Open Access Article
Open Access ArticleRisk assessment of an organochlorine pesticide mixture in the surface waters of Qingshitan Reservoir in Southwest China
Honghu Zeng†
abc,
Xin Fua,
Yanpeng Liang† *abc,
Litang Qin*abc and
Lingyun Mobc
*abc,
Litang Qin*abc and
Lingyun Mobc
aCollege of Environmental Science and Engineering, Guilin University of Technology, Guilin 541004, China. E-mail: ypliang1980@163.com
bGuangxi Key Laboratory of Environmental Pollution Control Theory and Technology, Guilin University of Technology, Guilin 541004, China. E-mail: qinsar@163.com
cCollaborative Innovation Center for Water Pollution Control and Water Safety in Karst Area, Guilin University of Technology, Guilin 541004, China
First published on 15th May 2018
Abstract
Risk assessment of single pollutants has been extensively studied. However, the co-exposure of pollutants in a real environment may pose a greater risk than single chemicals. In this study, concentration addition-based risk quotients were applied to the risk assessment of the 15 organochlorine pesticides (OCPs) mixtures (α-hexachlorocyclohexane (HCH), β-HCH, γ-HCH, δ-HCH, heptachlor, aldrin, heptachlor epoxide, chlordane, α-endosulfan, p,p′-dichloro-diphenyl-dichloroethylene, endrin, β-endosulfan, p,p′-dichloro-diphenyl-dichloroethane, p,p′-dichloro-diphenyl-trichloroethane, and methoxychlor) detected in the surface water (reservoirs, ponds, and streams) of Qingshitan Reservoir in Southwest China from 2014 to 2016 by summing up the toxic units (RQSTU) of the toxicity data from the individual chemicals. The RQSTU of the OCPs mixture exceeded 1 in 45.23% of the 283 surface water samples based on acute data and an assessment factor of 100, indicating a potential risk for the aquatic environment (fish). Methoxychlor and γ-HCH contributed the most toxicities in the pesticide mixtures toward Daphnia and fish and provided at least 50% of the mixture toxicity in all samples with RQSTU larger than 1. The most sensitive organism to realistic OCPs mixtures in the surface waters of Qingshitan Reservoir was fish, followed by Daphnia and algae. The values of the maximum cumulative ratio for all samples indicated that the risk assessment based on single chemicals underestimated the pesticide mixture toxicities, which shows that special consideration should be made for the ecological risk of pesticide mixtures in the aquatic environment.
1 Introduction
Organochlorine pesticides (OCPs) are typical persistent organic pollutants (POPs) that are widely used as broad-spectrum insecticides and are successfully used in agricultural pest control.1 OCPs have attracted global attention because of their resistance to environmental degradation, toxicity, and bioaccumulation potential.2 OCPs are internationally controlled by the 2004 Stockholm Convention because of their adverse human health effects, including cancer, reproductive defects, and endocrine, and immunological toxicities.3 OCPs can still be widely detected in the global environment because of their high chemical stability and migration properties,4–6 which may threaten ecological integrities and humans.Qingshitan Reservoir has a capacity of 6 × 108 m3 and is the largest hydrological basin in Guilin Southwest China (Fig. 1) with multiple functions, such as water supply, power generation, storage, transport, tourism, and aquaculture.7 Agriculture is the main industry in the region around Qingshitan Reservoir. Agricultural sewage flows into the reservoir, and cage-culture fishing reduces the environmental quality of the region. Several studies have been conducted on the environmental pollution in this region.8,9 OCPs were used in large-scale in the 1960–1980 in Guilin Southwest China.10 Experimental investigations have been recently conducted to describe the contamination characteristics of OCP residues in the multi-media environment of this region. Wang et al. investigated the concentration and distribution of OCPs in air from the Kast cave (Dayan cave) in Guilin and showed that OCPs were detected in the air from the Dayan cave despite of the cave's relatively closed space.11 Zhang et al. investigated the distribution and sources of OCPs in the surface sediments of Lijiang River and showed that the contamination of OCPs in the sediments was at a medium level compared to the other studied rivers, and the sources of hexachlorocyclohexane and dichlorodiphenyltrichloroethane are long-term migration and historical usage.12 In our previous study, the agricultural sewage carrying OCPs flows into the ponds around Qingshitan Reservoir and finally reaches the reservoir through the streams.13 OCPs accumulated in the sediment of Qingshitan Reservoir, which may released into the water body at a specific condition.9,14 Therefore, the OCPs in the water body (reservoirs, ponds and streams) of Qingshitan may pose a potential risk to aquatic organisms.
Ecosystems are usually exposed to chemical mixtures, rather than the ideal scenario of exposure to a single substance.15–17 Many potential hazard substances are released into the surface water and cause water pollution because of human activities.18 The traditional risk assessment only estimates the risk of a single type of chemical.19 However, chemical exposure below the no observed effect concentration can produce a combined effect.20–22 Therefore, risk assessment based on a single chemical may underestimate the risk of pollutants in the actual environment. The risk assessment based on multiple chemicals can accurately estimate the ecological risk of co-exposed pollutants.20
Although many mixtures are present in the actual environment, limited data on mixture toxicity are available. Managers evaluate the risk of mixtures based on the toxicity data of a single substance or a similar mixture with extrapolation technique.23 Two reference models, namely, concentration addition (CA) and independence action (IA), can be used to predict the combined effects using the concentration-response of single chemicals.24 Backhaus and Faust provided a tiered approach that applies CA as a precautious first tier and considers IA in the second tier for the environmental risk assessment of chemical mixtures.25 This approach can optimize the use of available exposure data and single chemical toxicity data to calculate the risk quotient (RQ) by summing up the measured environmental concentration/predicted no effect concentration (MEC/PNEC) ratios (RQMEC/PNEC) or toxic units (RQSTU). An additional IA model is suggested to be used in the risk assessment only if the RQSTU exceeds the threshold, and the error estimations indicate the possibility of substantial differences between the CA- and IA-based assessments.24
This study aims to conduct risk assessment of the pesticide mixtures detected in the three important surface waters of Qingshitan Reservoir (reservoirs, ponds, and streams) in Southwest China from 2014 to 2016. The samples were extracted by solid phase extraction (SPE), and the 15 OCPs were qualitative and quantitative analyzed by gas chromatography-mass spectrometry (GC-MS) and gas chromatography with a 63Ni electron capture detector (GC-ECD). The mixture RQ of 15 OCPs was calculated from the toxicities data of single compounds toward algae, Daphnia magna, and fish. The ecological risk of the OCP mixtures was assessed to determine the pesticide that is a potential threat to the aquatic organisms. The most sensitive organism among algae, Daphnia, and fish was determined, and the largest contribution to the mixture risk was calculated and presented using the toxic unit (TU) value.
2 Materials and methods
2.1 Sample collection
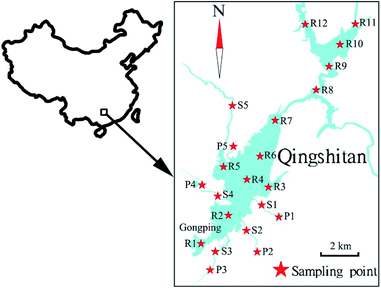
The study was conducted on the surface water of Qingshitan Reservoir in Guilin city, Southwest China. Water samples were collected in August 2014, November 2014, March 2015, June 2015, September 2015, December 2015, and June 2016. A total of 283 water samples were collected in these seven sampling campaigns from 2014 to 2016. The sampling sites of this study are shown in Fig. 1, and the global positioning system (GPS) information of the sampling points is shown in Table 1. The reservoir sampling sites (R1–R12) have multilevel depths, with sampling ports at 0.5, 5, and 10 m below the water surface. Water samples (P1–P5) were collected from five ponds close to the residential area and agricultural land. In addition, five points were sampled in the streams (S1–S5) around the Qingshitan Reservoir, which were connected to the ponds and reservoirs. Among these sites, R1 and R2 are located in the cage-culture fishing area, and the domestic sewage and/or industrial wastewater produced by Gongping town are poured into these two sites.| Sampling point | North latitude (N) | East longitude (E) |
|---|---|---|
| R1 | 25°28′23.6′′ | 110°10′02.0′′ |
| R2 | 25°29′21.5′′ | 110°10′32.8′′ |
| R3 | 25°30′03.2′′ | 110°11′22.9′′ |
| R4 | 25°30′15.4′′ | 110°11′03.8′′ |
| R5 | 25°30′20.7′′ | 110°10′05.5′′ |
| R6 | 25°30′50.1′′ | 110°11′10.4′′ |
| R7 | 25°31′03.6′′ | 110°11′24.4′′ |
| R8 | 25°32′19.1′′ | 110°12′12.9′′ |
| R9 | 25°33′15.7′′ | 110°13′23.4′′ |
| R10 | 25°34′07.4′′ | 110°13′41.4′′ |
| R11 | 25°34′53.6′′ | 110°14′05.8′′ |
| R12 | 25°34′47.5′′ | 110°12′10.8′′ |
| P1 | 25°28′47.8′′ | 110°10′52.2′′ |
| P2 | 25°27′30.4′′ | 110°10′05.1′′ |
| P3 | 25°27′17.6′′ | 110°09′39.1′′ |
| P4 | 25°29′27.1′′ | 110°09′42.3′′ |
| P5 | 25°30′49.2′′ | 110°10′00.6′′ |
| S1 | 25°28′46.5′′ | 110°10′51.0′′ |
| S2 | 25°27′32.5′′ | 110°10′03.4′′ |
| S3 | 25°27′18.4′′ | 110°09′39.5′′ |
| S4 | 25°29′35.1′′ | 110°09′26.1′′ |
| S5 | 25°30′21.9′′ | 110°10′78.6′′ |
Glass amber bottles with capacity of 2.5 L and covered with tinfoil were deployed. The glass amber bottles and tinfoil were precleaned with hexane. In each sampling site, 2.5 L of water was collected. To facilitate the extraction for target substances and to inhibit the growth of microorganisms, the collected water was added with 10% of methanol, and its pH was immediately adjusted to less than 2 using hydrogen nitrate. The samples were transported to the laboratory and refrigerated at 4 °C. Solid phase extraction (SPE) was conducted within 24 hours.
2.2 Extraction and analysis
The 15 OCPs were extracted by SPE. A 0.45 μm glass fiber filter was used to filter the water samples, and 1 L of the filtered water sample was taken for solid phase extraction. The extraction steps are as follows, (1) activation: the C18 column was activated with 10 mL of ethyl acetate and dichloromethane mixed solution (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 10 mL of methanol and 10 mL of ultrapure water in sequence; (2) sample loading: 1 L water sample was continuously passed through a C18 column at a flow rate of 10 mL min−1. After loading, the column was rinsed with 10 mL of ultrapure water, and the C18 column was dried with nitrogen for 40 min; (3) elution: the target fraction was eluted twice with a mixture of 6 mL of ethyl acetate and dichloromethane mixed solution (v/v = 1
1), 10 mL of methanol and 10 mL of ultrapure water in sequence; (2) sample loading: 1 L water sample was continuously passed through a C18 column at a flow rate of 10 mL min−1. After loading, the column was rinsed with 10 mL of ultrapure water, and the C18 column was dried with nitrogen for 40 min; (3) elution: the target fraction was eluted twice with a mixture of 6 mL of ethyl acetate and dichloromethane mixed solution (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The eluent was blown to near dryness with high purity nitrogen, and n-hexane was brought to 1 mL for instrument determination.
1). The eluent was blown to near dryness with high purity nitrogen, and n-hexane was brought to 1 mL for instrument determination.
The compounds in the standard OCPs mixed solution were determined by GC-MS to determine the retention time of different compounds. The compounds were quantified through GC-ECD using an external calibration method and a 7-point curve (the correlation coefficient value of the calibration curve was above 0.999).9
After one week, a set of calibration standards were run to check for interference and cross contamination. Procedural and solvent blank were examined by same procedure adopted for original sample analysis. All experiments were conducted in duplication. The method detection limits (MDLs) of OCPs were 0.02–2.03 ng L−1, blank spike recovery rate is 73.20–117.00%, and relative standard deviation is 1.19–15.40%.
2.3 Exposure data and characterization of risk
Exposure data were obtained from 283 water samples collected at 22 sampling sites, which represent the environmental status of the water body of reservoirs, ponds, and streams during the main period of agricultural practices from 2014 to 2016. The concentration and detection frequency for the 15 OCPs, namely, hexachlorocyclohexane (β-HCH, α-HCH, γ-HCH, δ-HCH), heptachlor, aldrin, heptachlor epoxide, chlordane, α-endosulfan, β-endosulfan, endrin, dichlorodiphenyltrichloroethane (p,p′-DDE, p,p′-DDD, p,p′-DDT), and methoxychlor, analyzed in the water body of reservoirs, ponds, and streams are presented in Table 2. The detection frequencies of the 15 OCPs in the water body of reservoirs, ponds, and streams were 61.59–100%, 88.57–100%, and 74.29–100%, respectively, showing the widespread exposure of OCP in the study area.| Organochlorine pesticides | Reservoir | Pond | Stream | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range (ng L−1) | Mean (ng L−1) | Detection ration | Range (ng L−1) | Mean (ng L−1) | Detection ration | Range (ng L−1) | Mean (ng L−1) | Detection ration | |
| a nd is non-detected. | |||||||||
| β-HCH | 7.72–95.13 | 47.11 | 100.00% | 50.49–125.36 | 91.96 | 100.00% | 9.07–62.32 | 42.62 | 100.00% |
| α-HCH | nda–35.63 | 16.99 | 99.53% | nd–69.42 | 40.11 | 97.14% | nd–34.15 | 17.09 | 97.14% |
| γ-HCH | nd–26.10 | 11.25 | 97.18% | 3.94–48.95 | 28.54 | 100.00% | nd–29.76 | 10.99 | 97.14% |
| δ-HCH | nd–37.94 | 7.59 | 92.49% | nd–45.75 | 19.06 | 97.14% | nd–27.14 | 10.69 | 94.29% |
| Heptachlor | nd–25.63 | 11.68 | 93.43% | nd–40.85 | 18.95 | 97.14% | nd–28.04 | 12.37 | 94.29% |
| Aldrin | nd–14.37 | 1.76 | 61.59% | nd–23.26 | 11.14 | 94.29% | nd–17.65 | 7.34 | 77.14% |
| Heptachlor-epoxide | nd–5.70 | 1.35 | 92.02% | nd–6.63 | 1.57 | 88.57% | nd–3.96 | 1.32 | 97.14% |
| Chlordane | nd–7.18 | 1.60 | 96.24% | 0.50–10.34 | 2.41 | 100.00% | nd–9.65 | 2.59 | 94.29% |
| α-Endosulfan | nd–6.34 | 1.43 | 92.02% | nd–8.52 | 2.04 | 88.57% | nd–4.07 | 1.49 | 91.43% |
| p,p′-DDE | nd–4.08 | 1.32 | 90.14% | nd–8.95 | 1.92 | 94.29% | nd–4.48 | 1.31 | 85.71% |
| Endrin | nd–14.59 | 1.28 | 96.24% | nd–6.96 | 1.39 | 97.14% | nd–11.90 | 1.43 | 88.57% |
| β-Endosulfan | nd–8.01 | 1.61 | 94.37% | 0.42–10.65 | 2.26 | 100.00% | nd–8.53 | 2.39 | 94.29% |
| p,p′-DDD | nd–15.87 | 7.16 | 97.18% | 0.48–41.51 | 14.92 | 100.00% | nd–23.02 | 10.47 | 91.43% |
| p,p′-DDT | nd–13.12 | 3.09 | 73.24% | nd–24.64 | 7.79 | 94.29% | nd–15.14 | 4.17 | 74.29% |
| Methoxychlor | nd–13.90 | 2.33 | 94.37% | nd–13.64 | 2.37 | 88.57% | nd–13.41 | 2.25 | 94.29% |
| ΣHCHs | 14.86–149.88 | 82.49 | — | 65.56–248.45 | 179.66 | — | 14.3–117.12 | 81.39 | — |
| ΣDDTs | 0.65–23.55 | 11.56 | — | 5.80–62.84 | 24.63 | — | nd–33.92 | 15.95 | — |
| ΣOCPs | 21.07–218.06 | 117.55 | — | 77.84–314.42 | 246.40 | — | 25.2–180.17 | 128.52 | — |
The toxicity data (Table 3) of half effect concentrations (EC50) of algae and Daphnia and the half lethal concentration (LC50) of fish for the 15 OCPs were taken from the PPDB pesticide properties database26 and ref. 27–29 The lowest acute toxicity values were calculated with the assessment factor (AF) of 100 proposed by Lepper to obtain the PNEC values for the 15 OCPs (Table 3).30
| Organochlorine pesticides | Algae EC50 (72 h) | Crustaceans (Daphnia sp.) EC50 (48 h) | Fish LC50 (96 h) | PNEC (μg L−1) |
|---|---|---|---|---|
| a Refers no data.b Data collected from ref. 27–29 the rest toxicity data collected from PPDB database, the unit for EC50/LC50 of algae, Daphnia, and fish is mg L−1. | ||||
| β-HCH | —a | — | 1.52b | 15.2 |
| α-HCH | 10 | 0.37 | 0.82 | 3.7 |
| γ-HCH | 2.5 | 1.6 | 0.0029 | 0.029 |
| δ-HCH | — | — | 1.58b | 15.8 |
| Heptachlor | 0.027 | 0.042 | 0.007 | 0.07 |
| Aldrin | — | 0.028 | 0.0046 | 0.046 |
| Heptachlor epoxide | 200 | 0.24 | 0.02 | 0.2 |
| Chlordane | 0.362b | 0.59 | 0.09 | 0.9 |
| α-Endosulfan | 2.15 | 0.44 | 0.002 | 0.02 |
| p,p′-DDE | — | 0.001 | 0.032 | 0.01 |
| Endrin | — | 0.0042 | 0.0073 | 0.042 |
| β-Endosulfan | — | 0.962b | 0.0028b | 0.028 |
| p,p′-DDD | — | 0.009 | 0.07 | 0.09 |
| p,p′-DDT | 100b | 0.005 | 7 | 0.05 |
| Methoxychlor | 0.6 | 0.00078 | 0.052 | 0.0078 |
2.4 Mixture risk assessment
CA was a basis for the risk assessment of the pesticide mixture.25 First, the risk quotient (RQ) for each pesticide was calculated as the ratio between the measured environmental concentration and the predicted no effect concentration (MEC/PNEC).31,32 Second, the mixture RQMEC/PNEC (eqn (1)) for a scenario was obtained by summing up the MEC/PNEC ratios for each pesticide. Three risk levels were classified according to the individual RQs (or mixture RQMEC/PNEC): 0.01 < RQ (RQMEC/PNEC) < 0.1, low risk; 0.1 < RQ (RQMEC/PNEC) < 1, medium risk; and 1 < RQ (RQMEC/PNEC), high risk.32,33 Previous studies proved that RQMEC/PNEC will always be higher than RQSTU (RQMEC/PNEC is slightly overestimated than RQSTU).25 If RQMEC/PNEC exceeds one, which indicates a potential risk, then RQSTU can be calculated in the next step. The mixture RQSTU (eqn (2)) was calculated by the sum of toxic units (STU) of the most sensitive organism group (the highest PEC/E(L)C50 value) for each trophic level and the corresponding AF of 100.
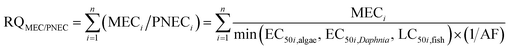 | (1) |
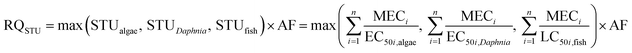 | (2) |
3 Results and discussion
3.1 Exposure concentrations
The mean concentration and detection frequencies of the 15 pesticides (β-HCH, α-HCH, γ-HCH, δ-HCH, heptachlor, aldrin, heptachlor epoxide, chlordane, α-endosulfan, p,p′-DDE, endrin, β-endosulfan, p,p′-DDD, p,p′-DDT, and methoxychlor) based on the monitoring data from reservoirs, ponds, and streams of Qingshitan Reservoir (Guilin Southwest China) are listed in Table 2. The total OCP concentrations in reservoirs, ponds, and streams ranged from 21.07 ng L−1 to 218.06 ng L−1, 77.84 ng L−1 to 314.42 ng L−1, and 25.24 ng L−1 to 180.17 ng L−1 (mean 117.55 ng L−1, 246.40 ng L−1, and 128.52 ng L−1), respectively. The order of residual levels of OCPs of Qingshitan Reservoir is as follows: ponds > streams > reservoirs. According to Table 2, HCHs and DDTs are the two major groups of OCPs in the water. The total concentrations of HCHs in reservoirs, ponds, and streams were 14.86–149.88 ng L−1, 65.56–248.45 ng L−1, and 14.37–117.12 ng L−1 (mean values: 82.94 ng L−1, 179.66 ng L−1, and 81.31 ng L−1), respectively. The concentrations of the total HCHs accounted for 47.82–92.37%, 59.63–88.70%, 43.60–95.86% of the total OCPs in reservoirs, ponds, and streams, respectively. Moreover, HCHs have been detected in all samples collected from Qingshitan Reservoir. Thus, HCHs were the main components of OCPs in the water body, and β-HCH was the dominant HCH isomer. The total DDT concentrations in reservoirs, ponds, and streams were 0.65–23.55 ng L−1, 5.80–62.84 ng L−1, and nd–33.92 ng L−1 (mean 11.56 ng L−1, 24.63 ng L−1, and 15.95 ng L−1), respectively. The contents of DDTs were much lower than those of HCHs in the surface water of the reservoirs, ponds, and streams of Qingshitan Reservoir. This may be attributed to the different natures of HCHs and DDTs, that is, the hydrophobicity of DDT is much higher than that of HCH, such as the water solubility of β-HCH is approximately 500 times that of p,p′-DDD, and the octanol–water partition coefficient of p,p′-DDD is approximately 1000 times that of β-HCH.34 On the other hand, the differences of HCHs and DDTs residual in surface water may be due to the application difference of OCPs in the region.The comparison of the amount of OCPs in surface waters collected at the Qingshitan Reservoir with those of other regions, such as China (Nansi lake,35 Guanting reservoir,36 Chaohu lake,37 Yangchaihu lake,38 Haihe river39), Pakistan (Indus river,40 Chenab river41), India (Gomti river42), Italy (Tiber river,43 Sarno river44), Spain (Ebro river45), Greece (Vistonida lake46), Russia (Moscow river47), Malaysia (Sembrong lake48), and Chile (Cochamo river49) (Table 4), shows that the HCHs levels in the samples from the Qingshitan Reservoir were lower than those at the Haihe river in China but higher than those of the other regions, as presented in Table 4. The average concentration of HCHs in the surface water of Qingshitan Reservoir is approximately 500 times that of the concentration of HCHs in the Tiber river and Sarno river in Italy, and approximately 30 times that of Chenab river in Pakistan. Compared with other polluted waters, the DDTs in the samples from Qingshitan Reservoir were significantly higher than those from Tiber river, Sarno river in Italy and Moscow river in Russia but lower than those presented in the Haihe river in China; Indus river in Pakistan; Cochamo river in Chile; and Sembrong lake in Malaysia, while close to those found in other regions presented in Table 4. In addition, methoxychlor was not detected in Chaohu lake, China; Gomti river, India; Moscow river, Russia; and Tiber river, Italy. The detection rate of methoxychlor in the surface water samples of Qingshitan Reservoir was 94.37%, but the average concentration of methoxychlor was lower than that in the Guanting reservoir, China; and Sembrong lake, Malaysia.
| Sampling location | ΣHCHs (mean) | ΣDDTs (mean) | Methoxychlor (mean) | Reference |
|---|---|---|---|---|
| a Refers no data.b nd is no detection. | ||||
| Nansi lake (China) | 19.57–21.98 (22.15) | 14.66–24.57 (18.23) | —a | 35 |
| Guanting reservoir (China) | 6.20–12.81 (9.80) | 10.94–14.04 (12.06) | ndb–9.18 (9.00) | 36 |
| Chaohu lake (China) | 14.00–44.00 (25.70) | 18.10–28.4 (22.20) | nd | 37 |
| Yangchaihu lake (China) | 4.10–40.67 (8.84) | 0.01–11.70 (2.02) | — | 38 |
| Haihe river (China) | 300.00–1070.00 (600.00) | 20.00–148.00 (90.17) | — | 39 |
| Indus river (Pakistan) | 9.10–115.00 (44.60) | 7.30–226.00 (60.12) | — | 40 |
| Chenab river (Pakistan) | 0.33–11.9 (3.31) | 1.90–20.6 (9.07) | — | 41 |
| Gomti river (India) | 1.63–368.70 (46.69) | nd–74.95 (5.97) | nd | 42 |
| Tiber river (Italy) | 0.003–1.37 (0.21) | 0.004–1.78 (0.25) | nd | 43 |
| Sarno river (Italy) | 0.006–0.85 (0.24) | 0.23–1.18 | — | 44 |
| Ebro river (Spain) | 0.22–28.58 (3.38) | 1.97–6.77 (3.10) | — | 45 |
| Vistonida lake (Greece) | nd–17.00 | nd–18.00 | nd–56.00 | 46 |
| Moscow river (Russia) | — | nd | nd | 47 |
| Cochamo river (Chile) | nd–104.00 | 6.00–73.00 | — | 48 |
| Sembrong lake (Malaysia) | 2.25–7.84 (4.21) | nd–312.20 (40.25) | nd–51.4 (14.03) | 49 |
| Qingshitan Reservoirs (China) | 14.86–149.88 (82.94) | 0.65–23.55 (11.56) | nd–13.89 (2.33) | This study |
| Qingshitan ponds (China) | 65.56–248.45 (179.66) | 5.80–62.84 (24.63) | nd–13.64 (2.37) | This study |
| Qingshitan streams (China) | 14.37–117.12 (81.31) | nd–33.92 (15.95) | nd–13.41 (2.25) | This study |
Thus, the contaminations of HCHs in the surface waters of Qingshitan Reservoir were at a relatively high level compared to those of the other studied regions, while the contamination of DDTs and methoxychlor were at a medium level. The regional difference in the amount of OCPs might be attributed to the industrial production and agricultural application in areas where OCPs is not inhibited. The Qingshitan Reservoir and Indus river are located in agriculturally developed areas, and the Haihe river located in highly industrialized areas, which have high intensities of human activity. With the properties of anti-degradation, the OCPs residual levels in the surface water in these regions are relatively high.
3.2 Risk assessment for pesticide mixtures
The RQ of 15 OCPs was calculated based on the concentration detected in Qingshitan Reservoir, in which a single pesticide scenario was assumed (Table 5). The values of RQs in the surface water of reservoirs, ponds, and streams were less than 1 for all OCPs, indicating the absence of risk (or at a very low level) for the aquatic environment resulted from pesticide at the single pesticide scenario.| Pesticide compounds | Reservoirs | Ponds | Streams |
|---|---|---|---|
| a RQ: risk quotient. | |||
| RQβ-HCH | 0.0031 | 0.0060 | 0.0028 |
| RQα-HCH | 0.0046 | 0.0108 | 0.0046 |
| RQγ-HCH | 0.3878 | 0.9842 | 0.3789 |
| RQδ-HCH | 0.0005 | 0.0012 | 0.0007 |
| RQHeptachlor | 0.1669 | 0.2707 | 0.1767 |
| RQAldrin | 0.0383 | 0.2423 | 0.1596 |
| RQHeptachlor Epoxide | 0.0068 | 0.0078 | 0.0066 |
| RQChlordane | 0.0018 | 0.0027 | 0.0029 |
| RQα-endosulfan | 0.0713 | 0.1020 | 0.0743 |
| RQp,p′-DDE | 0.1316 | 0.1922 | 0.1306 |
| RQEndrin | 0.0305 | 0.0331 | 0.0342 |
| RQβ-endosulfan | 0.0574 | 0.0805 | 0.0854 |
| RQp,p′-DDD | 0.0796 | 0.1658 | 0.1164 |
| RQp,p′-DDT | 0.0618 | 0.1557 | 0.0834 |
| RQmethoxychlor | 0.2991 | 0.3035 | 0.2879 |
However, the real environment is always exposed to pesticide mixtures. To evaluate the ecological risk of OCP mixtures, the CA model was used to predict the risk quotient of mixtures (RQMEC/PNEC) that consist of 15 OCPs detected in the real environment based on the acute toxicity data of a single substance to algae, Daphnia magna, and fish. The risk quotient of mixtures was calculated from the MEC and PNEC of a single compound, where methoxychlor has a minimum PNEC value of 0.0078 μg L−1 and a relatively low PNEC value for p,p′-DDE (0.01 μg L−1), α-endosulfan (0.02 μg L−1), β-endosulfan (0.028 μg L−1), and γ-HCH (0.029 μg L−1) (Table 3). The frequency of exceedance of RQMEC/PNEC (>1) for the OCPs detected in the waters of the reservoirs, ponds, and streams are listed in Table 6, which shows that the frequency of RQMEC/PNEC > 1 ranges from 69.01% (reservoir) to 85.71% (pond). The samples with RQMEC/PNEC > 1 indicate a potential risk and warrants concern. RQSTU was calculated in a next step. This result is consistent with the residual level of OCPs at reservoirs, ponds, and streams. Using the measured concentrations of each sample, we calculated the frequency of samples with RQSTU > 1 for the reservoirs, ponds, and streams. The results showed that the RQSTU of the 128 samples exceeded 1 and accounted for 45.23% of the total sample.
| Water area | No. of samples | Freq. RQMEC/PNECa (%) |
|---|---|---|
| a Frequency of exceedance of RQMEC/PNEC = n/N, where n is the number of samples with RQMEC/PNEC ratios above 1, and N is the total number of samples with analytical measurements for the pesticide compounds. | ||
| Reservoirs | 213 | 69.01 |
| Ponds | 35 | 85.71 |
| Streams | 35 | 80.00 |
Table 7 lists the statistical data for RQSTU and the sum of the toxic units (STU). It shows that the surface water samples with RQSTU above 1 account for up to 62.44% (mean value of reservoirs, ponds, and streams) of the surface water samples with RQMEC/PNEC above 1, indicating the RQMEC/PNEC was slightly overestimated for the pesticide mixture in real environment. A previous research proved that the ratio between RQMEC/PNEC and RQSTU was smaller than the number of considered trophic levels (three levels in this study).25 The maximum ratio between RQMEC/PNEC and RQSTU reached 1.91, which similar to the result (a ratio < 1.3) observed in seven European sewage treatment plant (STP) effluents.50 This is because the toxic components that dominate the mixtures have similar ecotoxicological profiles.51 Therefore, the RQSTU above 1 for the OCP mixtures in 45.23% of the 283 surface water samples indicates a high potential risk to the aquatic environment.
| Water area | No. of samples | Freq. RQSTUa (%) | Freq. maxb (%) | ||
|---|---|---|---|---|---|
| STUalgae | STUDaphnia | STUfish | |||
| a Frequency of exceedance of RQSTU = n/N, where n is the number of samples with RQSTU ratios above 1, and N is the total number of samples with RQMEC/PNEC ratios above 1.b Frequency of maxSTU = n/N, where n is the number of samples with maxSTU for algae, Daphnia and fish, and N is the total number of samples with RQMEC/PNEC ratios above 1. | |||||
| Reservoirs | 147 | 53.74 | 0.00 | 48.10 | 51.90 |
| Ponds | 30 | 100.00 | 0.00 | 10.00 | 90.00 |
| Streams | 28 | 67.86 | 0.00 | 36.84 | 63.16 |
Table 7 shows that the proportion of STUfish in the sum of the maximum toxic unit of the sample with RQPEC/PNEC above 1 is 51.90–90.00%, while STUDaphnia accounts for 10.00–48.10% and STUalgae accounts for 0%. In the three trophic levels, fish is more likely to obtain the maximum sum of toxic unit (maxSTU). Table 8 shows that in the 128 samples with RQSTU above 1, fish and Daphnia accounted for 62.50% and 37.50% of maximum toxic unit (mTU), respectively. Compared with Daphnia and algae, fish is more susceptible to OCPs. Overall, the data indicate that fish has the highest sensitivity to OCPs at three trophic levels, followed by Daphnia and algae.
| Regions | Organism | Methoxychlor | γ-HCH |
|---|---|---|---|
| Reservoirs no. mTU | Algae | 0 | 0 |
| Daphnia | 38 | 0 | |
| Fish | 0 | 41 | |
| Ponds no. mTU | Algae | 0 | 0 |
| Daphnia | 3 | 0 | |
| Fish | 0 | 27 | |
| Streams no. mTU | Algae | 0 | 0 |
| Daphnia | 7 | 0 | |
| Fish | 0 | 12 |
Maximum cumulative ratio (MCR, eqn (3)) is defined as the ratio of the toxicity received by an individual from exposures to multiple chemicals to the largest toxicity received by the individual from any one chemical (maximum toxicity),52 which could be used to measure whether individuals' cumulative exposures are dominated by a single chemical or are the result of the contribution of many chemicals.
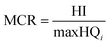 | (3) |
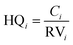 | (4) |
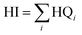 | (5) |
Fig. 2 presents the scatter plots of MCR versus RQSTU for Daphnia and fish for the pesticide compounds detected in the surface water of the reservoirs, ponds, and streams. Fig. 2 shows that the number of samples with RQSTU above 1 and MCR values smaller than 2 in the reservoirs, ponds and streams of Qingshitan Reservoir accounts for 91.14%, 83.33%, and 52.63% (mean: 82.30%) of the samples with RQSTU greater than 1, respectively, indicating that one OCP compound provides at least 50% of the whole mixture toxicity in most samples with RQSTU above 1. In other words, a small number of pesticide compounds dominate the total toxicity. For the 23 samples with RQSTU > 1 and MCR > 2, 9 samples were from the reservoirs, 5 from the ponds, and 9 from the streams. The MCR values had an average of 2.432 and ranged from 2.073 to 3.396 in the 23 samples, which indicate that the fraction of toxicity from the most toxic pesticide compound averages 41% and ranges from 29% to 49%. However, 51% to 71% (mean: 59%) of the pesticide mixture toxicity in 23 surface water samples resulted from the other 14 OCPs. Therefore, the real water environment is always contaminated by multiple compounds, and considering a single OCP will underestimate the risk to aquatic organism (the toxic will underestimate about 59% for 23 samples with RQSTU > 1 and MCR > 2, even though the most toxic OCP was considered).
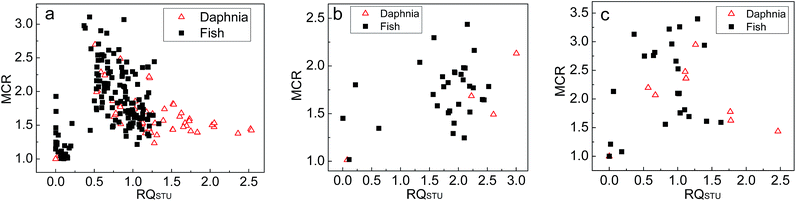 | ||
| Fig. 2 Scatter plots of MCR versus RQSTU for Daphnia and fish for the pesticide compounds detected in waters of the reservoirs (a), ponds (b), and streams (c). | ||
The joint MCR values had an average of 1.519 for the two pesticides with the highest TU values and ranged from 1.237 to 1.787 in the 23 samples with RQSTU > 1 and MCR > 2 (Table 9). The results indicate that the two pesticides with the highest toxicities from the 23 samples contribute 66% (ranged from 56% to 81%) of the entire mixture toxicity. Obviously, the mixture toxicity of the OCPs in the water body of Qingshitan Reservoir agricultural wetland to aquatic animal mainly results from a few components, but considering only one or two OCPs will cause risk underestimations.
| Pesticide | No. algae | No. crustaceans | No. fisha | Joint MCRb |
|---|---|---|---|---|
| a No. fish is the numbers of samples with organic chlorine pesticides contributed to STUfish at maximum exception of methoxychlor and γ-HCH.b Joint MCR is the combined MCR with the most maximum two compounds contribute to STU. | ||||
| Heptachlor | 0 | 0 | 7 | 1.337–1.688 |
| Aldrin | 0 | 0 | 5 | 1.490–1.767 |
| α-Endosulfan | 0 | 0 | 1 | 1.293 |
| p,p′-DDE | 0 | 4 | 0 | 1.237–1.441 |
| Endrin | 0 | 1 | 1 | 1.570–1.773 |
| β-Endosulfan | 0 | 0 | 3 | 1.581–1.787 |
| p,p′-DDT | 0 | 1 | 0 | 1.600 |
Table 8 presents the details of the organic chlorine pesticides in the samples with RQSTU larger than 1 for algae, Daphnia, and fish that contributed to mTU in the surface water of the reservoirs, ponds, and streams in Qingshitan Reservoir. Table 8 indicates that two OCPs, methoxychlor and γ-HCH, account for the highest risk of toxicity in 128 samples with RQSTU above 1 in the reservoirs, ponds, and streams of the Qingshitan Reservoir agriculture wetland. Methoxychlor contributes the highest risk to Daphnia magna and γ-HCH accounts for the highest risk to fish. The methoxychlor and γ-HCH compounds are associated with the previous mention that one OCP compound provides at least 50% of the whole mixture toxicity in the samples with RQSTU > 1. This is because of the highest acute toxicity of methoxychlor toward Daphnia magna and γ-HCH to fish, and their high detection rate and concentrations in the real environment. In addition, p,p′-DDE, endrin, and p,p′-DDT pose a certain risk to Daphnia magna. Heptachlor, aldrin, α-endosulfan, β-endosulfan, and endrin have a certain risk to fish (Table 9). Though β-HCH was the predominant pesticide in the surface water of Qingshitan Reservoir, the higher values of EC50 (LC50) eventually resulted in lower environment risk in the surface water.
Overall, γ-HCH and methoxychlor are the two predominant risk providers to the OCP mixture in the real aquatic environment of Qingshitan Reservoir, and risk underestimation occurs when the possible mixture effects with other OCPs is ignored. Under certain circumstances, considering a single substance will cause greater undervaluation of real pesticide mixtures. However, toxic pesticides that are more polar, thermally unstable, and volatile were not considered in the scope of this study, which may lead to the underestimation of the overall water toxicity.
4 Conclusions
The result of the mixture risk assessment based on the RQSTU showed that OCPs residues at the monitored sites pose threaten to aquatic ecosystems. The risk posed by OCPs mixtures were presented in 128 samples out of 283 analyzed samples. The risk of the samples was dominated by only one OCP (methoxychlor or γ-HCH), accounts for at least 50% of the whole mixture toxicity. With respect to the 15 OCPs detected in Qingshitan, methoxychlor showed the highest toxicity to Daphnia magna and γ-HCH presented the highest toxicity to fish. p,p′-DDE, endrin, and p,p′-DDT pose a certain risk toward Daphnia magna, while chlordane, aldrin, α-endosulfan, endrin, and β-endosulfan pose certain risks to fish but much less than methoxychlor and γ-HCH. Fish is the most sensitive to OCPs, and algae is the least sensitive. In summary, the mixtures of 15 OCPs pose a potential risk to the ecological system. Therefore, a better control of the OCPs in Qingshitan Reservoir in Southwest China should be considered to improve the water quality.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to all members of the laboratory for helpful discussions during the course of these studies. This work was supported by grants from the National Natural Science Foundation of China (No. 51578171, 21407032, and 21667013), Special Funding for Guangxi ‘BaGui Scholar’ Construction Projects, Science Research and Technology Development Project of Guangxi (Guikehe1599005-2-2), and Science Research and Technology Development Project of Guilin (2016012505).References
- M. H. Wong, A. O. W. Leung, J. K. Y. Chan and M. P. K. Choi, Chemosphere, 2005, 60, 740–752 CrossRef PubMed.
- J. Liu, S. H. Qi, J. Yao, D. Yang, X. L. Xing, H. X. Liu and C. K. Qu, Chemosphere, 2016, 163, 35–43 CrossRef PubMed.
- E. J. Mrema, F. M. Rubino, G. Brambilla, A. Moretto, A. M. Tsatsakis and C. Colosio, Toxicology, 2013, 307, 74–88 CrossRef PubMed.
- N. L. Devi, I. C. Yadav, P. Raha, S. H. Qi and Y. Dan, Environ. Sci. Pollut. Res., 2015, 22, 20154–20166 CrossRef PubMed.
- H. Karadeniz and S. Yenisoy-Karakas, Environ. Monit. Assess., 2015, 187, 94 CrossRef PubMed.
- A. Mehmood, A. Mahmood, S. A. M. A. S. Eqani, M. Ishtiaq, A. Ashraf, N. Bibi, A. Qadir, J. Li and G. Zhang, Hum. Ecol. Risk Assess., 2017, 23, 1–13 CrossRef.
- R. N. Li, Q. W. Chen, D. Tonina and D. S. Cai, Ecol. Eng., 2015, 76, 75–83 CrossRef.
- L. Chen, J. P. Qian, L. Zhang, Z. Z. Zhu and Z. Lin, Guangdong Agric. Sci., 2013, 40, 160–164 Search PubMed.
- Y. P. Liang, G. N. Chen, H. H. Zeng, L. T. Qin and L. Y. Mo, Toxicol. Environ. Chem., 2016, 98, 658–668 CrossRef.
- A. S. Muhayimana, S. H. Qi, Y. H. Wang, X. S. Kong, J. O. Odhiambo and J. P. Zhang, J. Am. Sci., 2009, 5, 35–43 Search PubMed.
- Y. H. Wang, S. J. Guo, Y. Y. Xu, W. S. Wang, S. H. Qi, X. L. Xing and D. X. Yuan, Environ. Geochem. Health, 2012, 34, 493–502 CrossRef PubMed.
- D. Zhang, Y. H. Wang, K. F. Yu, P. Y. Li, R. J. Zhang and Y. Y. Xu, Bull. Environ. Contam. Toxicol., 2014, 93, 580–585 CrossRef PubMed.
- Y. Song, Y. P. Liang, H. H. Zeng, L. T. Qin and L. Y. Mo, Industrial Safety and Environmental Protection, 2016, 40, 68–71 Search PubMed.
- G. H. Dai, X. H. Liu, G. Liang and W. W. Gong, Environ. Sci. Pollut. Res. Int., 2014, 21, 4516–4526 CrossRef PubMed.
- T. Backhaus, Philos. Trans. R. Soc. London, Ser. B, 2014, 369, 1656 CrossRef PubMed.
- S. S. Liu, J. Zhang, Y. H. Zhang and L. T. Qin, Acta Chim. Sin., 2012, 70, 1511–1517 CrossRef.
- L. T. Qin, J. Wu, L. Y. Mo, H. H. Zeng and Y. P. Liang, Environ. Sci. Pollut. Res., 2015, 22, 12759–12768 CrossRef PubMed.
- R. Verro, A. Finizio, S. Otto and M. Vighi, Environ. Sci. Technol., 2009, 43, 530–537 CrossRef PubMed.
- A. Kienzler, S. K. Bopp, S. van der Linden, E. Berggren and A. Worth, Regul. Toxicol. Pharmacol., 2016, 80, 321–334 CrossRef PubMed.
- T. Backhaus, AAPS J., 2016, 18, 804–813 CrossRef PubMed.
- M. Breitholtz, J. R. Nyholm, J. Karlsson and P. L. Andersson, Chemosphere, 2008, 72, 1242–1249 CrossRef PubMed.
- H. Walter, F. Consolaro, P. Gramatica, M. Scholze and R. Altenburger, Ecotoxicology, 2002, 11, 299–310 CrossRef PubMed.
- K. R. Solomon, T. C. M. Brock, D. D. Zwart, S. D. Dyer, L. Posthuma, S. M. Richards, H. Sanderson, P. K. Sibley and P. J. van den Brink, J. Healthc. Eng., 2008, 3, 649–661 Search PubMed.
- E. Silva and M. J. Cerejeira, Environ. Sci. Pollut. Res. Int., 2015, 22, 6756–6765 CrossRef PubMed.
- T. Backhaus and M. Faust, Environ. Sci. Technol., 2012, 46, 2564–2573 CrossRef PubMed.
- Pesticide Properties Database (PPDB), http://sitem.herts.ac.uk/aeru/iupac/index.htm, Accessed 02 Sep 2016.
- T. Bednarz, Acta Hydrobiol., 1981, 23, 155–172 Search PubMed.
- E. C. Oliveira-Filho and F. J. R. Paumgartten, Bull. Environ. Contam. Toxicol., 1997, 59, 984–988 CrossRef PubMed.
- M. T. Wan, J. N. Kuo, C. Buday, G. Schroeder, G. Van Aggelen and J. Pasternak, Environ. Toxicol. Chem., 2005, 24, 1146–1154 CrossRef PubMed.
- P. Lepper, Manual on the Methodological Framework to Derive Environmental Quality Standards for Priority Substances in accordance with Article 16 of the Water Framework Directive (2000/60/EC), Fraunhofer-Institute Molecular Biology and Applied Ecology, Schmallenberg, Germany, 2005 Search PubMed.
- M. H. Nie, C. X. Yan, W. B. Dong, M. Liu, J. L. Zhou and Y. Yang, Chemosphere, 2015, 127, 109–116 CrossRef PubMed.
- L. L. Yao, Y. X. Wang, L. Tong, Y. M. Deng, Y. G. Li, Y. Q. Gan, W. Guo, C. J. Dong, Y. H. Duan and K. Zhao, Ecotoxicol. Environ. Saf., 2017, 135, 236–242 CrossRef PubMed.
- M. D. Hernando, M. Mezcua, A. R. Fernandez-Alba and D. Barcelo, Talanta, 2006, 69, 334–342 CrossRef PubMed.
- L. Shen and F. Wania, J. Chem. Eng. Data, 2005, 50, 742–768 CrossRef.
- G. Z. Zhang, Z. K. Pan, A. Y. Bai, J. Li and X. M. Li, Environ. Monit. Assess., 2014, 186, 2039–2051 CrossRef PubMed.
- L. L. Liu, J. Li, M. R. Li, S. J. Ren, Y. F. Wang and Y. D. Tang, J. Beijing Norm. Univ., Nat. Sci., 2014, 50, 657–661 Search PubMed.
- S. Jiang, B. H. Sun, B. Xu, H. L. Wu and J. H. Li, Environ. Chem., 2016, 35, 1228–1236 Search PubMed.
- Y. Hu, L. X. Yuan, S. H. Qi, H. X. Liu and X. L. Xing, Environ. Sci. Pollut. Res. Int., 2014, 21, 9376–9384 CrossRef PubMed.
- T. Wang, Z. L. Zhang, J. Huang, H. Y. Hu, G. Yu and F. S. Li, Environmental Science, 2007, 28, 730–735 Search PubMed.
- U. Ali, A. Bajwa, M. J. Iqbal Chaudhry, A. Mahmood, J. H. Syed, J. Li, G. Zhang, K. C. Jones and R. N. Malik, Ecotoxicol. Environ. Saf., 2016, 126, 177–185 CrossRef PubMed.
- A. Mahmood, R. N. Malik, J. Li and G. Zhang, Ecotoxicology, 2014, 23, 1713–1721 CrossRef PubMed.
- A. Malik, P. Ojha and K. P. Singh, Environ. Monit. Assess., 2009, 148, 421–435 CrossRef PubMed.
- P. Montuori, S. Aurino, F. Garzonio and M. Triassi, Sci. Total Environ., 2016, 571, 1001–1016 CrossRef PubMed.
- P. Motuori, T. Cirillo, E. Fasano, A. Nardone, F. Esposito and M. Triassi, Environ. Sci. Pollut. Res. Int., 2014, 21, 5023–5035 CrossRef PubMed.
- M. A. Fernández, C. Alonso, M. J. González and L. M. Hernández, Chemosphere, 1999, 38, 33–43 CrossRef.
- S. K. Golfinopoulos, A. D. Nikolaou, M. N. Kostopoulou, N. K. Xilourgidis, M. C. Vagi and D. T. Lekkas, Chemosphere, 2003, 50, 507–516 CrossRef PubMed.
- N. Eremina, A. Paschke, E. A. Mazlova and G. Schüürmann, Environ. Pollut., 2016, 210, 409–418 CrossRef PubMed.
- Z. Sharip, N. Hashim and S. Suratman, Environ. Monit. Assess., 2017, 189, 560 CrossRef PubMed.
- J. A. Placencia and S. Contreras, Mar. Pollut. Bull., 2018, 126, 389–395 CrossRef PubMed.
- T. Backhaus and M. Karlsson, Water Res., 2014, 49, 157–165 CrossRef PubMed.
- J. C. Liu, G. H. Lu, Z. X. Xie, Z. H. Zhang, S. Li and Z. H. Yan, Sci. Total Environ., 2015, 511, 54–62 CrossRef PubMed.
- P. S. Price and X. L. Han, Int. J. Environ. Res. Public Health, 2011, 8, 2212–2225 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |