Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silica-coated magnetic iron oxide functionalized with hydrophobic polymeric ionic liquid: a promising nanoscale sorbent for simultaneous extraction of antidiabetic drugs from human plasma prior to their quantitation by HPLC†
Sahar Badragheha,
Mohsen Zeeb *b and
Mohamad Reza Talei Bavil Olyaib
*b and
Mohamad Reza Talei Bavil Olyaib
aDepartment of Chemistry, Karaj Branch, Islamic Azad University, Karaj, Iran
bDepartment of Applied Chemistry, Faculty of Science, Islamic Azad University, South Tehran Branch, Tehran, Iran. E-mail: zeeb.mohsen@gmail.com; m_zeeb@azad.ac.ir; Fax: +982133717140; Tel: +982133722831-7
First published on 29th August 2018
Abstract
Herein, silica-coated iron oxide nanoparticles modified with imidazolium-based polymeric ionic liquid (Fe3O4@SiO2@PIL) were fabricated as a sustainable sorbent for magnetic solid-phase extraction (MSPE) and simultaneous determination of trace antidiabetic drugs in human plasma by high-performance liquid chromatography-ultraviolet detection (HPLC-UV). The Fe3O4 core was functionalized by silica (SiO2) and vinyl layers where the ionic liquid 1-vinyl-3-octylimidazolium bromide (VOIM-Br) was attached through a free radical copolymerization process. In order to achieve hydrophobic magnetic nanoparticles and increase the merits of the sorbent, Br− anions were synthetically replaced with PF6−. The properties and morphology of the sorbent were characterized by various techniques and all the results illustrated the prosperous synthesis of Fe3O4@SiO2@PIL. A comprehensive study was carried out to investigate and optimize various parameters affecting the extraction efficiency. The limit of detection (LOD, S/N = 3) for empagliflozin, metformin and canagliflozin was 1.3, 6.0 and 0.8 ng mL−1, respectively. Linearity (0.997 ≥ r2 ≥ 0.993) and linear concentration ranges of 5.0–1200.0, 20.0–1800.0 and 5.0–1000.0 ng mL−1 were obtained for empagliflozin, metformin and canagliflozin, respectively. Intra-assay (3.8–7.5%, n = 9) and inter-assay (3.2–8.5%, n = 12) precisions as well as accuracies (≤9.1%) displayed good efficiency of the method. Finally, the method was applied for the quantitation of antidiabetic drugs in human plasma after oral administration and main pharmacokinetic data including Tmax (h), Cmax (ng mL−1), AUC0–24 (ng h mL−1), AUC0–∞ (ng h mL−1), and T1/2 (h) were evaluated.
1. Introduction
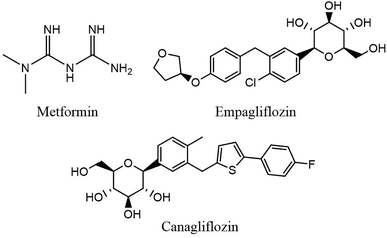
Type 2 diabetes mellitus (T2DM) and its abnormalities constitute a major metabolic disease affecting human health seriously in modern society.1 T2DM is a heterogeneous group of disorders characterized by abnormalities in carbohydrate, protein, and lipid metabolism which can cause serious health complications including ketoacidosis, kidney failure, heart disease, stroke and metabolic syndrome (dyslipidemia, hypertension). Furthermore, for many patients with T2DM, monotherapy with an oral antidiabetic agent is not adequate and multiple drugs may be necessary to achieve glycemic control in the long term. Thus, a combination formulation which includes drugs with different and complementary mechanisms of action would potentially offer increased terms of convenience and patient compliance.2–4 Therapeutic drug monitoring is essential to ensure the measurement of plasma concentration for pharmacokinetic studies, bioequivalence assessment of commercially available tablet formulation, optimization of novel dosage forms and dosing regimen in combination therapy for diagnostic purposes.5,6 Thus, development of reliable, sensitive and rapid analytical methods is required to simultaneously determine trace amounts of antidiabetic drugs in human plasma. The structures of three antidiabetic drugs chosen for this study including metformin (MET), empagliflozin (EMPA) and canagliflozin (CANA) are shown in Fig. 1.Due to the complex matrix of plasma, sample preparation methods are often required prior to chromatographic analysis. There are several techniques described in the literature for extraction of drugs in biological matrices such as liquid–liquid extraction (LLE), dispersive liquid–liquid microextraction (DLLME), solid phase extraction (SPE), micro-solid-phase extraction (μ-SPE), solid-phase membrane micro-tip extraction (SPMMTE), etc.7–10 In this case, Saha and coauthors have described probe electrospray ionization mass spectrometry to detection of drugs from plasma samples with no or minimal sample pre-treatment.11 Recent attempts in sample preparation are particularly focused on improving the quality of analytical results and introducing new technological expansions such as simplification, miniaturization and automation of the whole analytical method.
Due to some notable properties of iron-oxide nanoparticles including the intrinsic magnetism, low toxicity, facile preparation and high surface area-to-volume ratio, they are the most frequently used sorbents in MSPE.12–15 So far, various kinds of surface modifier such as carbon nanotubes (CNTs),16 graphene,17 polymer18 and cyclodextrins19 have been utilized as exterior shell material to adjust the properties of sorbents. Core–shell structure is a common existing form for MSPE sorbents, and lately it has received great attention for different screening purposes.20 Among these magnetic hybrid materials, modifying Fe3O4 with ionic liquids (ILs) on the shell moieties has been attracting growing attention.21,22
Beyond the excellent physicochemical and thermal properties of the ILs, they are also recurrently recognized by their excellent solvation ability for a wide range of compounds.23–26 Lately, ILs have been extremely used as green extraction media in some enrichment methods for different monitoring purposes.27 In our previous works, ILs were successfully used in micro-scale extraction methods to determine trace levels of atenolol and zinc in human plasma and food samples, respectively.28,29 Physically immobilization of ILs on the surface of Fe3O4 particles can merge the advantages of ILs and magnetic materials which resolves this serious drawback of ILs. Chen and his coworkers indicated that physically modification of Fe3O4@SiO2 particles with [OMIM]PF6 could provide practical sorbent for extraction of bisphenol A.30 However, physically adsorption of ILs on the surface of nanoparticles is truly unstable and results in poor reusability.31–33 For this presently reason, ILs have been covalently bonded to magnetite to significantly improve the stability and minimize ILs loss during extraction procedure.34
Literature survey revealed that polymeric bonds offer strong adhesion between the IL coating and the magnetic nanoparticles (MNPs), which prevents ionic liquids leakage.35–38
In this study, silica-coated iron oxide nanoparticles were functionalized with PIL (poly-1-vinyl-3-octylimidazolium bromide (VOIM-Br)) by a free radical copolymerization process in order to fabricate an efficient magnetic recyclable sorbent. Br− anions of PIL were synthetically changed with PF6− anions to increase hydrophobic character of the designed solid phase. The new sorbent possesses the advantages of both PILs and the magnetic iron oxide. Three antidiabetic drugs involving EMPA, MET and CANA were selected as the model compounds to ascertain the feasibility of this sorbent for simultaneous extraction and preconcentration prior to quantification by HPLC-UV. To the best of our knowledge, this work is the first report on the application of Fe3O4@SiO2@PIL-PF6 for trace determination of antidiabetic drugs in human plasma. The predominant factors affecting the extraction efficiency of the analytes were evaluated in detail and optimized with one at a time approach. Ultimately, the optimum conditions were successfully used to investigate the applicability of the proposed method for the determination of antidiabetic drugs in 8 healthy fed participants and main pharmacokinetic data were achieved.
2. Experimental
2.1. Chemicals
1-Vinylimidazole and potassium hexafluorophosphate (KPF6) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) chloride tetrahydrate (FeCl2·4H2O), ammonia, tetraethyl orthosilicate (TEOS), 1-vinyltriethoxysilane (VTES), 2,2′-azobisisobutyronitrile (AIBN), 1-bromooctane and triethylamine were obtained from Merck Chemicals (Darmstadt, Germany). HPLC grades of methanol, acetonitrile, acetone, potassium dihydrogen phosphate and sodium dodecyl sulfate were acquired from Merck (Darmstadt, Germany). Standards of CANA and EMPA were supplied from MSN Life Science Private Limited Unit-2, (India) and Emeishan Hongsheng Pharmaceutical Co. (China), respectively. Standard of MET kindly donated by Mahban Chemi Co. (Tehran, Iran). Fixed-dose combination Invokamet tablets (50 mg CANA/500 mg MET) and Jardiance tablets (25 mg EMPA) were purchased from Janssen (USA). Fresh plasma samples were obtained from Iranian Blood Transfusion Organization (Tehran, Iran) and stored at −18 °C until being used. Ultrapure water (Millipore, Bedford, MA, USA) was used throughout the whole experiments.2.2. Apparatus
Fourier transform infrared (FT-IR) spectral studies were carried out by a Vector 22 FT-IR spectrometer (Bruker, Germany) over the wavenumber range of 400–4000 cm−1. The 1H NMR spectra of synthesized IL were recorded with a Avance DRX-500 MHz spectrometer instrument (Bruker, Germany). X-ray diffraction (XRD) patterns were obtained with a D8 Advance X-ray diffractometer (Bruker, Germany) using Cu Kα radiation source (λ = 1.54059 Å). Thermal gravimetric analysis (TGA) were carried out using a Pyris 1 TGA instruments (Perkin Elmer, USA) operated with a heating rate of 15 °C min−1 from 25 °C to 800 °C under oxygen atmosphere. The size and morphology of the as-synthesized nanoparticles were studied using a SIGMA VP-500 field emission scanning electron microscope (FESEM) (Zeiss, Germany) and a Tecni F20 transmission electron microscopy (FEI, Hillsboro, OR, US). The elemental composition was characterized by an energy dispersive X-ray fluorescence spectroscopy (EDXRF) (Oxford Instrument, UK) attached to the Zeiss FESEM. Magnetization measurement was performed using a vibrating sample magnetometer (VSM/AGFM Meghnatis Daghigh Kavir Co., Kashan) at room temperature by cycling the field from −10 to 10 kOe. A Sonorex ultrasonic water bath (Misonix-S3000, USA) equipped with a digital timer and a temperature controller, a WTW Inolab pH meter (Germany), a CL centrifuge (International Equipment Co, USA) and a Heidolph heating magnetic stirrer (Schwabach, Germany) were used.2.3. HPLC condition
The chromatographic analysis was conducted using a Waters alliance e2695, (Massachusetts, USA) system which was equipped with a Waters 2487 dual wavelength detector and C18 TMS endcapping/reversed phase column (Luna 5 μm C18 100 A HPLC column 250 × 4.6 mm id, Phenomenex Co, Torrance, CA) at 40 °C. The mobile phase which being used in an isocratic mode was composed of potassium dihydrogen phosphate and sodium dodecyl sulfate (0.01 M, pH 6): acetonitrile (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, v/v) and passed through column with flow rate of 1 mL min−1 (the mobile phase was filtered and degassed daily prior to use in HPLC). In the case of metformin, the retention time of this drug was too short and overlapped with solvent peak which was due to its charge. In order to retain this drug in the column and obtain better separation condition a surfactant like dodecyl sulfate was used as an ion-pairing agent. The column effluent was monitored at UV wavelength of 215 nm and the injection volume was 20.0 μL. The mobile phase was filtered by passing it through a 0.2 μm membrane filter (Millipore, Bedford, MA, USA).
45, v/v) and passed through column with flow rate of 1 mL min−1 (the mobile phase was filtered and degassed daily prior to use in HPLC). In the case of metformin, the retention time of this drug was too short and overlapped with solvent peak which was due to its charge. In order to retain this drug in the column and obtain better separation condition a surfactant like dodecyl sulfate was used as an ion-pairing agent. The column effluent was monitored at UV wavelength of 215 nm and the injection volume was 20.0 μL. The mobile phase was filtered by passing it through a 0.2 μm membrane filter (Millipore, Bedford, MA, USA).
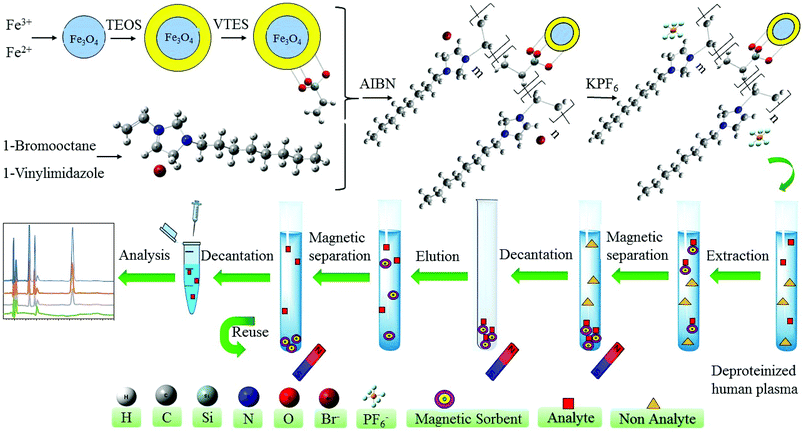
2.4. Synthesis of hydrophobic Fe3O4@SiO2@PIL-PF6 MNPs
The synthesis of the Fe3O4@SiO2@PIL-PF6 was carried out following six well-defined steps, which are explained below:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (FeCl3·6H2O (10.8 g) and FeCl2·4H2O (4.0 g)) were dissolved in 250.0 mL of ultrapure water. The solution was vigorously stirred at 80 °C for 30 min. Later on, 20.0 mL of ammonia solution (25% (w/w)) was added dropwise into the mixture. The resultant solution was vigorously stirred at 80 °C for 60 min and N2 was used as the protective gas in the whole experiment. After completion of the reaction and cooling to room temperature, the obtained black precipitate was separated from the reaction media using an external magnetic field. Next, it was washed with water and ethanol in order to remove the unreacted chemicals. Finally, the resulting material was dried in vacuum.
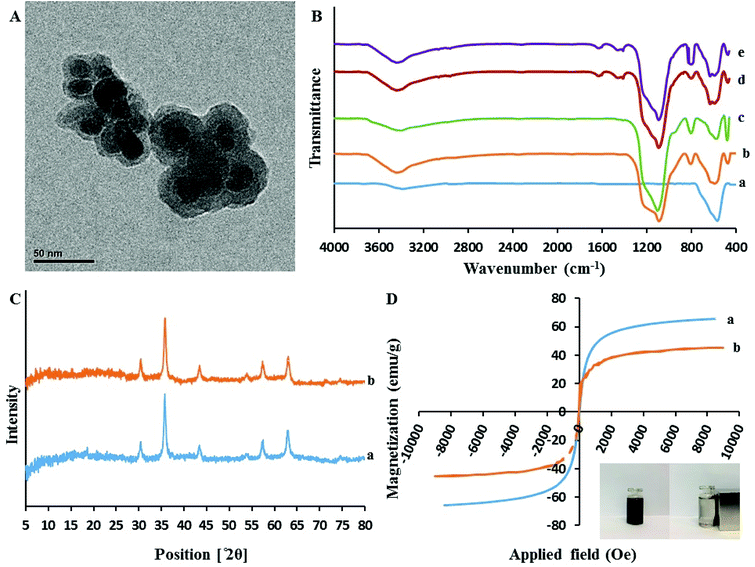
1 (FeCl3·6H2O (10.8 g) and FeCl2·4H2O (4.0 g)) were dissolved in 250.0 mL of ultrapure water. The solution was vigorously stirred at 80 °C for 30 min. Later on, 20.0 mL of ammonia solution (25% (w/w)) was added dropwise into the mixture. The resultant solution was vigorously stirred at 80 °C for 60 min and N2 was used as the protective gas in the whole experiment. After completion of the reaction and cooling to room temperature, the obtained black precipitate was separated from the reaction media using an external magnetic field. Next, it was washed with water and ethanol in order to remove the unreacted chemicals. Finally, the resulting material was dried in vacuum.In this step, according to our scientific experience, the chemical stability of Fe3O4 and Fe3O4@SiO2 MNPs were analyzed via comparative acid corrosion experiment to be certain about the successful silica coating of iron nanoparticles. For Fe3O4 the black color of the solution was changed upon the addition of concentrated HCl (turned to yellow) and mixture limpid immediately but no significant change occurred for Fe3O4@SiO2 (Fig. S1†). This observation confirms triumphant coating of Fe3O4 and resistance enhancement of the fabricated core against oxidation and corrosion using TEOS.
2.5. Preparation of standard solutions and quality control samples
Stock standard solutions of CANA, EMPA and MET (100.0 mg L−1) were prepared separately by dissolving proper amounts of each drug in HPLC grade methanol. These solutions were substituted every week with new ones to prevent decomposition of the drugs. The working standard solutions were daily prepared by appropriate stepwise dilution of the stock standard solutions with deionized water to the desired concentration. Standards for plotting calibration curve were provided by spiking the working solutions into human plasma. Quality control (QC) samples of each drug at concentration levels of 25.0, 500.0 and 1000.0 ng mL−1 were prepared for evaluating the accuracy and precision of the method. All the solutions were stored in a dark brown glass vials under refrigeration at −18 °C and brought to ambient temperature just prior to use.2.6. Plasma sample preparation
Every frozen human plasma were firstly thawed at room temperature and placed into a sample glass tube. Preparation of plasma before MSPE method was based on protein precipitation with aid of acetonitrile. In this regard, the plasma (1.9 mL) was fortified with 100.0 μL of required concentrations of analytes followed by mixing with 2.0 mL ACN. The obtained solution was vortexed for 1 min and centrifuged for 5 min at 3500 rpm. Then, ACN content of the clear supernatant was evaporated under nitrogen stream and the remaining sample was transferred into 4.0 mL buffer solution at pH 4.0. Sample volume after evaporation under nitrogen stream was about 2 mL and total sample volume after transferring to 4 mL buffer solution was about 6 mL. 5.0 mL of the deproteinized samples containing target drugs were subjected to the extraction procedure for subsequent analysis.2.7. Recommended MSPE procedure
The experimental MSPE setup is shown in Fig. 2. Initially, 5.0 mL of the deproteinized human plasma (initial volume of spiked plasma sample 2.0 mL) was placed into a glass tube and 10.0 mg of magnetic Fe3O4@SiO2@PIL was added to the sample. The mixture was sonicated for 4 min to totally disperse the magnetic sorbent through the matrix and complete the extraction of target drugs at room temperature. Analyte-loaded sorbent was collected from the sample solution by exposing the glass tube to a powerful neodymium–iron–boron (Nd–Fe–B) magnet (5 × 5 × 4 cm, 0.8 tesla), and the upper phase was poured out. Later, the adsorbed analytes were eluted by 1.0 mL of acetonitrile (0.5 mL every time and washed two times) to desorb the analytes. Elution process was completed during 2 min in the ultrasonic bath. The magnet was used again to separate the sorbent and then the total volume of eluted solution was collected and evaporated to dryness under stream of the nitrogen. The dry residue was re-dissolved in 100.0 μL of mobile phase and stirred for 1 min. Finally, 20.0 μL of this solution was injected into the HPLC-UV system for analysis.3. Results and discussion
3.1. Characterization of the Fe3O4@SiO2@PIL
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) and they found to be 18.15 and 17.78 nm, respectively which is in agreement with TEM results.
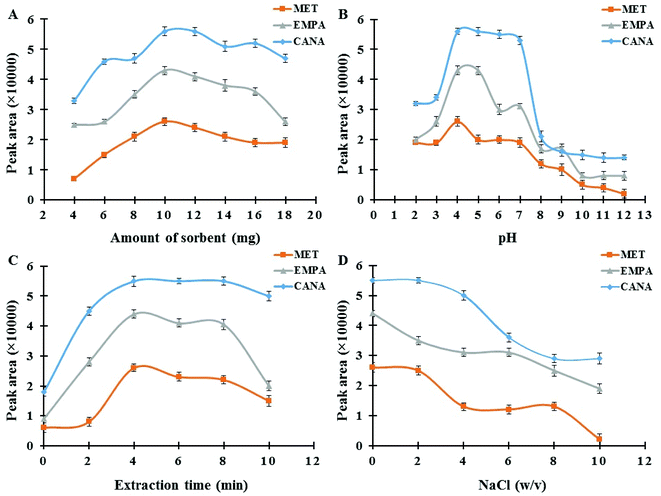
θ) and they found to be 18.15 and 17.78 nm, respectively which is in agreement with TEM results.3.2. Optimization of extraction conditions
3.3. Reusability of the sorbent
The magnetic nanoscale sorbent can disperse in the plasma medium, and in the presence of magnetic field it can be separated easily. After the extraction of analytes, the sorbent could be conveniently regenerated by rinsing the MNPs sequentially with 2.0 mL of ACN and 2.0 mL double-distilled water during sonication time of 4 min. After drying, the sorbent was applied for subsequent MSPE. The extraction recovery of analytes decreased approximately 10% after 14 cycles of MSPE procedure which indicated a slight loss of the sorption capacity.3.4. Method validation
| ER% = EF × (VFinal volume/VInitial volume of plasma) × 100. | (1) |
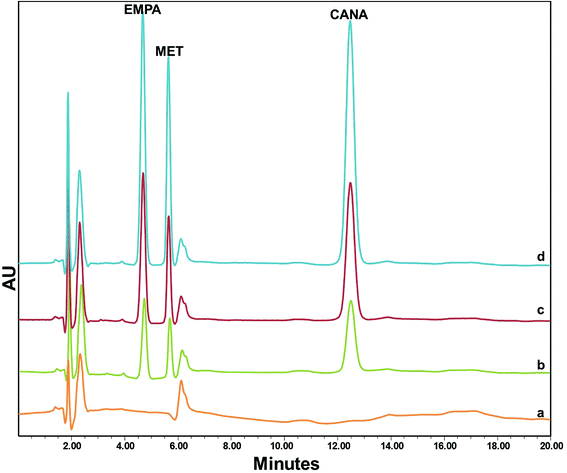
The analytical performance data are summarized in Table 1. The calibration curves were linear over the concentration ranges of 5.0–1200.0 ng mL−1 for EMPA, 20.0–1800.0 ng mL−1 for MET and 5.0–1000.0 ng mL−1 for CANA while correlation coefficient varied from 0.993 to 0.997. The LODs based on S/N = 3 were 1.3 ng mL−1, 6.0 ng mL−1 and 0.8 ng mL−1 for EMPA, MET and CANA, respectively. The chromatograms for blank and spiked human plasma are exhibited in Fig. 6 and indicated that there was no remarkable interference in the whole analytical protocol.
| Analyte | LDR (ng mL−1) | Linear equation | r2 | LOD (ng mL−1) | LOQ (ng mL−1) | EF | ER% (n = 3) |
|---|---|---|---|---|---|---|---|
| a LDR: Linear dynamic range; r2: correlation coefficient; LOD: limit of detection; LOQ: limit of quantification; EF: enrichment factor; ER: extraction recovery (250.0 ng mL−1 of each drug was used). | |||||||
| EMPA | 5.0–1200.0 | Y = 173X + 195 | 0.996 | 1.3 | 5.0 | 18.4 | 92.0 |
| MET | 20.0–1800.0 | Y = 105X + 76 | 0.993 | 6.0 | 20.0 | 17.3 | 86.5 |
| CANA | 5.0–1000.0 | Y = 225X + 270 | 0.997 | 0.8 | 5.0 | 18.5 | 92.5 |
| Drug | Concentration (ng mL−1) | Intra-day, n = 9 | Inter-day, n = 12 | ||||
|---|---|---|---|---|---|---|---|
| Found value ± SD (ng mL−1) | RSD (%) | Accuracy (%) | Found value ± SD (ng mL−1) | RSD (%) | Accuracy (%) | ||
| a RSD (%) = 100 × SD/mean; accuracy (%) = (mean concentration found − known concentration)/(known concentration); intraday (n = 12) = triplicate samples within a series of six measurements on different days. | |||||||
| EMPA | 25.0 | 26.8 ± 1.3 | 4.8 | 7.2 | 22.9 ± 1.2 | 5.2 | −8.4 |
| 500.0 | 529.0 ± 21.7 | 4.1 | 5.8 | 545.5 ± 27.3 | 5.0 | 9.1 | |
| 1000.0 | 1036.0 ± 39.4 | 3.8 | 3.6 | 958.0 ± 42.1 | 4.4 | −4.2 | |
| MET | 25.0 | 26.6 ± 1.5 | 5.6 | 6.4 | 26.8 ± 1.7 | 6.3 | 7.2 |
| 500.0 | 530.0 ± 38.2 | 7.2 | 6.0 | 543.1 ± 36.9 | 6.8 | 8.6 | |
| 1000.0 | 1044.0 ± 42.8 | 4.1 | 4.4 | 1050.0 ± 33.6 | 3.2 | 5.0 | |
| CANA | 25.0 | 26.5 ± 1.3 | 4.9 | 6.0 | 27.0 ± 2.3 | 8.5 | 8.0 |
| 500.0 | 520.5 ± 39.0 | 7.5 | 4.1 | 526.5 ± 41.1 | 7.8 | 5.3 | |
| 1000.0 | 1039.0 ± 41.6 | 4.0 | 3.9 | 1066.0 ± 42.9 | 4.0 | 6.6 | |
3.5. Application of the method to pharmacokinetic assessment
In order to evaluate the capability and validity of the proposed method, it was applied for determination of EMPA, MET and CANA in human plasma and main pharmacokinetic data were calculated. Eight healthy fed male volunteers within the age range of 28–42 years old were enrolled in these studies. Oral administration of a fixed-dose combination Invokamet tablet (50 mg CANA/500 mg MET) was performed to four volunteers and the blood samples were collected at 0, 1, 2, 3, 4, 5, 6, 8, 10, 12 and 24 h after drug administration. The plasma samples were immediately transferred into a clean polypropylene tube and stored at −18 °C until being used. All assessments were approved by “The Committee For Research Ethics” of Department of Pharmacy and Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences. In the next experiments, a single oral dose administration of Jardiance tablet (25 mg EMPA) was performed to four volunteers and the blood samples were collected and treated as the previous experiments. Each real sample was extracted at optimal conditions by the proposed procedure and the mean plasma concentration–time curve was obtained. The main pharmacokinetic parameters including Tmax, Cmax, AUC0–t, AUC0–∞, and T1/2 are summarized in Table 3. These data demonstrate the flexibility of the method in multipurpose analytical applications and its validity for trace drug monitoring in complex biological matrix.| Pharmacokinetic parameters | Mean ± SD | ||
|---|---|---|---|
| CANA | MET | EMPA | |
| a Tmax: Time required for reaching maximum plasma concentration, Cmax: maximum plasma concentration, AUC0–24: area under curve, AUC0–∞: area under curve at infinite time, T1/2 (h): time required for reaching to half concentration. | |||
| Tmax (h) | 3.2 ± 0.3 | 4.1 ± 0.5 | 2.9 ± 0.3 |
| Cmax (ng mL−1) | 390.4 ± 50.2 | 859.7 ± 91.2 | 215.6 ± 44.1 |
| AUC0–24 (ng h mL−1) | 3862.8 ± 426.9 | 6627.1 ± 699.3 | 1398.7 ± 122.5 |
| AUC0–∞ (ng h mL−1) | 3973.6 ± 508.5 | 7022.8 ± 703.6 | 1426.0 ± 150.8 |
| T1/2 (h) | 12.1 ± 1.4 | 6.3 ± 0.8 | 7.2 ± 1.1 |
3.6. Comparison with other methods
Comparison of the proposed method with different existing methods for extraction and determination of antidiabetic drugs is provided in Table 4. It was revealed that along with its simplicity, present combination benefits from some advantages such as low LODs, wide linearity ranges, high sensitivities and reasonable recoveries of target analytes with an important emphasis on the reusability of the sorbent which is comparable with existing techniques. These advantages confirm applicability of the present extraction/preconcentration procedure for trace monitoring of antidiabetic drugs in complex plasma matrices.| Sorbent/extraction method | Drug | LOD (ng mL−1) | DLR (ng mL−1) | r2 | RSD (%) | Separation/detection system | Ref. |
|---|---|---|---|---|---|---|---|
| a Sequential hollow-fiber liquid phase microextraction.b Ion-pair vortex assisted liquid–liquid microextraction.c Ion pair solid phase extraction.d Solid phase extraction.e High performance liquid chromatography-electrospray ionization multi-stage mass spectrometry.f Liquid–liquid extraction. | |||||||
| Sequential HF-LPMEa | MET | 1 | 5–2500 | 0.999 | <8.4% | HPLC-UV | 48 |
| IP-VALLLMEb | MET | 1400 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2000000 000–2000000 |
0.9988 | <10.8 | HPLC-UV | 49 |
| IPSPEc | MET | 3 | 50–2000 | ≥0.997 | <9% | HPLC-UV | 50 |
| SPE cartridge | MET | 1480 | — | ≥0.9992 | <13.4 | HPLC-ESI-MSne | 51 |
| SPEd cartridge | CANA | — | 10.3–6019 | ≥0.99 | — | LC-MS/MS | 52 |
| LLEf | EMPA | — | — | 0.9997 | <6.99 | LC-MS/MS | 53 |
| MSPE | EMPA | 1.3 | 5.0–1200.0 | 0.996 | ≤5.2 | HPLC-UV | This work |
| MET | 6.0 | 20.0–1800.0 | 0.993 | ≤6.8 | |||
| CANA | 0.8 | 5.0–1000.0 | 0.997 | ≤8.5 | |||
4. Conclusion
The aim of present study was to develop and validate a reliable MSPE method based on PIL-coated Fe3O4@SiO2 nanoparticles for simultaneous extraction, preconcentration and determination of antidiabetic drugs from human plasma. Chemical immobilization of PIL on the surface of Fe3O4@SiO2 core–shell through polymeric bonds offers strong adhesion between the IL and MPNs which prevents ionic liquids leakage. Furthermore, the later modification merges the interesting advantages of MNPs with individual characteristics of ILs resulting significant improvements in stability, reusability, mass transfer capacity and diffusion routes of the nanoscale sorbent. The successful pharmacokinetic study with low dose administration of antidiabetic drugs demonstrated that the current enrichment procedure in combination with HPLC-UV is a valid means for screening purposes. According to these criteria, the validated method has notable potential for further toxicodynamic and bioequivalence studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to express their gratitude to Karaj Islamic Azad University, Islamic Azad University South Tehran Branch, Nik Azma Pars Alborz laboratory and Padideh Shimi Jam Co. (specially Dr Ramin Mostafalou) for supporting of this work. The authors also greatly appreciate Mr Alireza Hajikazemi for cooperating to perform HPLC analysis and all volunteers for helping us to study pharmacokinetic features.References
- P. Sengupta, U. Bhaumik, A. Ghosh, A. K. Sarkar, B. Chatterjee, A. Bose and T. K. Pal, Chromatographia, 2009, 69, 1243–1250 CrossRef.
- C. Hess, F. Musshoff and B. Madea, Anal. Bioanal. Chem., 2011, 400, 33–41 CrossRef PubMed.
- K. S. Lakshmi and T. Rajesh, J. Iran. Chem. Soc., 2011, 8, 31–37 CrossRef.
- M. d. O. Viana, P. d. P. R. Lima, C. D. V. Soares and C. Fernandes, J. Pharm. Biomed. Anal., 2014, 96, 241–248 CrossRef PubMed.
- H. N. Mistri, A. G. Jangid and P. S. Shrivastav, J. Pharm. Biomed. Anal., 2007, 45, 97–106 CrossRef PubMed.
- G.-P. Zhong, H.-c. Bi, S. Zhou, X. Chen and M. Huang, J. Mass Spectrom., 2005, 40, 1462–1471 CrossRef PubMed.
- H. Sereshti and S. Bakhtiari, RSC Adv., 2016, 6, 75757–75765 RSC.
- I. Ali, U. Kulsum, Z. A. Al-Othman, A. Alwarthan and K. Saleem, J. Sep. Sci., 2016, 39, 2678–2688 CrossRef PubMed.
- A. A. Rajabi, Y. Yamini, M. Faraji and S. Seidi, Med. Chem. Res., 2013, 22, 1570–1577 CrossRef.
- B. Fresco-Cala, S. Cárdenas and J. M. Herrero-Martínez, Microchim. Acta, 2017, 184, 1863–1871 CrossRef.
- S. Saha, M. K. Mandal and K. Hiraoka, Anal. Methods, 2013, 5, 4731–4738 RSC.
- M. Ebrahimi, A. Ebrahimitalab, Z. Es'haghi and A. Mohammadinejad, Arch. Environ. Contam. Toxicol., 2015, 68, 412–420 CrossRef PubMed.
- A. Taghvimi, H. Hamishehkar and M. Ebrahimi, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1009–1010, 66–72 CrossRef PubMed.
- V. Moreno, M. Zougagh and Á. Ríos, Microchim. Acta, 2016, 183, 871–880 CrossRef.
- H. Bagheri, A. Roostaie and M. Y. Baktash, Anal. Chim. Acta, 2014, 816, 1–7 CrossRef PubMed.
- M. Luo, D. Liu, L. Zhao, J. Han, Y. Liang, P. Wang and Z. Zhou, Anal. Chim. Acta, 2014, 852, 88–96 CrossRef PubMed.
- J. Wu, H. Zhao, D. Xiao, P.-H. Chuong, J. He and H. He, J. Chromatogr. A, 2016, 1454, 1–8 CrossRef PubMed.
- M. Zeeb and H. Farahani, Chem. Pap., 2017, 72, 15–27 CrossRef.
- G. H. Gaber Ahmed, R. Badía Laíño, J. A. García Calzón and M. E. Díaz García, Microchim. Acta, 2014, 181, 941–948 CrossRef.
- V. Romero, I. Costas-Mora, I. Lavilla and C. Bendicho, J. Anal. At. Spectrom., 2013, 28, 923–933 RSC.
- J. Cheng, X. Ma and Y. Wu, Food Anal. Methods, 2014, 7, 1083–1089 CrossRef.
- J. Zou, X. Ma, Y. Dang and Y. Chen, J. Anal. At. Spectrom., 2014, 29, 1692–1697 RSC.
- N. Fasih Ramandi and F. Shemirani, Talanta, 2015, 131, 404–411 CrossRef PubMed.
- F. Zare, M. Ghaedi and A. Daneshfar, RSC Adv., 2015, 5, 70064–70072 RSC.
- Ş. Tokalıoğlu, E. Yavuz, H. Şahan, S. G. Çolak, K. Ocakoğlu, M. Kaçer and Ş. Patat, Talanta, 2016, 159, 222–230 CrossRef PubMed.
- M. D. Joshi and J. L. Anderson, RSC Adv., 2012, 2, 5470–5484 RSC.
- N. Papaiconomou, I. Billard and E. Chainet, RSC Adv., 2014, 4, 48260–48266 RSC.
- M. Zeeb, H. Farahani and M. K. Papan, J. Sep. Sci., 2016, 39, 2138–2145 CrossRef PubMed.
- M. Zeeb and M. Sadeghi, Microchim. Acta, 2011, 175, 159 CrossRef.
- S. Chen, J. Chen and X. Zhu, Microchim. Acta, 2016, 183, 1315–1321 CrossRef.
- J. Chen and X. Zhu, Food Chem., 2016, 200, 10–15 CrossRef PubMed.
- H. He, D. Yuan, Z. Gao, D. Xiao, H. He, H. Dai, J. Peng and N. Li, J. Chromatogr. A, 2014, 1324, 78–85 CrossRef PubMed.
- J. Chen and X. Zhu, Spectrochim. Acta, Part A, 2015, 137, 456–462 CrossRef PubMed.
- S. Chen, X. Qin, W. Gu and X. Zhu, Talanta, 2016, 161, 325–332 CrossRef PubMed.
- J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009–1036 CrossRef.
- S. Wang, H.-L. Ma, J. Peng, Y. Zhang, J. Chen, L. Wang, L. Xu, J. Li and M. Zhai, Dalton Trans., 2015, 44, 7618–7625 RSC.
- C. Liu, Y. Liao and X. Huang, Talanta, 2017, 172, 23–30 CrossRef PubMed.
- S. Zhou, N. Song, X. Lv and Q. Jia, Microchim. Acta, 2017, 184, 3497–3504 CrossRef.
- D. Guzmán-Lucero, O. Olivares-Xometl, R. Martínez-Palou, N. V. Likhanova, M. A. Domínguez-Aguilar and V. Garibay-Febles, Ind. Eng. Chem. Res., 2011, 50, 7129–7140 CrossRef.
- J. Zou, Y.-G. Peng and Y.-Y. Tang, RSC Adv., 2014, 4, 9693–9700 RSC.
- M. H. Sherahi, M. Shadaei, E. Ghobadi, F. Zhandari, Z. Rastgou and S. M. B. Hashemi, Food Hydrocolloids, 2018, 79, 81–89 CrossRef.
- H. Abdolmohammad-Zadeh and Z. Javan, Microchim. Acta, 2015, 182, 1447–1456 CrossRef.
- A. A. Asgharinezhad, N. Mollazadeh, H. Ebrahimzadeh, F. Mirbabaei and N. Shekari, J. Chromatogr. A, 2014, 1338, 1–8 CrossRef PubMed.
- A. E. Bretnall and G. S. Clarke, Metformin hydrochloride, In Analytical profiles of drug substances, ed. K. Florey, Academic Press, San Diego (CA), 1998, pp. 243–291 Search PubMed.
- D. Desai, B. Wong, Y. Huang, Q. Ye, D. Tang, H. Guo, M. Huang and P. Timmins, J. Pharm. Sci., 2014, 103, 920–926 CrossRef PubMed.
- A. A. Asgharinezhad, S. Karami, H. Ebrahimzadeh, N. Shekari and N. Jalilian, Int. J. Pharm., 2015, 494, 102–112 CrossRef PubMed.
- M. A. Daryakenary and M. Zeeb, RSC Adv., 2017, 7, 53210–53218 RSC.
- G. M. Ben-Hander, A. Makahleh, B. Saad, M. I. Saleh and K. W. Cheng, Talanta, 2015, 131, 590–596 CrossRef PubMed.
- A. Alshishani, A. Makahleh, H. F Yap, E. A. Gubartallah, S. M. Salhimi and B. Saad, Talanta, 2016, 161, 398–404 CrossRef PubMed.
- S. AbuRuz, J. Millership and J. McElnay, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 798, 203–209 CrossRef.
- Y. Zhou, F. Wang, Z. Wang, H. Zhan, W. Liu and Z. Meng, Sepu, 2018, 36, 159–166 Search PubMed.
- S. V. Saibaba, N. R. Pilli, B. P. K. Bimireddy and P. S. Pandiyan, Future Journal of Pharmaceutical Sciences, 2018 DOI:10.1016/j.fjps.2017.12.003.
- B. M. Ayoub, S. Mowaka, E. S. Elzanfaly, N. Ashoush, M. M. Elmazar and S. A. Mousa, Sci. Rep., 2017, 7, 2583–2593 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02109k |
| This journal is © The Royal Society of Chemistry 2018 |