Open Access Article
Open Access ArticleGated photochromic molecules with AIEgen: turn-on the photochromism with an oxidation reagent†
Leyu Wang,
Tao Yu*,
Zongliang Xie,
Eethamukkala Ubba,
Tianya Zhan,
Zhiyong Yang,
Yi Zhang * and
Zhenguo Chi
* and
Zhenguo Chi *
*
PCFM Lab, GD HPPC Lab, Guangdong, Engineering Technology Research Center for High-performance Organic, Polymer Photoelectric Functional Films, State Key Laboratory of Optoelectronic Material and Technologies, School of Chemistry, Sun Yat-sen University, Guangzhou, 510275, P. R. China. E-mail: yutao33@mail.sysu.edu.cn; ceszy@mail.sysu.edu.cn; chizhg@mail.sysu.edu.cn
First published on 22nd May 2018
Abstract
A couple of gated photochromic molecules TrPEP and TrPEPO with AIEgen have been rationally designed and synthesized. No photochromism is detected for TrPEP whilst TrPEPO shows obvious photochromic properties in the solution state. By adding equimolar H2O2 aqueous solution to the TrPEP solution, the photochromic properties would be quickly turned on. The oxidation reagent acts as a gate to switch the photochromic properties by switching the triphenylphosphine group to a triphenylphosphine oxide group. Both TrPE and TrPEO display typical AIE phenomena. Different intensive emission bands with the emission maxima of 500 nm and 455 nm are detected before (TrPEP) and after (TrPEPO) oxidization in solid states. Combining the AIEgens, photochromic ON/OFF states can be easily indicated by the different emission colors in the solid state. Single crystal analyses and TD-DFT calculations were carried out to further investigate the photophysical and photochromic properties of these compounds. These new triphenylethylene derivatives provide a new strategy to achieve gated photochromic materials with simple chemical structures and gate indicators.
Introduction
The concept of Aggregation-Induced Emission (AIE) was first raised by Tang in 2001.1 Different from the common aggregation-caused quenching material, the emission intensities of molecules with AIEgen are highly increased after aggregation due to the restriction of their intermolecular rotations.2 With great potential applications in the areas of OLED materials,3–5 chemosensing,6,7 biolabeling8–10 and piezochromic materials.11,12 AIE materials have become a hotspot both in the industrial and academic areas. Lately, several AIE systems including hexaphenylsilole derivatives, tetraphenylethene derivatives, triphenylethylene derivatives and others have been designed and developed.1–16 The AIEgen for triphenylethylene derivatives was firstly reported by our group.13 Later, it is revealed that the color and emission bands of these molecules could be drastically changed after ground and the piezochromic properties of these molecules were investigated.11 In very recent, some triphenylethylene derivatives were designed as fast-response and simple-structured photochromic materials by utilizing intramolecular ring-closure reactions.17,18Photochromic materials have attacked enormous attention for their potential applications in the areas of anti-fake, photo-switchable molecular devices, bio-labeling and optical memory storage systems.19–29 Many photochromic systems with excellent photochromic properties have been designed and synthesized such as spiropyran,30–35 azo-containing compounds,36–39 and dithienylethene derivatives.40–46 Beside promoted the photochromic properties, combining gated properties to photochromic molecules which could easily switch the photochromism was also an important issue. Several chemical or physical methods such as oxidation/reduction reagents,47,48 acidity,49,50 hydrogen bonds,51 ion-binding,52,53 and mechano-simulations18 have been investigated as switches to manipulating the photochromic properties. Enlarging the library of gated photochromic molecules, especially with simple chemical structures, is still quite urgent. In addition, realizing real-time monitoring for the photochromic ON/OFF states also becomes an important issue to be solved.18,47–53 As mentioned above, triphenylethylene derivatives were considered as a series of simple-structured and easily-modified photochromic system. Attaching electron withdrawing groups to the triphenylethylene derivatives could promote the photochromism by stabilizing the ring-closure structures. Therefore, manipulation the electron withdrawing abilities of the functional groups on triphenylethylene moieties might realize the gated photochromic properties. Phosphine and phosphine oxide moieties are an oxidation–reduction pair with tremendous differences in electron donating abilities and steric configurations.54,55 Therefore, gated photochromic materials might be achieved by combining triphenylphosphine moieties into the triphenylethylene structures. Besides, the AIEgen for triphenylethylene derivatives could realize intense emission with distinct colors for their ON/OFF states respectively. In this regard, a triphenylethylene derivative with triphenylphosphine moieties named TrPEP was rational designed and synthesized (Fig. 1). The AIE-active TrPEP display strong bluish green emission but no photochromism. By adding equimolar H2O2, TrPEP was easily oxide to TrPEPO which show obvious photochromism and different emission color. AIE properties and gated photochromic properties for these compounds were studied. Single-crystal analyses and TD-DFT calculations were also carried out to investigate the photophysical and photochromic properties. This study suggested a reliable way to achieve gated photochromic materials with real-time monitoring properties.
Results and discussion
Synthesis and characterization
The synthetic routes and the synthetic details for TrPEP and TrPEPO are shown in Scheme S1 in the ESI.† The chemical structures of TrPEP and TrPEPO were characterized by 1H-NMR spectroscopy, High-resolution EI mass spectrometry, and elemental analysis (see ESI†).Aggregation-induced emission studies
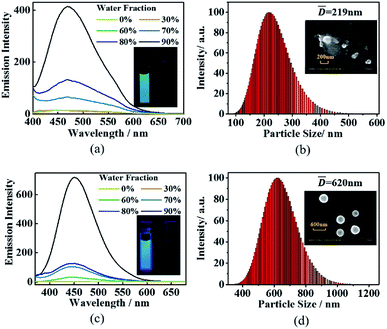
Triphenylethylene structure was used as a typical unit for achieving light-emitting molecules with AIE properties.13 Indeed, both TrPEP and TrPEPO show obvious AIE properties. Fig. 2(a) features the emission spectra of compound TrPEP in THF/water mixed solvent system containing different of water fractions. In pure THF solution, the emission band of TrPEP is hardly detectable, which is mainly ascribed to intramolecular rotations for the triphenylethylene moieties according to previous reports for the AIE molecules with similar structures.2 When the water fractions were increased, the emission intensities were boosted up with the emission maximum of ca. 471 nm. Similarly, TrPEPO was non-emissive in ethanol solution but become strongly emissive by enhancing the water fractions as shown in Fig. 2(c). The emission maximum for TrPEPO obvious blue-shifted compared with TrPEP (from 471 nm to 450 nm). The luminescent quantum yields for TrPEP and TrPEPO in both mixed solvent systems with different water fractions (v/v) were measured. For TrPEP, the luminescence quantum yields were increased from 0% (when the water fraction is below 60%) to 18.1% in THF/water mixed solvent systems with the enhancement of water fractions. TrPEPO display quantum yields ranging from 0% (when the water fraction is below 30%) to 29.3% in ethanol/water mixed solvent systems. The luminescent quantum yields for TrPEP and TrPEPO in mixed solvent systems were summarized in Table S1.† The obvious distinct emission properties between TrPEP and TrPEPO were mainly caused by different emission originations. For TrPEP, the bluish green emission was assignable as intramolecular charge transfer emission from the triphenylphosphine moiety to chloro-substituted triphenylethylene moieties according to the compounds with similar structures in previous reports.56,57 While for TrPEPO, the emission band might be mainly originated from π–π* transitions due to the switching from the electron-donating triphenylphosphine unit to the electron-withdrawing triphenylphosphine oxide unit. The emission originations for TrPEP and TrPEPO were further demonstrated by TD-DFT calculations vide infra. Dynamic light scattering studies were performed for TrPEP and TrPEPO in THF/water and ethanol/water mixed solvent systems containing 90% water (v/v) respectively. As shown in Fig. 2(b) and (d), particle size analyses revealed that average particles sizes were 219 nm and 620 nm for TrPEP and TrPEPO respectively. To further investigate the morphologies of the nanoparticles, scanning electron microscopy (SEM) studies were performed with the same solutions for DLS studies. The SEM images were shown in the inset pictures of Fig. 2(b) and (d). The SEM results for both of the two samples were in accordance with their DLS data. It is noticed that nano-sized balls were formed for TrPEPO in ethanol/water mixed solvent systems containing 90% water (v/v).Gated photochromic properties
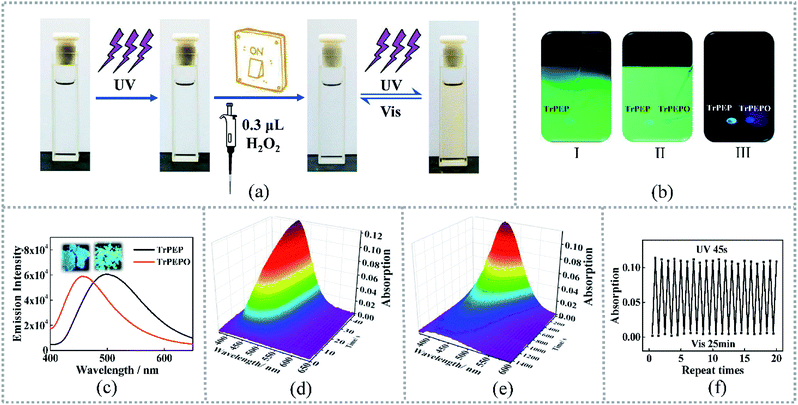
As mentioned before, photochromic processes for triphenylethylene derivatives were occurred by fulfilled stilbene-type 6-π electron ring closures reactions. For compound TrPEP, the photochromism was not observed in both solid state and solution state. But after addition of equimolar H2O2 aqueous solution (0.3 μL 30 wt%) to 3 mL TrPEP THF solution (1 × 10−3 mol L−1), the photochromism was obviously turned on. The photochromism pictures for a TrPEP solution (1 × 10−3 mol L−1) before and after adding H2O2 aqueous solution were listed in Fig. 3(a). After oxidation to TrPEPO, the color of the solution was changed to yellow upon UV-irradiation and reverted to transparent upon white light irradiation. Transformation yield for the oxidation reaction within 5 minutes was confirmed by comparing the 1H NMR spectra for the reaction residue, TrPEP and TrPEPO (Fig. S3†). It is revealed that almost all the TrPEP was oxidized to TrPEPO within 5 min during the photochromic turn-on process. As mention before, TrPEP and TrPEPO show diverse emission colours in aggregation states. Therefore, the photochromic ON/OFF state could be easily distinguished by spotting the solution on a TLC plate as shown in Fig. 3(b). Under UV light, different emissive spots could be clearly detected within 5 seconds. A blue emissive spot indicates the photochromic ON state, while green emissive spot indicates the photochromic OFF state. To further confirm the gated photochromic properties, TrPEPO was synthesized and purified (in ESI†) to study its photochromic properties. Fig. 3(c) features the emission spectra and pictures of TrPEP and TrPEPO in solid states upon 365 nm UV-irradiation. TrPEP displayed a bluish green emission with the emission maximum at ca. 500 nm while TrPEPO showed a sky-blue emission at ca. 455 nm. The diverse emission colors between the two compounds promoted the easy indication for the ON/OFF states of the photochromism. The luminescent quantum yields for TrPEP and TrPEPO in solid states were 91.3% and 77.4% respectively (Table S2†). Degassed THF solutions of TrPEPO (1 × 10−3 mol L−1) displayed obvious color changes from colorless to yellow after UV-irradiation (365 nm). As mention before, the color change was attributed to the ring-closure reaction. Time-dependent UV-vis absorption spectra for TrPEPO were measured during the photochromic and photochromic bleaching processes and the specification of the UV-light and white-light sources were listed in ESI.† As shown in Fig. 3(d), new absorption bands with the maxima at ca. 460 nm were detected for the TrPEPO THF solutions with the concentration of 1 × 10−3 mol L−1, upon UV irradiation. After irradiation for 45 seconds, the lower-energy absorption bands for the ring-closure structures of TrPEPO were no longer enhanced. With subsequent white-light irradiation, the color of TrPEPO solution was faded. The mismatch of the exposure wavelengths between the photochromic and photochromic bleaching processes indicates the lower lying energy states for the ring-open state than the ring-closures state which was often observed in photochromic systems. The photochromic bleaching process for TrPEPO takes ca. 1400 s (Fig. 3(e)). To investigate the reversibility for the photochromic process, 20 photochromic and bleaching cycles were carried out for TrPEPO solution obtained through H2O2-gated reaction. After 20 photochromic and bleaching cycles, negligible fatigue could be detected in the solution (Fig. 3(f)). Good recyclability of TrPEPO indicates its potential applications as rewritable and reversible responsive materials. Different from the other photochromic triphenylethylene derivatives, TrPEPO display obvious photochromic properties in solution states but not in solid states. It is might be caused by the bulky triphenylphosphine oxide moieties which resisted the ring-closure reactions in aggregation states. To further prove this assumption, single crystal analyses were carried out in the following part.Single crystal analyses for TrPEP and TrPEPO
Single crystals for TrPEP and TrPEPO were obtained by recrystallization from hexane–dichloromethane mixed solvent system. The CCDC numbers for these two single crystal structures are 1832435 and 1832437 respectively. Single crystals of TrPEP and TrPEPO are based on P21/c and Pc space groups and the data for bond distances and angles for these two crystals are listed in Table S3 to S6 in the ESI.† The single crystal structures for TrPEP and TrPEPO were listed in Fig. 4(a) and (b). For both of the two compounds, three aryl moieties for the triphenylethylene adopted twisted conformations, which can diminish the steric hindrance effects and reduce the energies for their aggregation states. For TrPEPO crystals structure, the dihedral angles between the two phenyl rings which involve in the ring-closure reactions are 63.2° and 61.3° for the two types of conformations. To fulfill the photochromic ring-closure reactions, triphenylphosphine oxide moieties have to rotate at least 61.3° relative to the other phenyl ring involving in the ring-closure reaction. However, the rotation for the triphenylphosphine oxide moieties are quite difficult in aggregation state due to large steric hindrance effect as shown in Fig. 4(c). Therefore, adding the triphenylphosphine groups on the triphenylethylene derivatives could promoted the gated photochromic properties, however, the rigidity of the functional group would affect the photochromism of TrPEPO in aggregation states.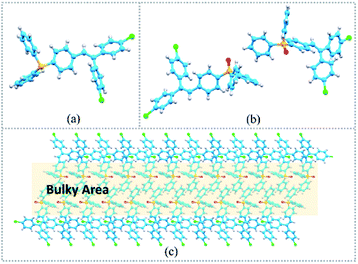 | ||
| Fig. 4 (a) Single crystal structure for compound TrPEP; (b) single crystal structure for compound TrPEPO; (c) molecular packing for TrPEPO molecules viewed down in b axis. | ||
DFT calculations
In order to gain further insights into the photophysical properties for TrPEP and TrPEPO, density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations at the B3LYP/6-31G* level were performed for TrPEP and TrPEPO. The calculations were performed according to their single-crystal structures. The HOMO and LUMO electronic distributions, the HOMO and LUMO energy levels, bandgaps and vertical excitation wavelengths for these two compounds are listed in Table 1. For TrPEP, the HOMO mainly delocalized at the triphenylphosphine moieties while the LUMO mainly delocalized at the dichloro-substituted triphenylethylene moieties. Therefore, the emission for TrPEP was mainly attributed the charge transfer state from the triphenylphosphine moieties to the dichloro-substituted triphenylethylene moieties. The electron-withdrawing ability was obviously enhanced by switching the triphenylphosphine group to triphenylphosphine oxide group. Thus, both the HOMO and LUMO for TrPEPO were delocalized at the dichloro-substituted triphenylethylene moieties. Therefore, the emission for TrPEPO was mainly originated from π–π* transitions. In accordance with the blue-shifted emission after oxidation, the energy gap between HOMO and LUMO was enhanced and the vertical excitation wavelength for TrPEPO was also blue-shifted by switching the TrPEP to TrPEPO.Conclusions
In summary, triphenylphosphine and triphenylphosphine oxide triphenylethylene derivatives TrPEP and TrPEPO have been logically designed and successfully synthesized. Both of the two compounds display AIE-active emission with distinct emission colors. No photochromic properties could be observed for TrPEP, whilst TrPEPO shows obvious photochromism. TrPEP could be easily and quickly oxidized to TrPEPO by adding equimolar H2O2. Therefore, oxidation reagents could act as gates to turn-on the photochromic properties. In relation to the AIEgen, different intense emission bands for TrPEP and TrPEPO could play as indicators to monitor the ON/OFF states of the photochromism. Photochromic and photophysical properties of these compounds were further investigated by single crystal analyses and TD-DFT calculations. These phosphine and phosphine oxide triphenylethylene derivatives with AIEgens feature a new strategy for designing gated photochromic materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the NSF of China (51733010, 51703253, 21672267), the Fundamental Research Funds for the Central Universities, Guangdong Science and Technology Plan (2015B090913003, 2017As3010295) and Educational Commission of Guangdong Province (2016KQNCX007).Notes and references
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 18, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 29, 4332–4353 RSC.
- G. Yu, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng, Z. Shuai, B. Z. Tang, D. Zhu, W. Fang and Y. Luo, J. Am. Chem. Soc., 2005, 127, 6335–6346 CrossRef PubMed.
- H.-C. Su, O. Fadhel, C.-J. Yang, T.-Y. Cho, C. Fave, M. Hissler, C.-C. Wu and R. Réau, J. Am. Chem. Soc., 2006, 128, 983–995 CrossRef PubMed.
- C. Y. K. Chan, Z. Zhao, J. W. Y. Lam, J. Liu, S. Chen, P. Lu, F. Mahtab, X. Chen, H. H. Y. Sung, H. S. Kwok, Y. Ma, I. D. Williams, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 378–389 CrossRef.
- Z. Li, Y. Dong, B. Mi, Y. Tang, M. Häussler, H. Tong, Y. Dong, J. W. Y. Lam, Y. Ren, H. H. Y. Sung, K. S. Wong, P. Gao, I. D. Williams, H. S. Kwok and B. Z. Tang, J. Phys. Chem. B, 2005, 109, 10061–10066 CrossRef PubMed.
- Y. Liu, Y. Tang, N. N. Barashkov, I. S. Irgibaeva, J. W. Y. Lam, R. Hu, D. Birimzhanova, Y. Yu and B. Z. Tang, J. Am. Chem. Soc., 2010, 132, 13951–13953 CrossRef PubMed.
- Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298–3302 CrossRef PubMed.
- S. Gui, Y. Huang, F. Hu, Y. Jin, G. Zhang, L. Yan, D. Zhang and R. Zhao, Anal. Chem., 2015, 87, 1470–1474 CrossRef PubMed.
- J. Qian and B. Z. Tang, Chem, 2017, 3, 56–91 Search PubMed.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- G. Li, X. Ren, G. Shan, W. Che, D. Zhu, L. Yan, Z. Su and M. R. Bryce, Chem. Commun., 2015, 51, 13036–13039 RSC.
- Z. Yang, Z. Chi, T. Yu, X. Zhang, M. Chen, B. Xu, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2009, 19, 5541–5546 RSC.
- Y.-S. Zheng and Y.-J. Hu, J. Org. Chem., 2009, 74, 5660–5663 CrossRef PubMed.
- K. Kokado and Y. Chujo, Macromolecules, 2009, 42, 1418–1420 CrossRef.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- D. Ou, T. Yu, Z. Yang, T. Luan, Z. Mao, Y. Zhang, S. Liu, J. Xu, Z. Chi and M. R. Bryce, Chem. Sci., 2016, 7, 5302–5306 RSC.
- T. Yu, D. Ou, L. Wang, S. Zheng, Z. Yang, Y. Zhang, Z. Chi, S. Liu, J. Xu and M. P. Aldred, Mater. Chem. Front., 2017, 01, 1900–1904 RSC.
- R. M. Kellogg, M. B. Greon and H. Wynberg, J. Org. Chem., 1967, 32, 3093–3100 CrossRef.
- G. M. Tsivgoulis and J.-M. Lehn, Angew. Chem., Int. Ed., 1995, 34, 1119–1122 CrossRef.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef PubMed.
- S. Higgins, Chem. Br., 2003, 39, 26–29 Search PubMed.
- F. M. Raymo and M. Tomasulo, Chem. Soc. Rev., 2005, 34, 327–336 RSC.
- K. Uchida, N. Nishikawa, N. Izumi, S. Yamazoe, H. Mayama, Y. Kojima, S. Yokojima, S. Nakamura, K. Tsujii and M. Irie, Angew. Chem., Int. Ed., 2010, 49, 5942–5944 CrossRef PubMed.
- D. Kitagawa, I. Yamashita and S. Kobatake, Chem. Commun., 2010, 46, 3723–3725 RSC.
- D. Kitagawa and S. Kobatake, Chem. Sci., 2012, 3, 1445–1449 RSC.
- F. Meng, Y.-M. Hervault, L. Norel, K. Costuas, C. V. Dyck, V. Geskin, J. Cornil, H. H. Hng, S. Rigaut and X. Chen, Chem. Sci., 2012, 3, 3113–3118 RSC.
- J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef PubMed.
- D. Kitagawa, H. Tsujioka, F. Tong, X. Dong, C. J. Bardeen and S. Kobatake, J. Am. Chem. Soc., 2018, 140, 4208–4212 CrossRef PubMed.
- J. B. Flannery Jr, J. Am. Chem. Soc., 1968, 90, 5660–5671 CrossRef.
- D. Levy and D. Avnir, J. Phys. Chem., 1988, 92, 4734–4738 CrossRef.
- J. L. Bahr, G. Kodis, L. Garza, S. Lin, A. L. Moore, T. A. Moore and D. Gust, J. Am. Chem. Soc., 2001, 123, 7124–7133 CrossRef PubMed.
- H. R. Allcock and C. Kim, Macromolecules, 1991, 24, 2846–2851 CrossRef.
- N. Tamai and H. Miyasaka, Chem. Rev., 2000, 100, 1875–1890 CrossRef PubMed.
- Q. Qi, C. Li, X. Liu, S. Jiang, Z. Xu, R. Lee, M. Zhu, B. Xu and W. Tian, J. Am. Chem. Soc., 2017, 139, 16036–16039 CrossRef PubMed.
- H. Dürr, Angew. Chem., Int. Ed., 1989, 28, 413–431 CrossRef.
- X. Zhu, F. Yin, H. Zhao, S. Chen and Z. Bian, RSC Adv., 2017, 7, 46344–46353 RSC.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef PubMed.
- J. Lv, Y. Liu, J. Wei, E. Chen, L. Qin and Y. Yu, Nature, 2016, 537, 179 CrossRef PubMed.
- A. Fernández-Acebes and J. M. Lehn, Adv. Mater., 1998, 10, 1519–1522 CrossRef.
- S. Chen, X. Li and L. Song, RSC Adv., 2017, 7, 29854–29859 RSC.
- T. Nakashima, M. Goto, S. Kawai and T. Kawai, J. Am. Chem. Soc., 2008, 130, 14570–14575 CrossRef PubMed.
- V. Lemieux, M. D. Spantulescu, K. K. Baldridge and N. R. Branda, Angew. Chem., Int. Ed., 2008, 120, 5112–5115 CrossRef.
- J. Zhang, W. Tan, X. Meng and H. Tian, J. Mater. Chem., 2009, 19, 5726–5729 RSC.
- B. Li, Y.-H. Wu, H.-M. Wen, L.-X. Shi and Z.-N. Chen, Inorg. Chem., 2012, 51, 1933–1942 CrossRef PubMed.
- J. C.-H. Chan, H.-L. Wong, W.-T. Wong and V. W.-W. Yam, Chem.–Eur. J., 2015, 21, 6936–6948 CrossRef PubMed.
- M. Irie, O. Miyatake, K. Uchida and T. Eriguchi, J. Am. Chem. Soc., 1994, 116, 9894–9900 CrossRef.
- X. Li, Y. Ma, B. Wang and G. Li, Org. Lett., 2008, 10, 3639–3642 CrossRef PubMed.
- S. H. Kawai, S. L. Gilat and J.-M. Lehn, Eur. J. Org. Chem., 1999, 9, 2359–2366 CrossRef.
- K. Yumoto, M. Irie and K. Matsuda, Org. Lett., 2008, 10, 2051–2054 CrossRef PubMed.
- M. Irie, O. Miyatake and K. Uchida, J. Am. Chem. Soc., 1992, 114, 8715–8716 CrossRef.
- Z. Shi, Y. Tu and S. Pu, RSC Adv., 2018, 8, 6727–6732 RSC.
- C.-T. Poon, W. H. Lam and V. W.-W. Yam, J. Am. Chem. Soc., 2011, 133, 19622–19625 CrossRef PubMed.
- K. M. Middlemiss and D. P. Santry, J. Chem. Phys., 1974, 61, 5400–5403 CrossRef.
- T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681–4727 CrossRef PubMed.
- T. Han, Y. Hong, N. Xie, S. Chen, N. Zhao, E. Zhao, J. W. Y. Lam, H. H. Y. Sung, Y. Dong, B. Tong and B. Z. Tang, J. Mater. Chem. C, 2013, 1, 7314–7320 RSC.
- Q. Huang, T. Yu, Z. Xie, W. Li, L. Wang, S. Liu, Y. Zhang, Z. Chi, J. Xu and M. P. Aldred, J. Mater. Chem. C, 2017, 5, 11867–11872 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details of new compounds, experimental details, details of single crystals, photophysical data, supplemental figures and Crystallographic details (CIF). CCDC 1832435 and 1832437. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra02828a |
| This journal is © The Royal Society of Chemistry 2018 |