC (rms = 0.78 nm) at sites other than the aggregate (Fig. S14, ESI†) was larger than that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (rms = 0.59 nm) (Fig. S8, ESI†). These results indicate a poorly developed donor–acceptor bicontinuous network structure. Furthermore, despite the regioisomerically pure structure of Et2-α-1-α-[70]PBC, the SCLC electron mobility of PCDTBT
Et2-cis-1-[70]PBC (rms = 0.59 nm) (Fig. S8, ESI†). These results indicate a poorly developed donor–acceptor bicontinuous network structure. Furthermore, despite the regioisomerically pure structure of Et2-α-1-α-[70]PBC, the SCLC electron mobility of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC (5.1 × 10−5 cm2 V−1 s−1) was not improved relative to that of PCDTBT
Et2-α-1-α-[70]PBC (5.1 × 10−5 cm2 V−1 s−1) was not improved relative to that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (5.9 × 10−5 cm2 V−1 s−1) and was one order magnitude lower than that of PCDTBT
Et2-cis-1-[70]PBC (5.9 × 10−5 cm2 V−1 s−1) and was one order magnitude lower than that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (6.9 × 10−4 cm2 V−1 s−1) (Table 3). In sharp contrast with the BIE-[70]fullerene bis-adducts,38 the regioisomer isolation of Et2-α-1-α-[70]PBC from Et2-cis-1-[70]PBC deteriorated the OPV device performance because of decrease in its solubility and higher tendency to form its aggregates.
[70]PCBM (6.9 × 10−4 cm2 V−1 s−1) (Table 3). In sharp contrast with the BIE-[70]fullerene bis-adducts,38 the regioisomer isolation of Et2-α-1-α-[70]PBC from Et2-cis-1-[70]PBC deteriorated the OPV device performance because of decrease in its solubility and higher tendency to form its aggregates.
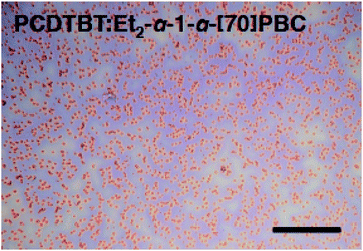 | ||
Fig. 6 Optical microscopy image of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC on ITO/PEDOT:PSS substrate. Scale bar represents 50 μm. Et2-α-1-α-[70]PBC on ITO/PEDOT:PSS substrate. Scale bar represents 50 μm. | ||
Conclusion
cis-1 Bis-adduct fullerenes were utilized as electron acceptors in OPV devices for the first time. Propylene-tethered cis-1 bismethano[70]fullerenes (R2-cis-1-[70]PBC) with two methyl, ethyl, phenyl, and thienyl groups were designed rationally and prepared to examine the substitution effect on the film structures and OPV performances of the blend films with an amorphous polymer, PCDTBT. R2-cis-1-[70]PBC with ethyl groups (Et2-cis-1-[70]PBC) showed the highest PCE (4.60%) as a result of good miscibility with PCDTBT. Owing to the elevated LUMO level, VOC of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC-based device (0.844 V) was slightly higher than that of the PCDTBT
Et2-cis-1-[70]PBC-based device (0.844 V) was slightly higher than that of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM-based one (0.831 V). The device performance of PCDTBT
[70]PCBM-based one (0.831 V). The device performance of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC was inferior to that of PCDTBT
Et2-cis-1-[70]PBC was inferior to that of PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (PCE = 5.55%) due to lower electron mobility and charge collection efficiency. Isolation of the α-1-α isomer (Et2-α-1-α-[70]PBC) from the corresponding isomer mixture, Et2-cis-1-[70]PBC was also conducted, but PCE of the OPV device decreased (3.25%) despite removing the inhomogeneity of structure and electronic properties of the fullerene bis-adducts. The decreased solubility and enhanced aggregation behavior of Et2-α-1-α-[70]PBC relative to Et2-cis-1-[70]PBC resulted in deteriorated OPV performance. These results obtained suggest that the optimization of substituent structure, considering their solubility, aggregation, and electron mobility as well as packing density, are indispensable for taking full advantage of regioisomerically pure cis-1 bis-adducts of [70]fullerene as electron-accepting materials. This model study provides important information for further research to modulate the fundamental properties of [70]fullerene bis-adduct materials, which will play a key role in making advance of OPV devices.
[70]PCBM (PCE = 5.55%) due to lower electron mobility and charge collection efficiency. Isolation of the α-1-α isomer (Et2-α-1-α-[70]PBC) from the corresponding isomer mixture, Et2-cis-1-[70]PBC was also conducted, but PCE of the OPV device decreased (3.25%) despite removing the inhomogeneity of structure and electronic properties of the fullerene bis-adducts. The decreased solubility and enhanced aggregation behavior of Et2-α-1-α-[70]PBC relative to Et2-cis-1-[70]PBC resulted in deteriorated OPV performance. These results obtained suggest that the optimization of substituent structure, considering their solubility, aggregation, and electron mobility as well as packing density, are indispensable for taking full advantage of regioisomerically pure cis-1 bis-adducts of [70]fullerene as electron-accepting materials. This model study provides important information for further research to modulate the fundamental properties of [70]fullerene bis-adduct materials, which will play a key role in making advance of OPV devices.
Experimental
Instruments
1H NMR and 13C NMR spectra were measured with a JEOL JNM-EX400 NMR spectrometer. High-resolution mass spectra were measured on a JEOL JMS-700 MStation spectrometer. Attenuated total reflectance (ATR) FT-IR spectra were recorded on a ThermoFisher Scientific Nicolet 6700 FT-IR. Purification of fullerene derivatives was conducted by Shimadzu Prominence Modular HPLC with Nacalai Tesque Buckyprep (20 × 250 mm); eluent, toluene; flow rate, 10 mL min−1; temperature, 40 °C; detection, 330 nm. Regioisomer separation of Et2-cis-1-[70]PBC was conducted by Shimadzu Prominence Modular HPLC with Nacalai Tesque Cosmosil 5PBB (20 × 250 mm); eluent, toluene; flow rate, 10 mL min−1; temperature, 40 °C; detection, 330 nm. UV-vis absorption spectra were obtained on a Perkin Elmer Lambda 900 UV/vis/NIR spectrometer. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed using an ALS 630A electrochemical analyzer in an o-dichlorobenzene/acetonitrile mixture (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.1 M tetrabutylammonium hexafluorophosphate (Bu4NPF6) as a supporting electrolyte. Optical micrographs were recorded using KH-7700 (Hirox). Atomic force microscopy (AFM) analyses were carried out with an Asylum Technology MFP-3D-SA in the AC mode. X-ray diffraction (XRD) analyses of film samples were performed with a Rigaku SmartLab 9 kW using Cu Kα radiation. Samples for the X-ray measurements were prepared by spin-coating the polymer
1) containing 0.1 M tetrabutylammonium hexafluorophosphate (Bu4NPF6) as a supporting electrolyte. Optical micrographs were recorded using KH-7700 (Hirox). Atomic force microscopy (AFM) analyses were carried out with an Asylum Technology MFP-3D-SA in the AC mode. X-ray diffraction (XRD) analyses of film samples were performed with a Rigaku SmartLab 9 kW using Cu Kα radiation. Samples for the X-ray measurements were prepared by spin-coating the polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene solution on the glass substrate. Steady-state fluorescence spectra were recorded on a HORIBA NanoLog-TCSPC.
fullerene solution on the glass substrate. Steady-state fluorescence spectra were recorded on a HORIBA NanoLog-TCSPC.
Photocurrent–voltage characteristics were measured by Keithley 2400 SourceMeter under a nitrogen atmosphere and simulated solar light (100 mW cm−2, AM1.5) with OTENTO-SUN III solar simulator (Bunkoukeiki). Photocurrent action spectra were recorded with CEP-2000RR (Bunkoukeiki). Current–voltage characteristics of the electron- and hole-only devices for space-charge-limited current (SCLC) measurements were conducted using Keithley 2400 SourceMeter under a nitrogen atmosphere.
Materials
Ph2-cis-1-[70]PBC and PCDTBT were prepared according to the reported procedure.16,43 Nonane-3,7-dione,49 heptane-2,6-dione,50 and 1,5-di(thiophen-2-yl)pentane-1,5-dione51 were prepared by the reaction between N1,N5-dimethoxy-N1,N5-dimethylglutaramide and the corresponding Grignard reagent. C70 (98.0%) and phenyl C71-butyric acid methyl ester ([70]PCBM, >99.0%) were purchased from MTR Ltd. and American Dye Source, Inc, respectively. All other solvents and chemicals were of reagent-grade quality, purchased commercially, and used without further purification unless otherwise noted.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 (HPLC peak area ratio). 1H NMR (400 MHz, o-dichlorobenzene-d4, ppm): δ 2.55 (m, 6H); δ 2.49 (m, 6H); δ 2.27 (m, 4H); δ 2.27 (m, 4H); δ 1.95 (m, 16H); δ 1.79 (m, 1H); δ 1.57 (m, 1H); δ 1.16 (m, 1H); δ 1.02 (t, 21H, J = 7.4 Hz); δ 0.79 (t, 3H, J = 7.6 Hz). 13C NMR (100 MHz, o-dichlorobenzene-d4, ppm): δ 155.59, 152.03, 151.24, 151.10, 150.96, 150.72, 150.39, 149.50, 149.18, 148.96, 148.84, 148.45, 148.25, 148.07, 147.94, 147.72, 147.43, 147.16, 146.72, 146.22, 146.08, 145.94, 145.81, 145.51, 145.33, 144.58, 144.48, 144.22, 144.02, 143.81, 143.69, 143.45, 143.14, 142.96, 142.60, 142.30, 141.45, 141.33, 141.21, 140.85, 139.74, 139.32, 139.15, 138.83, 71.83, 69.40, 65.46, 36.68, 35.99, 34.47, 28.28, 27.08, 20.14, 19.48, 18.93, 12.69. IR (ATR, cm−1): νmax 3003, 2897, 1429, 1380, 867, 691, 677, 643, 594, 524, 510. HRMS (APCI) m/z calcd for [C79H16 − H]+ 961.1017, found 961.1009. Melting point: >300 °C.
22 (HPLC peak area ratio). 1H NMR (400 MHz, o-dichlorobenzene-d4, ppm): δ 2.55 (m, 6H); δ 2.49 (m, 6H); δ 2.27 (m, 4H); δ 2.27 (m, 4H); δ 1.95 (m, 16H); δ 1.79 (m, 1H); δ 1.57 (m, 1H); δ 1.16 (m, 1H); δ 1.02 (t, 21H, J = 7.4 Hz); δ 0.79 (t, 3H, J = 7.6 Hz). 13C NMR (100 MHz, o-dichlorobenzene-d4, ppm): δ 155.59, 152.03, 151.24, 151.10, 150.96, 150.72, 150.39, 149.50, 149.18, 148.96, 148.84, 148.45, 148.25, 148.07, 147.94, 147.72, 147.43, 147.16, 146.72, 146.22, 146.08, 145.94, 145.81, 145.51, 145.33, 144.58, 144.48, 144.22, 144.02, 143.81, 143.69, 143.45, 143.14, 142.96, 142.60, 142.30, 141.45, 141.33, 141.21, 140.85, 139.74, 139.32, 139.15, 138.83, 71.83, 69.40, 65.46, 36.68, 35.99, 34.47, 28.28, 27.08, 20.14, 19.48, 18.93, 12.69. IR (ATR, cm−1): νmax 3003, 2897, 1429, 1380, 867, 691, 677, 643, 594, 524, 510. HRMS (APCI) m/z calcd for [C79H16 − H]+ 961.1017, found 961.1009. Melting point: >300 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 (HPLC peak area ratio). 1H NMR (400 MHz, CDCl3, ppm): δ 2.13–1.96 (m, 6H); δ 1.81 (s, 6H). No clear signal was observed in 13C NMR spectrum. IR (ATR, cm−1): νmax 2928, 2901, 1428, 1359, 1131, 671, 579. HRMS (ESI) m/z calcd for [C77H12 + H]+ 935.0861, found 935.0834. Melting point: >300 °C.
33 (HPLC peak area ratio). 1H NMR (400 MHz, CDCl3, ppm): δ 2.13–1.96 (m, 6H); δ 1.81 (s, 6H). No clear signal was observed in 13C NMR spectrum. IR (ATR, cm−1): νmax 2928, 2901, 1428, 1359, 1131, 671, 579. HRMS (ESI) m/z calcd for [C77H12 + H]+ 935.0861, found 935.0834. Melting point: >300 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (HPLC peak area ratio). 1H NMR (400 MHz, CDCl3/CS2 (3/7), ppm): δ 7.44–7.42 (m, 2H); δ 7.34–7.29 (m, 5H); δ 7.23–7.20 (m, 1H); δ 7.12–7.06 (m, 1H); δ 7.01 (t, 3H, J = 4.4 Hz); δ 6.75–6.70 (m, 1H); δ 3.44–3.39 (m, 1H); δ 3.28–3.09 (m, 3H); δ 3.00–2.80 (m, 4H); δ 1.90–1.77 (m, 2H); δ 0.93–0.81 (m, 1H). No clear signal was observed in 13C NMR spectrum. IR (ATR, cm−1): νmax 2920, 2854, 1430, 1232, 903, 731, 698, 645, 630, 585. HRMS(APCI) m/z calcd for [C83H12S2 + Cl]– 1107.0074, found 1107.0063. Melting point: >300 °C.
40 (HPLC peak area ratio). 1H NMR (400 MHz, CDCl3/CS2 (3/7), ppm): δ 7.44–7.42 (m, 2H); δ 7.34–7.29 (m, 5H); δ 7.23–7.20 (m, 1H); δ 7.12–7.06 (m, 1H); δ 7.01 (t, 3H, J = 4.4 Hz); δ 6.75–6.70 (m, 1H); δ 3.44–3.39 (m, 1H); δ 3.28–3.09 (m, 3H); δ 3.00–2.80 (m, 4H); δ 1.90–1.77 (m, 2H); δ 0.93–0.81 (m, 1H). No clear signal was observed in 13C NMR spectrum. IR (ATR, cm−1): νmax 2920, 2854, 1430, 1232, 903, 731, 698, 645, 630, 585. HRMS(APCI) m/z calcd for [C83H12S2 + Cl]– 1107.0074, found 1107.0063. Melting point: >300 °C.Theoretical calculations
Geometry optimization, electronic structure calculations and molecular volume estimations for the fullerene compounds were performed using density functional theory (DFT) at the RB3LYP/6-31G(d) level. Calculations were carried out using the Gaussian 09 program.52 All structures were fully optimized without any symmetry restriction. Optimized structures of fullerene derivatives in packed systems were calculated under periodic boundary conditions at PM6-D3 based on MOPAC. Degree of packing was calculated by Nv0/V, where N is number of molecules in unit cell, v0 is volume of a molecule, and V is volume of unit cell.46Device fabrications
OPV devices were prepared on patterned indium tin oxide (ITO) substrates that were cleaned by ultra-sonication in deionized water, CHCl3, acetone, and tetramethylammonium hydroxide aqueous solution for 15 min each, and then deionized water for 25 min, followed by 2-propanol and ethanol for 15 min each. They were subsequently dried under nitrogen flow, and treated in a UV–ozone cleaner for 25 min. A thin layer of poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) was spin-coated on substrates at 1000 rpm for 60 s, followed by 4000 rpm for 10 s. The PEDOT:PSS layer was dried at 200 °C for 10 min, and then transferred into a glove box filled with dried N2 gas to coat the active layer. A blended solution of PCDTBT and fullerene (total concentration, 28 mg mL−1 for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC (1
Et2-cis-1-[70]PBC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w) and PCDTBT
3, w/w) and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC (1
Ph2-cis-1-[70]PBC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w); 21 mg mL−1 for PCDTBT
3, w/w); 21 mg mL−1 for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC (1
Et2-α-1-α-[70]PBC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w); 14 mg mL−1 for PCDTBT
2, w/w); 14 mg mL−1 for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC (1
Th2-cis-1-[70]PBC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w); 35 mg mL−1 for PCDTBT
1, w/w); 35 mg mL−1 for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM (1
[70]PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, w/w) was prepared in ODCB and filtered with a 0.45 μm porous filter. The active layer was spin-coated (at 1500 rpm for 60 s for PCDTBT
4, w/w) was prepared in ODCB and filtered with a 0.45 μm porous filter. The active layer was spin-coated (at 1500 rpm for 60 s for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-cis-1-[70]PBC, PCDTBT
Et2-cis-1-[70]PBC, PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2-α-1-α-[70]PBC, PCDTBT
Et2-α-1-α-[70]PBC, PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th2-cis-1-[70]PBC and PCDTBT
Th2-cis-1-[70]PBC and PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ph2-cis-1-[70]PBC; at 1000 rpm for 60 s for PCDTBT
Ph2-cis-1-[70]PBC; at 1000 rpm for 60 s for PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [70]PCBM) on the top of the PEDOT:PSS layer and then dried at 70 °C for 1 h. For the fabrication of the buffer layer, a solution of titanium isopropoxide in ethanol was spin-coated at 4000 rpm for 20 s onto the ITO/PEDOT:PSS/PCDTBT
[70]PCBM) on the top of the PEDOT:PSS layer and then dried at 70 °C for 1 h. For the fabrication of the buffer layer, a solution of titanium isopropoxide in ethanol was spin-coated at 4000 rpm for 20 s onto the ITO/PEDOT:PSS/PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene. The samples were dried in a desiccator at r.t. for 20 min, and finally transferred to an evaporation chamber for Al deposition (∼100 nm) before extracting their J–V characteristics under AM1.5 conditions.
fullerene. The samples were dried in a desiccator at r.t. for 20 min, and finally transferred to an evaporation chamber for Al deposition (∼100 nm) before extracting their J–V characteristics under AM1.5 conditions.
The electron-only devices for the SCLC measurements were fabricated as follows. A 50 nm Al film was first thermally deposited onto the glass substrate. The PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene blend film with the same ratio as in the PSC device was spin-coated at 800 rpm. Then, the active layer was capped by a 100 nm Al electrode.
fullerene blend film with the same ratio as in the PSC device was spin-coated at 800 rpm. Then, the active layer was capped by a 100 nm Al electrode.
Solubility tests
The solubilities of fullerene derivatives were estimated as follows.53 Saturated solutions of the fullerene materials were prepared by adding an excess amount of the fullerenes to ODCB, followed by sonication at room temperature for 1 min. Then, the saturated solutions were filtered through a membrane filter (Cosmonice Filter S, COSMOSIL, pore size: 0.45 μm) to remove the aggregates. The amounts of the fullerene materials dissolved in the filtrates were determined by weighing the solid contents that remained after evaporation of the solvent and thorough drying under vacuum.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI (Grant Number JP25220501 to H. I. and JP26708023 and JP15H00737 to T. U.). XRD measurement was supported by Micro/Nano Fabrication Hub in Kyoto University of “Low-Carbon Research Network” funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. K. M. and K. Y. thank the support by MEXT as “Priority Issue on Post-K computer” (Development of new fundamental technologies for high-efficiency energy creation, conversion/storage and use).References
- T. Umeyama and H. Imahori, J. Mater. Chem. A, 2014, 2, 11545 Search PubMed.
- K. A. Mazzio and C. K. Luscombe, Chem. Soc. Rev., 2015, 44, 78 RSC.
- S. Xiao, Q. Zhang and W. You, Adv. Mater., 2017, 29, 1601391 CrossRef PubMed.
- Y. Cai, L. Huo and Y. Sun, Adv. Mater., 2017, 29, 1605437 CrossRef PubMed.
- R. Ganesamoorthy, G. Sathiyan and P. Sakthivel, Sol. Energy Mater. Sol. Cells, 2017, 161, 102 CrossRef.
- H. Imahori, Bull. Chem. Soc. Jpn., 2007, 80, 621 CrossRef.
- C. B. Nielsen, S. Holliday, H.-Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803 CrossRef PubMed.
- S. Li, Z. Zhang, M. Shi, C.-Z. Li and H. Chen, Phys. Chem. Chem. Phys., 2017, 19, 3440 RSC.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148 CrossRef PubMed.
- Z. Fei, F. Eisner, X. Jiao, M. Azzouzi, J. Röhr, Y. Han, M. Shahid, A. Chesman, C. Easton, C. McNeill, T. Anthopoulus, J. Nelson and M. Heeney, Adv. Mater., 2018, 30, 1705209 CrossRef PubMed.
- M. M. Wienk, J. M. Kroon, W. J. H. Verhees, J. Knol, J. C. Hummelen, P. A. van Hal and R. A. J. Janssen, Angew. Chem., Int. Ed., 2003, 42, 3371 CrossRef PubMed.
- Y. He, G. Zhao, B. Peng and Y. Li, Adv. Funct. Mater., 2010, 20, 3383 CrossRef.
- Y. Li, Chem.–Asian J., 2013, 8, 2316 CrossRef PubMed.
- M. Lenes, G.-J. A. H. Wetzelaer, F. B. Kooistra, S. C. Veenstra, J. C. Hummelen and P. W. M. Blom, Adv. Mater., 2008, 20, 2116 CrossRef.
- Y. He, H.-Y. Chen, J. Hou and Y. Li, J. Am. Chem. Soc., 2010, 132, 1377 CrossRef PubMed.
- M. R. Cerón, M. Izquierdo, A. Aghabali, A. J. Valdez, K. B. Ghiassi, M. M. Olmstead, A. L. Balch, F. Wudl and L. Echegoyen, J. Am. Chem. Soc., 2015, 137, 7502 CrossRef PubMed.
- T. Umeyama, T. Miyata, A. C. Jakowetz, S. Shibata, K. Kurotobi, T. Higashino, T. Koganezawa, M. Tsujimoto, S. Gélinas, W. Matsuda, S. Seki, R. H. Friend and H. Imahori, Chem. Sci., 2017, 8, 181 RSC.
- T. Umeyama, S. Shibata, K. Igarashi, S. Takahara, T. Higashino, S. Seki and H. Imahori, Chem. Lett., 2017, 46, 1001 CrossRef.
- T. Umeyama, S. Shibata, T. Miyata, K. Igarashi, T. Koganezawa and H. Imahori, RSC Adv., 2017, 7, 45697 RSC.
- T. Umeyama, K. Igarashi, D. Sakamaki, S. Seki and H. Imahori, Chem. Commun., 2018, 54, 405 RSC.
- A. Herrmann, F. Diederich, C. Thilgen, H.-U. T. Meer and W. H. Müller, Helv. Chim. Acta, 1994, 77, 1689 CrossRef.
- N. Haruta, T. Sato and K. Tanaka, J. Org. Chem., 2012, 77, 9702 CrossRef PubMed.
- S. Kitaura, K. Kurotobi, M. Sato, Y. Takano, T. Umeyama and H. Imahori, Chem. Commun., 2012, 48, 8550 RSC.
- D. S. Sabirov, J. Phys. Chem. C, 2013, 117, 9148 Search PubMed.
- X. Meng, G. Zhao, Q. Xu, Z. a. Tan, Z. Zhang, L. Jiang, C. Shu, C. Wang and Y. Li, Adv. Funct. Mater., 2014, 24, 158 CrossRef.
- M.-H. Liao, Y.-Y. Lai, Y.-Y. Lai, Y.-T. Chen, C.-E. Tsai, W.-W. Liang and Y.-J. Cheng, ACS Appl. Mater. Interfaces, 2014, 6, 996 Search PubMed.
- R. Tao, T. Umeyama, K. Kurotobi and H. Imahori, ACS Appl. Mater. Interfaces, 2014, 6, 17313 Search PubMed.
- R. Tao, T. Umeyama, T. Higashino, T. Koganezawa and H. Imahori, Chem. Commun., 2015, 51, 8233 RSC.
- B. Zhang, J. Subbiah, Y. Y. Lai, J. M. White, D. J. Jones and W. W. H. Wong, Chem. Commun., 2015, 51, 9837 RSC.
- B. Zhang, J. M. White, D. J. Jones and W. W. H. Wong, Org. Biomol. Chem., 2015, 13, 10505 Search PubMed.
- D. S. Sabirov, A. O. Terentyev and R. G. Bulgakov, J. Phys. Chem. C, 2015, 119, 10697 Search PubMed.
- F. Zhao, X. Meng, Y. Feng, Z. Jin, Q. Zhou, H. Li, L. Jiang, J. Wang, Y. Li and C. Wang, J. Mater. Chem. A, 2015, 3, 14991 Search PubMed.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 Search PubMed.
- Z.-J. Li, S. Wang, S.-H. Li, T. Sun, W.-W. Yang, K. Shoyama, T. Nakagawa, I. Jeon, X. Yang, Y. Matsuo and X. Gao, J. Org. Chem., 2017, 82, 8676 CrossRef PubMed.
- T. Cao, N. Chen, G. Liu, Y. Wan, J. D. Perea, Y. Xia, Z. Wang, B. Song, N. Li, X. Li, Y. Zhou, C. J. Brabec and Y. Li, J. Mater. Chem. A, 2017, 5, 10206 Search PubMed.
- D. S. Sabirov, J. Phys. Chem. C, 2016, 120, 24667 Search PubMed.
- W. W. H. Wong, J. Subbiah, J. M. White, H. Seyler, B. L. Zhang, D. J. Jones and A. B. Holmes, Chem. Mater., 2014, 26, 1686 CrossRef.
- R. Tao, T. Umeyama, T. Higashino, T. Koganezawa and H. Imahori, ACS Appl. Mater. Interfaces, 2015, 7, 16676 Search PubMed.
- L.-L. Deng, X. Li, S. Wang, W.-P. Wu, S.-M. Dai, C.-B. Tian, Y. Zhao, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Sci. Bull., 2016, 61, 132 CrossRef.
- We follow the nomenclature for [70]fullerene bis-adducts proposed by Echegoyen, et al. in ref. 16. In this rule, Greek letters such as α, β, γ, and δ define the reacted [6,6]-bonds, and numbers represent the smallest number of bonds separating the addition sites. The same type of bonds on opposite semisphere across the δ-bonds are differentiated using a prime designation. In addition, we also apply the nomenclature of [60]fullerene bis-adducts to [70]fullerene ones; “cis-1” means that two addends exist at [6,6]-bonds in the same hexagon (e.g., α-1-α and α-1-β), and “cis-2” that two addends exist at [6,6]-bonds in the hexagons next to each other (e.g., α-2-α) (Fig. 1b).
- M. R. Cerón and L. Echegoyen, J. Phys. Org. Chem., 2016, 29, 613 CrossRef.
- S. Beaupré and M. Leclerc, J. Mater. Chem. A, 2013, 1, 11097 Search PubMed.
- T. Umeyama, Y. Watanabe, E. Douvogianni and H. Imahori, J. Phys. Chem. C, 2013, 117, 21148 Search PubMed.
- B. M. Savoie, A. Rao, A. A. Bakulin, S. Gelinas, B. Movaghar, R. H. Friend, T. J. Marks and M. A. Ratner, J. Am. Chem. Soc., 2014, 136, 2876 CrossRef PubMed.
- S. Shoaee, S. Subramaniyan, H. Xin, C. Keiderling, P. S. Tuladhar, F. Jamieson, S. A. Jenekhe and J. R. Durrant, Adv. Funct. Mater., 2013, 23, 3286 CrossRef.
- L. S. Barreto, O. L. Alves and R. A. Jackson, Phys. Chem. Glasses, 2002, 43C, 119 Search PubMed.
- The ratio of α-1-α
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-1-β in Ph2-cis-1-[70]PBC was reported to be 78
α-1-β in Ph2-cis-1-[70]PBC was reported to be 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 in ref. 16, whereas 68
22 in ref. 16, whereas 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 in Ph2-cis-1-[70]PBC obtained in this study. The slight difference in the composition ratio may result from the difference in the analysis method; the isolation yields were calculated after preparative thin layer chromatography in ref. 16, while peak area ratios in HPLC traces were used in this study.
32 in Ph2-cis-1-[70]PBC obtained in this study. The slight difference in the composition ratio may result from the difference in the analysis method; the isolation yields were calculated after preparative thin layer chromatography in ref. 16, while peak area ratios in HPLC traces were used in this study. - We tried to obtain X-ray single crystal structure of Et2-α-1-α-[70]PBC, but sufficiently large single crystal for the measurement was not obtained.
- C. Cardellicchio, V. Fiandanese, G. Marchese and L. Ronzini, Tetrahedron Lett., 1987, 28, 2053 CrossRef.
- R. Doi, M. Shibuya, T. Murayama, Y. Yamamoto and Y. Iwabuchi, J. Org. Chem., 2015, 80, 401 CrossRef PubMed.
- D. C. Owsley, J. M. Nelke and J. J. Bloomfield, J. Org. Chem., 1973, 38, 901 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- T. Umeyama, S. Shibata and H. Imahori, RSC Adv., 2016, 6, 83758 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02896f |
| This journal is © The Royal Society of Chemistry 2018 |