Open Access Article
Open Access ArticleAn antibacterial collagen membrane crosslinked by the inclusion complex of β-cyclodextrin dialdehyde and ofloxacin for bacterial keratitis†
Yawei Chenab,
Wenjing Song *ab,
Xuan Zhaoab,
Qianqian Hanc and
Li Ren
*ab,
Xuan Zhaoab,
Qianqian Hanc and
Li Ren *ab
*ab
aSchool of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: phsongwj@scut.edu.cn; psliren@scut.edu.cn
bNational Engineering Research Center for Tissue Restoration and Reconstruction, Guangzhou 510006, China
cDepartment of Biomaterials, National Institutes for Food and Drug Control, Beijing 102629, China
First published on 17th May 2018
Abstract
Infectious keratitis is one of the leading causes of blindness in the world, especially in developing countries. Corneal transplant surgery is a feasible treatment for bacterial keratitis when drug therapy cannot be effective. However, the amount of corneal donors is far from requirements of clinical treatment and thus, the prognosis of bacterial keratitis is not satisfactory. In this study, we developed a novel antibacterial corneal repair material (β-CD-DA/OFLX-Col) for bacterial keratitis. The inclusion complex of β-cyclodextrin dialdehyde (β-CD-DA) with ofloxacin formed by host–guest interaction was used as both a drug vector and a crosslinker for further reaction with the amino groups on lysine of collagen chains. Physical properties, cytocompatibility, and antibacterial property of β-CD-DA/OFLX-Col film were characterized. The results indicated that the film was mainly transparent and possessed superior mechanical properties. Moreover, human corneal epithelial cells could adhere to the film and proliferate normally, indicating that the β-CD-DA/OFLX-Col film was non-cytotoxic and had good biocompatibility. Most importantly, the β-CD-DA/OFLX-Col film exhibited prominent antibacterial effects against E. coli and S. aureus in vitro, which could minimize the risks of infection. The prepared β-CD-DA/OFLX-Col film could greatly increase bioavailability of drugs and reduce toxic side effects, thus displaying great potential in bacterial keratitis treatment.
1. Introduction
Corneal blindness is one of the major causes of visual deficiency after cataract, glaucoma, and age-related macular degeneration.1 Keratoplasty is considered to be an effective method to cure corneal blindness. More recently, keratoprosthesis has been developed for more routine cases of corneal blindness where a diseased cornea is replaced with an artificial cornea. However, implantation of an artificial cornea carries a high risk of infection. At present, the common treatment is the frequent use of high-concentration antibiotic eye drops. However, bioavailability of topical antibiotics is poor, especially in the presence of intact corneal epithelium.2–4 Once drug treatment is invalid and the infection develops rapidly, it may lead to corneal ulcer perforation and then, therapeutic corneal transplantation will be imperative. Therefore, it is necessary to develop a novel antibacterial corneal repair material to replace the traditional artificial cornea.Mehta et al.5 developed an anti-infective collagen-based artificial corneal scaffold. Subsequently, it was demonstrated that the addition of vancomycin in a collagen-based artificial corneal scaffold did not significantly alter biomechanical and optical properties of the implant. Moreover, the efficacy of antibiotic-loaded collagen hydrogel in preventing implantable device-associated S. aureus infections both in vitro and in vivo was also appreciable. Ren et al.6 developed a novel tobramycin-containing antibacterial collagen-based corneal repair material by chemical grafting. The tobramycin-containing collagen film possessed suitable physicochemical properties and showed an excellent antibacterial effect and biocompatibility in vitro and in vivo. However, there are still some problems that need to be solved for collagen-based materials such as poor mechanical performance,7 which may lead to an inability to maintain a stable release of the drug. Hence, developing an antibacterial corneal repair material with controllable drug release and excellent mechanical properties is very urgent.
We employed a new biocompatible crosslinking agent β-cyclodextrin dialdehyde (β-CD-DA)8 to enhance the mechanical strength of the collagen-based corneal repair material. Moreover, the internal cavity of β-CD-DA is relatively hydrophobic and can encapsulate a variety of compounds, especially medicines, based on host–guest interactions.9–11 Most antibiotic drugs used after keratoplasty are hydrophobic and likely to be delivered. Ofloxacin (OFLX) is a highly potent and broad-spectrum antibacterial agent used against common Gram-positive and Gram-negative pathogens to treat corneal diseases; it is hydrophobic and has poor bioavailability for cornea tissue, and it can be encapsulated by β-CD-DA.
In this study, we used β-CD-DA for encapsulating OFLX in the relatively hydrophobic internal cavity of β-CD-DA through host–guest interactions. The inclusion complex of β-CD-DA and OFLX (β-CD-DA/OFLX) was used to crosslink with collagen, and an ofloxacin-containing antibacterial collagen film (β-CD-DA/OFLX-Col) was prepared for corneal repair. When the antibacterial collagen film was applied onto the surface of the cornea, the encapsulated OFLX could escape from the collagen film through frequent physical shears caused by blinking, thus providing sustained drug release. The physicochemical properties, drug release profile, antibacterial effect, and biocompatibility of the β-CD-DA/OFLX-Col film were evaluated in vitro.
2. Materials and methods
2.1. Materials
β-Cyclodextrin was supplied by Shanghai Bio Science and Technology Co., Ltd., China. Ofloxacin was purchased from Shanghai Macklin Biochemical Co., Ltd, China. 2-Iodoxybenzoic acid (IBX) was provided by Shanghai Aladdin Bio-Chem Technology Co., Ltd., China. Type I collagen (HM Biotech Ltd., Guangzhou, China) was extracted from bovine tendon. Phosphate-buffered saline (PBS) was prepared from Gibco (Thermo Fisher Scientific, USA). Collagenase I from Clostridium histolyticum was supplied by Guangzhou Qiyun Biotechnology Co., Ltd, China. All cell-culture-related reagents were purchased from Sigma Chemical (St. Louis, MO, USA). Deionized water was obtained from a water purification system (Millipore S.A.S., France). Strains of Staphylococcus aureus (S. aureus, strain ATCC 29213) and Escherichia coli (E. coli, strain ATCC 15224) were provided by VWR International, LLC (Radnor, PA, USA).2.2. Preparation of inclusion complex
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
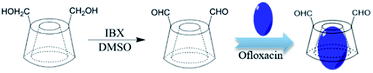
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 by different methods such as coprecipitation, lyophilization, and kneading. For ease of discussion, the optimized samples were prepared by lyophilization. First, 600 mg β-CD-DA was dissolved in 5.0 mL deionized water at 50 °C to obtain a saturated aqueous solution. Then, 60 mg OFLX was suspended in 0.2 mL ethanol, and this solution was added to the β-CD-DA solution. The above-mentioned mixed solution was continuously stirred for 1 h at 200 rpm at 50 °C. The reaction mixture was kept in a refrigerator for 4 h. During this time, a yellow precipitate was formed at the bottom of the bottle. After the mixed solution was lyophilized, the product was dissolved in acetic acid, filtered, and dried in an oven at 50 °C for 12 h. A yellow powder was eventually obtained. Scheme 1 displays the synthesis of β-CD-DA and inclusion complex.
10 by different methods such as coprecipitation, lyophilization, and kneading. For ease of discussion, the optimized samples were prepared by lyophilization. First, 600 mg β-CD-DA was dissolved in 5.0 mL deionized water at 50 °C to obtain a saturated aqueous solution. Then, 60 mg OFLX was suspended in 0.2 mL ethanol, and this solution was added to the β-CD-DA solution. The above-mentioned mixed solution was continuously stirred for 1 h at 200 rpm at 50 °C. The reaction mixture was kept in a refrigerator for 4 h. During this time, a yellow precipitate was formed at the bottom of the bottle. After the mixed solution was lyophilized, the product was dissolved in acetic acid, filtered, and dried in an oven at 50 °C for 12 h. A yellow powder was eventually obtained. Scheme 1 displays the synthesis of β-CD-DA and inclusion complex.
2.3. Characterization of the β-CD-DA/OFLX inclusion complex
| R (%) = (C × 50 × 10)/(m × X) × 100% | (1) |
| X (%) = (n1 × 361)/(n1 × 361 + n2 × 1131) × 100% | (2) |
Here, R is the inclusion rate (%), C is the concentration of OFLX (μg mL−1), m is mass of the inclusion complex (μg), X is the quantity percentage of OFLX (%), n1 is amount of OFLX before inclusion, n2 is amount of β-CD-DA before inclusion, and 1131 and 361 indicate molecular weights of β-CD-DA and OFLX, respectively.
2.4. Preparation of collagen films
Type I collagen solution (100 mL, 6.5 mg mL−1) was prepared by dissolving type I collagen in 0.01 mol L−1 HCl solution at 4 °C. The inclusion complex was dissolved in deionized water to achieve the concentration of 26 mg mL−1 and then, it was added to the collagen solution. The mixed solution (β-CD-DA/OFLX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) collagen = 1
collagen = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
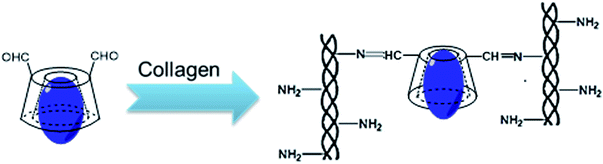
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, w/w) was thoroughly stirred at 4 °C for 24 h to obtain homogeneous blending and crosslinking. Then, the cross-linked collagen solution was dispensed into a specific mold and dried to form a cornea-shaped film. After that, the β-CD-DA/OFLX-Col film was rinsed three times with deionized water and dried again. For comparison, the collagen films crosslinked by EDC/NHS,15 β-CD-DA without OFLX, and a β-CD-DA-OFLX physical mixture were also prepared as the control group (Scheme 2).
5, w/w) was thoroughly stirred at 4 °C for 24 h to obtain homogeneous blending and crosslinking. Then, the cross-linked collagen solution was dispensed into a specific mold and dried to form a cornea-shaped film. After that, the β-CD-DA/OFLX-Col film was rinsed three times with deionized water and dried again. For comparison, the collagen films crosslinked by EDC/NHS,15 β-CD-DA without OFLX, and a β-CD-DA-OFLX physical mixture were also prepared as the control group (Scheme 2).
2.5. Characterization of physical and chemical properties of collagen films
2.6. Assessment of drug release in vitro
OFLX release trials were performed in vitro in a constant temperature shaker (Forma481, Thermo life sciences, USA) at 37 °C. For the trials, 400 mg of β-CD-DA/OFLX-Col and β-CD-DA-OFLX-Col films was immersed in 3 mL of PBS (pH 7.4) and then, all of the leached liquor was collected at regular time intervals (1, 2, 4, 8, 12, 24, 48, 72, 96, 120, 144, and 168 h). The volume of the medium was kept constant by adding the same amount of PBS. The concentrations of OFLX were measured using a quantified method described in Section 2.3.5. The percentage of OFLX release was calculated by dividing the amount of drug in the release medium by total OFLX loading on the films. The experiments of OFLX release were carried out in triplicate.2.7. Cytocompatibility in vitro
2.8. Antibacterial test
3. Results and discussion
3.1. Analysis of β-CD-DA/OFLX inclusion complex
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
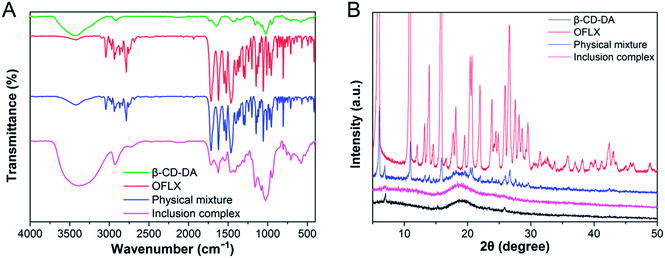
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and inclusion complexes are shown in Fig. 1A. The spectra of β-CD-DA and inclusion complex were similar, indicating that the skeletal structure of β-CD-DA had not changed. However, the peak of hydroxyl groups shifted from 3435 cm−1 to 3381 cm−1, indicating that the environment of β-CD-DA had changed, which caused the displacement of the hydroxyl telescopic peak. In the IR spectrum of OFLX, the peak at 3422 cm−1 was assigned to the hydroxyl groups, and the interactions of intermolecular hydrogen bonds formed a wide band. The peak at 1714 cm−1 was assigned to the vibration of the carboxyl group of OFLX, and this characteristic peak was also observed for the physical mixture and inclusion complexes. Furthermore, the peak at 1056 cm−1 was assigned to the telescopic vibration of C–F of OFLX, but this peak was not present for the inclusion complexes, thus showing that there were interactions between β-CD-DA and OFLX.
1), and inclusion complexes are shown in Fig. 1A. The spectra of β-CD-DA and inclusion complex were similar, indicating that the skeletal structure of β-CD-DA had not changed. However, the peak of hydroxyl groups shifted from 3435 cm−1 to 3381 cm−1, indicating that the environment of β-CD-DA had changed, which caused the displacement of the hydroxyl telescopic peak. In the IR spectrum of OFLX, the peak at 3422 cm−1 was assigned to the hydroxyl groups, and the interactions of intermolecular hydrogen bonds formed a wide band. The peak at 1714 cm−1 was assigned to the vibration of the carboxyl group of OFLX, and this characteristic peak was also observed for the physical mixture and inclusion complexes. Furthermore, the peak at 1056 cm−1 was assigned to the telescopic vibration of C–F of OFLX, but this peak was not present for the inclusion complexes, thus showing that there were interactions between β-CD-DA and OFLX.
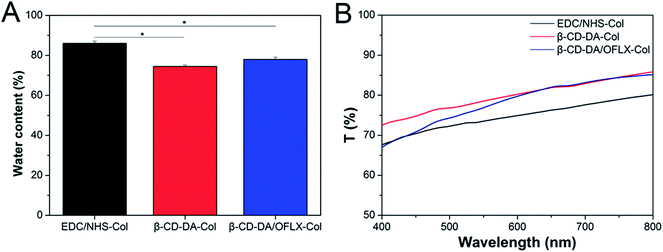
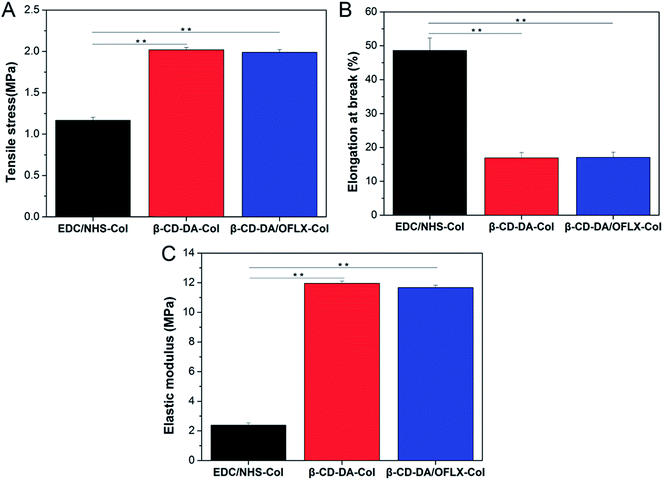
3.2. Analysis of physical and chemical properties of collagen films
3.3. Collagenase degradation in vitro
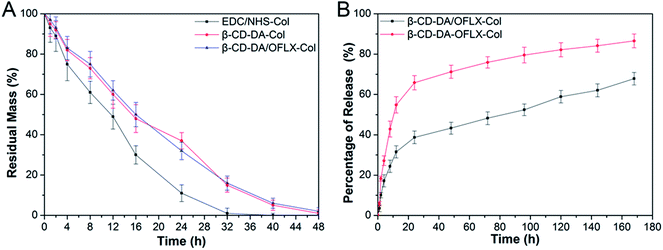
As shown in Fig. 6A, in general, an increase in crosslinking degree improves collagenase resistance in vitro. The degradation of the collagen films crosslinked with EDC/NHS was completed by about 32–36 h. The degradation of the collagen films crosslinked with β-CD-DA and β-CD-DA/OFLX required more than 48 h to complete. Our present results showed that the β-CD-DA crosslinked films had better anti-enzymatic stability than the EDC/NHS-crosslinked ones. The high crosslinking efficiency between β-CD-DA and collagen was due to strong interactions, which was the form of covalent bond between aldehyde group in β-CD-DA and amino group of collagen, and strong hydrogen bonds could be formed between hydroxyl and carboxyl groups, which significantly improved the resistance of the collagen-based films to collagenase.3.4. OFLX release in vitro
The results of the investigation of OFLX release (the cumulative release in vitro) from the collagen films crosslinked by β-CD-DA in the form of the physical mixture and inclusion complex are shown in Fig. 6B. The release profiles of both conditions exhibited an exponential tendency. The burst release of OFLX from the β-CD-DA/OFLX-Col inclusion complex in the first 16 h was about 30% of the total OFLX loading amount, and this was followed by a slower release of the drug. By contrast, the release profiles of collagen films crosslinked by β-CD-DA in the form of physical mixture of drugs were even more pronounced, and nearly 60% of OFLX was released in the first 16 h. On day 7, the total OFLX released from the physical mixture film was 90%, whereas that released from the inclusion complex film was less than 70%. Thus, the inclusion complex film could be used for a long-term drug release, which can ensure a certain antimicrobial activity.3.5. The morphology of HCECs
Fig. 7A shows the morphologies of HCECs on tissue culture plates and β-CD-DA-Col and β-CD-DA/OFLX-Col films at different time points. At day 1, the seeded cells had adhered to the surface of the film quite well, and there was no significant difference in the number of cells among them. At day 3, the morphology of HCECs gradually changed from a round shape to a spindle shape on two films, which was quite similar to that of HCECs growing on normal corneal tissues.24 Also, more round-shaped cells were observed on the tissue culture plates in comparison with those observed on the films, indicating that the seeded HCECs adhered better to the collagen films than tissue culture plates. After HCECs were seeded on the films, the cells proliferated rapidly. After 5 days, it was found that the films were almost completely covered with HCECs.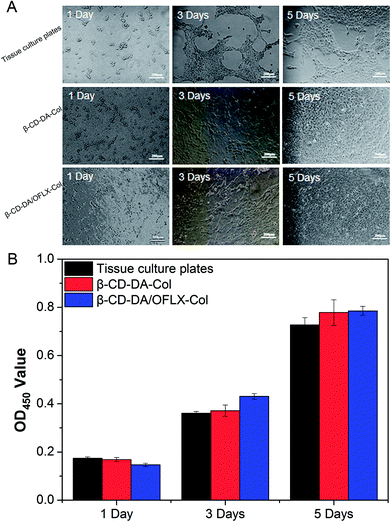 | ||
| Fig. 7 Morphology of HCECs seeded (A) and OD values (B) for tissue culture plates, β-CD-DA-Col film and β-CD-DA/OFLX-Col film for 1 day, 3 days and 5 days. | ||
In the field of tissue engineering, one of the important steps in the development of a novel scaffold is the evaluation of cytotoxicity. The CCK-8 test (Fig. 7B) showed the proliferation of HCECs on tissue culture plates and β-CD-DA-Col and β-CD-DA/OFLX-Col films. There was no significant difference in the toxicities of the three materials, indicating that the collagen membrane had excellent biocompatibility, and the addition of drugs did not increase the toxicity of the films.
3.6. Anti-bacterial effect
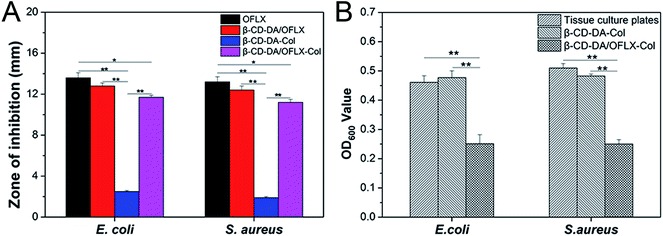
The antibacterial activities of the β-CD-DA/OFLX-Col films were analyzed against both Gram-positive and Gram-negative bacteria, and the results are presented in Fig. 8A and B. It can be seen from Fig. 8A that the β-CD-DA-Col film without OFLX failed to show any activity against E. coli (2.5 ± 0.1 mm) and S. aureus (1.9 ± 0.1 mm). The highest antimicrobial activity, which was obtained with the samples of pure OFLX, was due to their direct contact with the tested bacteria, and the inhibition zone diameters were 13.6 ± 0.5 and 13.2 ± 0.5 mm. For the β-CD-DA/OFLX-Col film, the maximum zone of inhibition of 11.7 ± 0.2 mm was found against E. coli, and the β-CD-DA/OFLX inclusion complex showed the maximum 12.8 ± 0.3 mm zone of inhibition against E. coli. Also, the maximum zone of inhibition of 11.2 ± 0.3 mm was found for the β-CD-DA/OFLX-Col film, and the maximum zone of inhibition of 12.4 ± 0.4 mm was observed for the β-CD-DA/OFLX inclusion complex against S. aureus. The difference in the size of zones among the β-CD-DA-Col film, pure OFLX, β-CD-DA/OFLX inclusion complex, and β-CD-DA/OFLX-Col film was significant (p < 0.01). Additionally, there were significant differences in the inhibition zones of pure OFLX and the β-CD-DA/OFLX-Col film (p < 0.05). OFLX is one of the newer quinolone antibiotics with a broad spectrum of antibacterial activity.25 In the results of the inhibition zone, the bacteriostasis effect on E. coli and S. aureus was basically consistent with the quantitative OFLX.Fig. 8B shows the bacteriostasis effects of tissue culture plates, β-CD-DA-Col film and β-CD-DA/OFLX-Col film in 24 h. Compared to the tissue culture plates and β-CD-DA-Col film, the β-CD-DA/OFLX-Col film exhibited significant difference in antimicrobial activity (p < 0.01). The results indicated that the β-CD-DA/OFLX-Col film had a remarkable antibacterial effect, which was enough to inhibit most Gram-positive and negative bacteria, even though the amount of drug release was less than 40% in 24 h. Therefore, this film not only helps avoid the trouble of frequent drug use, but also reduces the chance of drug resistance, and it can be effective for a long period of time.
4. Conclusions
In summary, we have successfully prepared β-CD-DA bio-crosslinker and β-CD-DA/OFLX inclusion complex by a convenient and efficient method. Then, β-CD-DA/OFLX was used to crosslink with collagen, and the properties of cross-linked collagen improved significantly. The test results suggested that the introduction of β-CD-DA could promote light transmittance and mechanical properties of the collagen-based corneal repair material. Meanwhile, the resulting film maintained the original lamellar structure and suitable water absorption. Cytocompatibility studies showed that the β-CD-DA/OFLX-Col film could promote cell proliferation compared to the control group. Moreover, it is worthy to note that the addition of drugs not only caused no loss of performance, but also showed eligible drug release rate and excellent antibacterial effect. Since the β-CD-DA/OFLX-Col film possesses suitable physicochemical properties and excellent antibacterial ability, it might provide a new opportunity for bacterial keratitis treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by National Natural Science Foundation of China (51603073, 51673071), Guangdong Scientific and Technological Project (2014B090907004), Natural Science Foundation of Guangdong Province (2017A030313294), Pearl River S&T Nova Program of Guangzhou (201710010195), the Fundamental Research Funds for the Central Universities.Notes and references
- WHO, Prevention of Blindness and Visual Impairment: Priority Eye Diseases, WHO, New York, 2015 Search PubMed.
- T. L. Du, V. Pillay, Y. E. Choonara, T. Govender and T. Carmichael, Expert Opin. Drug Delivery, 2011, 8, 71–94 CrossRef PubMed.
- D. Achouri, K. Alhanout, P. Piccerelle and V. Andrieu, Drug Dev. Ind. Pharm., 2012, 39, 1599–1617 CrossRef PubMed.
- L. Rabinovich-Guilatt, P. Couvreur, G. Lambert and C. Dubernet, J. Drug Targeting, 2004, 12, 623–633 CrossRef PubMed.
- A. K. Riau, D. Mondal, T. T. Aung, E. Murugan, L. Chen, N. C. Lwin, L. Zhou, R. W. Beuerman, B. Liedberg, S. S. Venkatraman and J. S. Mehta, ACS Biomater. Sci. Eng., 2015, 1, 1324–1334 CrossRef.
- Y. Liu, L. Ren, K. Long, L. Wang and Y. Wang, Acta Biomater., 2014, 10, 289–299 CrossRef PubMed.
- M. J. Wissink, R. Beernink, J. S. Pieper, A. A. Poot, G. H. Engbers, T. Beugeling, W. G. van Aken and J. Feijen, Biomaterials, 2001, 22, 151–163 CrossRef PubMed.
- J. Chen, X. Shi, L. Ren and Y. Wang, Carbon, 2016, 111, 18–27 CrossRef.
- K. Srinivasan and T. Stalin, Spectrochim. Acta, Part A, 2014, 130, 105–115 CrossRef PubMed.
- R. Krishnan, A. M. Rakhi and K. R. Gopidas, J. Phys. Chem. C, 2012, 116, 25004–25014 Search PubMed.
- S. M. Ali, K. Fatma and S. Dhokale, Beilstein J. Org. Chem., 2013, 9, 1917–1924 CrossRef PubMed.
- M. J. Cornwell, J. B. Huff and C. Bieniarz, Tetrahedron Lett., 1995, 36, 8371–8374 CrossRef.
- S. Liu, J. Cai, L. Ren, L. Wang and Y. Wang, RSC Adv., 2014, 4, 18608–18611 RSC.
- C. Mu, F. Liu, Q. Cheng, H. Li, B. Wu, G. Zhang and W. Lin, Macromol. Mater. Eng., 2010, 295, 100–107 Search PubMed.
- Z. Xuan, L. Yang, W. Li, L. Kai, W. Lin, S. Liu, Y. Wang and R. Li, Mater. Sci. Eng., C, 2015, 55, 201–208 CrossRef PubMed.
- C. Deng, F. Li, J. M. Hackett, S. H. Chaudhry, F. N. Toll, B. Toye, W. Hodge and M. Griffith, Acta Biomater., 2010, 6, 187–194 CrossRef PubMed.
- C. Zhang, Y. Liang, S. Deng, Z. Wang, R. Li and X. Sun, Clin. Ophthalmol., 2008, 2, 575–579 Search PubMed.
- W. R. Rd, V. Mahaguna and M. Sriwongjanya, Eur. J. Pharm. Biopharm., 1998, 46, 355–360 CrossRef.
- P. V. Demarco and A. L. Thakkar, J. Chem. Soc., Chem. Commun., 1970, 11, 2–4 RSC.
- A. Guerrero-Martínez, G. González-Gaitano, E. M. Murciano and G. Tardajos, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 251–256 CrossRef.
- J. Li and X. Zhang, J. Inclusion Phenom. Macrocyclic Chem., 2011, 69, 173–179 CrossRef.
- W. Liu, C. Deng, C. R. Mclaughlin, P. Fagerholm, N. S. Lagali, B. Heyne, J. C. Scaiano, M. A. Watsky, Y. Kato and R. Munger, Biomaterials, 2009, 30, 1551–1559 CrossRef PubMed.
- H. Tan, B. Wu, C. Li, C. Mu, H. Li and W. Lin, Carbohydr. Polym., 2015, 129, 17–24 CrossRef PubMed.
- J. Liu, G. Song, Z. Wang, B. Huang, Q. Gao, B. Liu, Y. Xu, X. Liang, P. Ma, N. Gao and J. Ge, Exp. Eye Res., 2007, 84, 599–609 CrossRef PubMed.
- J. B. Fourtillan, J. Granier, B. Saint-Salvi, J. Salmon, A. Surjus, D. Tremblay, M. V. D. Laurier and S. Beck, Infection, 1986, 14, 67–69 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02160k |
| This journal is © The Royal Society of Chemistry 2018 |