Open Access Article
Open Access ArticleImmediate separation of microneedle tips from base array during skin insertion for instantaneous drug delivery
Hyesun Juna,
Myun-Hwan Ahna,
In-Jeong Choia,
Seung-Ki Baeka,
Jung-Hwan Park*b and
Seong-O Choi *c
*c
aQuadMedicine R&D Centre, QuadMedicine Co., Ltd, Seongnam, Republic of Korea
bDepartment of BioNano Technology, Gachon BioNano Research Institute, Gachon University, Seongnam, Republic of Korea. E-mail: pa90201@gachon.ac.kr
cDepartment of Anatomy and Physiology, Nanotechnology Innovation Center of Kansas State, Kansas State University, Manhattan, KS, USA. E-mail: sochoi@ksu.edu
First published on 15th May 2018
Abstract
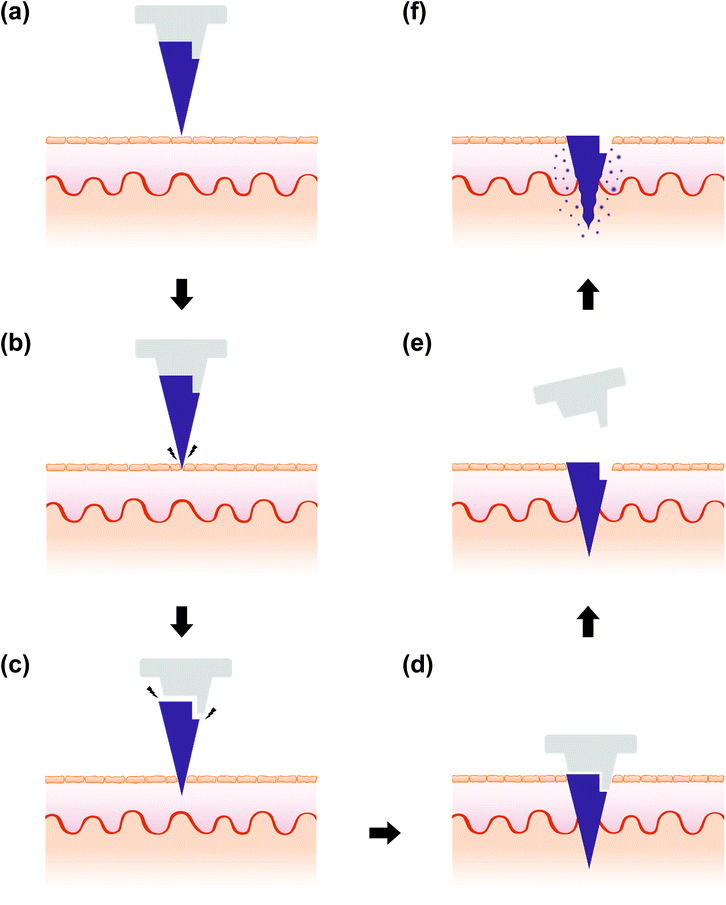
We have developed an insertion-responsive microneedle (IRMN) system that enables prompt drug delivery through the skin without attaching a skin patch. This system consists of square pyramidal hyaluronic acid (HA) microneedle tips and polycaprolactone (PCL) base arrays. During skin insertion, HA tips can be immediately separated from PCL base arrays due to the relatively weak adhesion strength between HA and PCL. Two base designs using truncated square pyramid stands, one without a wall (no-walled stand, NWS) and another with a wall on one side of the stand (single-walled stand, SWS), were prepared to study the effect of base geometry on the mechanical behavior of IRMNs. Ex vivo skin insertion tests showed successful separation of the tips from the base array upon insertion, regardless of the presence of a wall on the stand. However, only IRMNs-SWS were deeply embedded within the skin. Mechanical testing results demonstrated that the presence of a wall on the base enhanced the mechanical stability of the IRMNs. The wall also provided adequate adhesion between the tips and base, preventing the tips from breaking during insertion, while allowing the needle tip to separate upon removal. Histological examination confirmed that the tips were successfully separated from the base, embedded in the skin, and released fluorescent dyes within the skin. Our results suggest that the IRMN system is promising for the rapid and accurate delivery of various molecules through the skin, while improving user convenience by eliminating the need to attach microneedles to the skin.
1. Introduction
Microneedle (MN) systems provide minimally invasive approaches to transdermal drug delivery and have been widely studied over the last two decades. From the original solid MNs, which served as a pretreatment before topical drug application,1–5 drug-coated MNs have been developed to transport therapeutic agents into the skin during MN application.6–11 However, achieving precise and uniform drug coatings on three-dimensional microstructures is technically demanding, and the amount of drug that can be coated on MNs is limited due to their small surface area. Furthermore, drug-coated solid MNs leave biohazardous sharp waste after use, which could raise concerns about the reuse and safe disposal of MNs. The shortcomings of coated MNs have been addressed by constructing them from biocompatible polymers. For example, dissolving MNs, which are made of water-soluble polymers, have been shown to significantly improve drug loading capacity by incorporating drugs within the microneedle matrix and eliminate the risk of sharp waste, because the MNs dissolve away upon contact with bodily fluids in the skin.12–14 Although dissolving MNs have been successfully used to deliver various molecules,15–21 they still require firm and secure attachment to the skin with additional patches for a certain period of time to release the substances encapsulated in MNs, which could cause user inconvenience and skin irritation.22To overcome the aforementioned limitations, tip-separable MNs have been proposed,23,24 which have drug-containing microneedle tips mounted on supporting shafts. Once MNs are inserted in the skin, the “arrowhead” tips facilitate mechanical interlocking with the tissue, leading to the tips becoming embedded in the skin. These MNs showed reliable and deep skin penetration and improved drug delivery efficiency with relatively short application times in comparison with conventional MNs. For example, Chen et al.25 have demonstrated separable MNs composed of chitosan tips and polylactic acid (PLA) shafts. Upon insertion, the needle tips were successfully separated from the shaft array and embedded in the skin for sustained release of antigens encapsulated in the tips, demonstrating the possibility of inducing improved immune responses.
In this study, we present a new separable dissolving MN system, named insertion-responsive microneedles (IRMNs), which utilize the adhesion strength between the microneedle tips and bases as a separation mechanism. The IRMN concept is illustrated in Fig. 1. This study aimed to develop a MN system that exhibits secure insertion of drug-loaded dissolving tips into the skin, while allowing immediate tip separation within 1 s of needle insertion. This new system should have: (i) sufficient mechanical strength to penetrate the stratum corneum without losing structural integrity; (ii) immediate tip-base separation triggered by skin insertion only; and (iii) mechanical stability to prevent inappropriate tip separation during storage or transport. To achieve these features, we designed a microneedle base geometry to control adhesion between the tip and the base and investigated the separation principle during skin insertion by analyzing the mechanical characteristics of the IRMNs. The skin penetration and drug delivery capability of the IRMNs were also demonstrated using pig cadaver skin.
2. Materials and methods
2.1 Materials
Hyaluronic acid (HA, average Mw 832![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from SK Bioland (Cheonan, South Korea) and polydimethylsiloxane (PDMS, Sylgard 184) was purchased from Dow Corning (Midland, MI, USA). Polycaprolactone (PCL; average Mn, 80
000) was purchased from SK Bioland (Cheonan, South Korea) and polydimethylsiloxane (PDMS, Sylgard 184) was purchased from Dow Corning (Midland, MI, USA). Polycaprolactone (PCL; average Mn, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), Tween 80, gentian violet, and fluorescein sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA).
000), Tween 80, gentian violet, and fluorescein sodium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA).
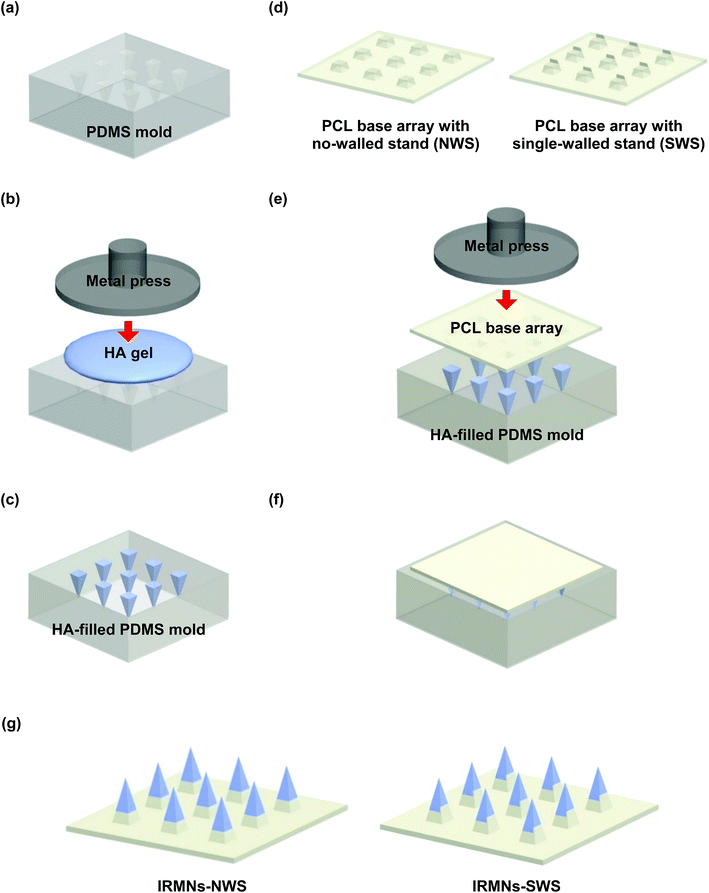
2.2 Fabrication of IRMNs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) was prepared and poured over the master structures. PDMS was cured at room temperature overnight, and the inverse-replica PDMS molds were separated from the master structures.
1 w/w) was prepared and poured over the master structures. PDMS was cured at room temperature overnight, and the inverse-replica PDMS molds were separated from the master structures.2.3 Mechanical characterization of IRMNs
2.4 Ex vivo porcine skin insertion test
To examine the penetration and separation capability of the IRMNs, skin insertion tests were performed on ex vivo pig skin. Full-thickness porcine cadaver skin (CRONEX Co., Ltd., Hwaseong, South Korea) was cut into a 2 × 2 cm patch, and the skin patch was stretched out and securely fixed on a flat surface. IRMN-NWS and IRMN-SWS were mounted on a top probe of the materials testing machine, displaced toward the porcine skin at a rate of 3 mm min−1 to a maximum force of 40 N, held in position for 10 s, and returned to their original position. The success rate of IRMN insertion and separation was examined by observing the skin penetration sites under an optical microscope.Skin penetration and drug delivery characteristics were further studied by histological examination. For clear visualization, fluorescein sodium salt was used as a model drug and incorporated into the HA tips of IRMNs (0.1% w/v). The porcine skin insertion test was performed as described above. The microneedle-treated skin region was then excised and embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, CA, USA). The skin sample was frozen in liquid nitrogen and sliced into 10 μm-thick sections using a cryostat microtome (Microm HM 550P, Thermo Scientific, Rockford, IL, USA) and placed on glass slides. The samples were imaged using an inverted fluorescence microscope (Nikon Eclipse 80i, Tokyo, Japan).
3. Results and discussion
3.1 Geometry of insertion-responsive microneedles
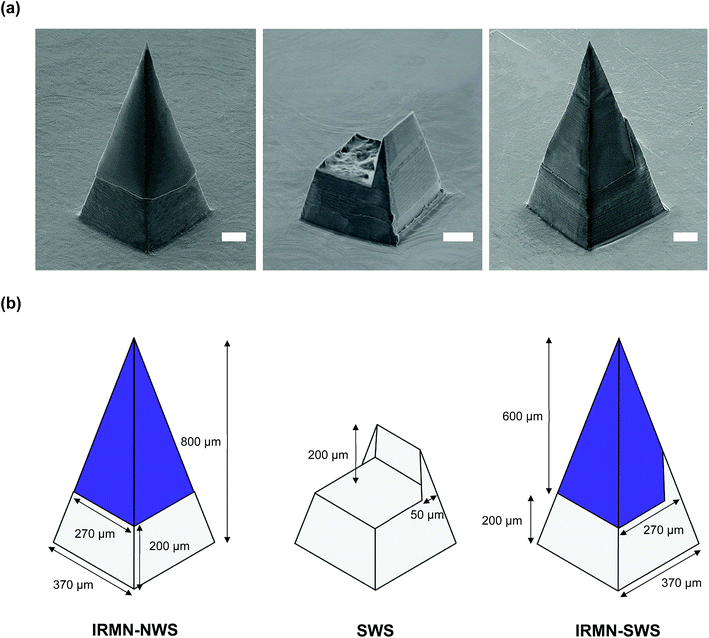
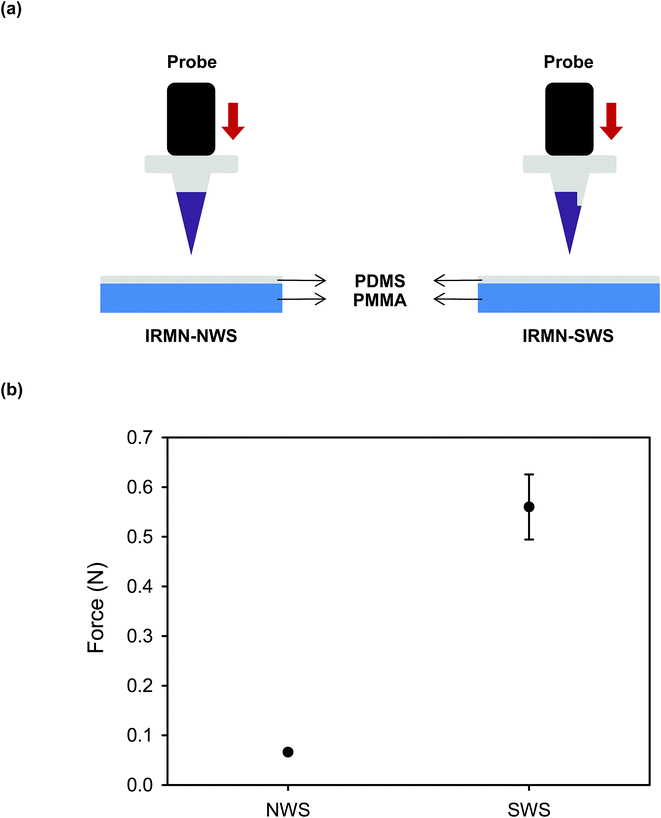
Polycaprolactone (PCL) base arrays with different geometries were prepared as shown in Fig. 3. One base was a truncated square pyramid without a wall (no-walled stand, NWS), while the other had a wall on one side of a truncated square pyramid (single-walled stand, SWS). The base dimensions were as follows: top width, 270 μm; bottom width, 370 μm; and height, 200 μm. For the SWS, the wall thickness and height were 200 μm and 50 μm, respectively. Pyramidal microneedles tips were constructed of hyaluronic acid (HA), a component widely distributed in body fluids and tissues, and had a base width of 270 μm and height of 600 μm. They were successfully integrated with the base array. The IRMN array comprised 57 microneedles in an area of 0.75 cm2.3.2 Mechanical properties of insertion-responsive microneedles
3.3 Ex vivo porcine skin insertion test
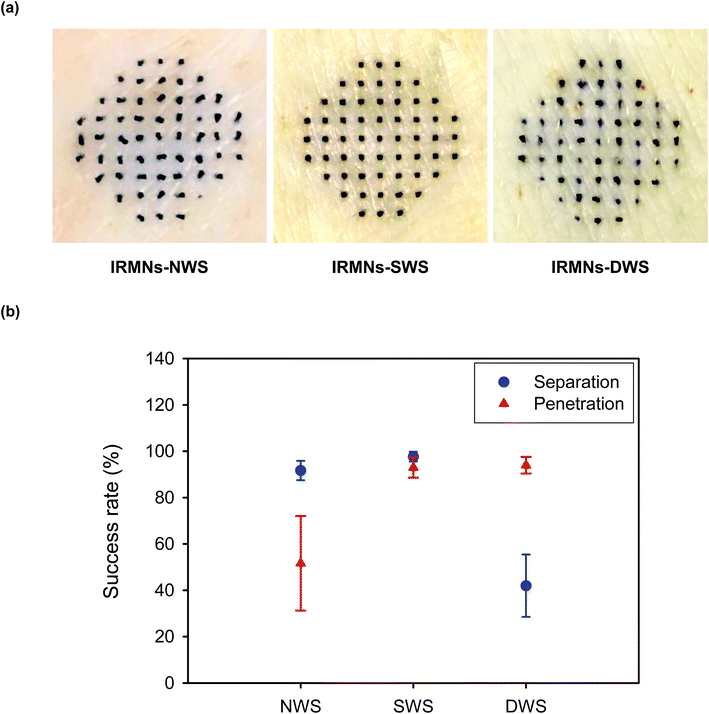
To evaluate the separation and penetration capabilities of IRMNs, the porcine skin insertion test was performed using IRMNs-NWS and IRMNs-SWS. During skin insertion, both types of IRMNs were successfully separated from the base array, regardless of the stand geometry. After insertion and removal of the IRMNs, the HA tips of IRMNs-SWS were deeply embedded into the porcine skin (Fig. 7(a) – middle). However, in the case of IRMNs-NWS, most HA tips were not fully inserted into the skin and a high proportion of the tips were lying on the skin surface (Fig. 7(a) – left), indicating that they were not able to penetrate the stratum corneum layer. During IRMN insertion into the skin, external forces in the vertical and horizontal directions were exerted on the microneedle tips, eventually leading to separation of the tips from the base array. To test the potential of IRMNs with a multi-walled stand, we also prepared IRMNs with a double-walled stand (DWS) and repeated the porcine skin insertion test. As shown in Fig. 7(a) – right, stained spots were observed after insertion of IRMNs-DWS into the skin, indicating that IRMNs-DWS successfully penetrated the skin. However, only 42% of the microneedle tips were embedded in the skin, with the remaining tips not separated from the base array after removal. This suggested that the DWS provided too-strong adhesion, which decreased the tip separation efficiency.3.4 Delivery of fluorescent molecules into skin using IRMNs
Drug delivery characteristics were analyzed after inserting IRMNs-SWS containing a model drug, fluorescein sodium salt, within a HA matrix into the porcine skin. The separated tips remained in the skin and the fluorescent molecule was distributed up to 350 μm below the skin layer, as shown in Fig. 8. This result demonstrated that this new IRMN system could deliver a drug encapsulated within the microneedle tips into the skin layer successfully and immediately without applying a microneedle patch on the skin for a certain amount of time.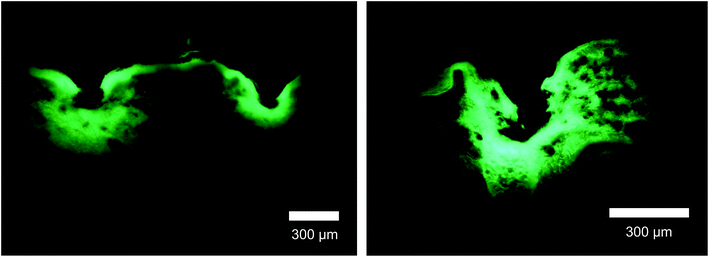 | ||
| Fig. 8 Cross-section views of the insertion sites of porcine skin applied with fluorescein-loaded IRMNs-SWS, as imaged by a fluorescence microscope. Scale bars represent 300 μm. | ||
4. Conclusion
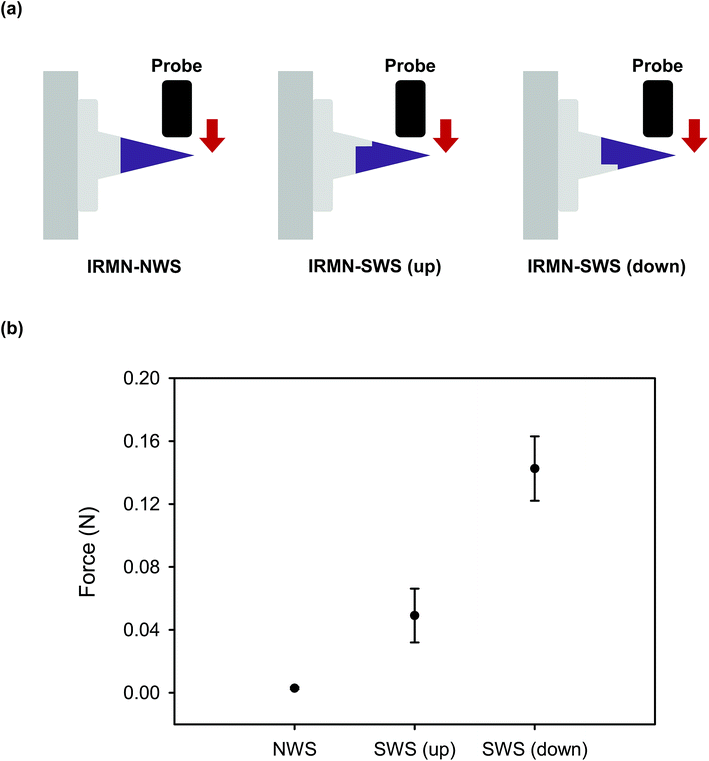
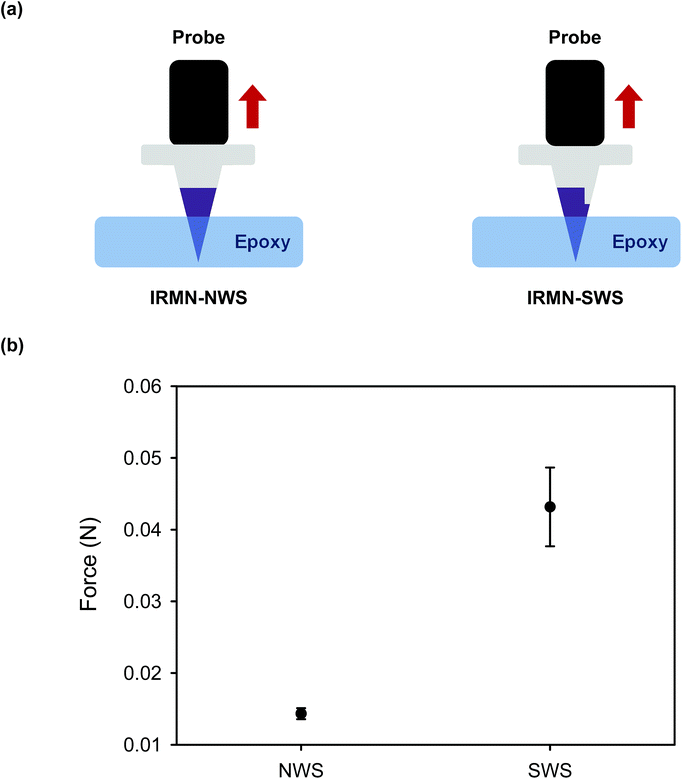
The IRMNs successfully penetrated the skin, and the HA tips were immediately separated from the PCL base by the external force that occurred during skin insertion. The separated HA tips remained embedded within the skin when the base was removed, and the fluorescent substance contained in the dissolving HA tips was delivered through the skin. For the IRMN with a single-walled stand (IRMN-SWS), the average separation force by lateral loads was 0.08 N, which was 27 times greater than that of IRMN with a no-walled stand (IRMN-NWS). This result indicated that, compared with IRMN-NWS, IRMN-SWS showed a markedly better mechanical stability to prevent undesired tip separation that could occur as a result of external stress during transport or storage. Under axial compression, the IRMN-SWS (0.56 N) showed 9.3 times greater mechanical resistance than the IRMN-NWS (0.06 N). Although IRMN-NWS had a low skin penetration rate, the tips of IRMN-SWS were successfully inserted into the skin. This result suggested that the additional wall structure was necessary to ensure consistent tip insertion while allowing successful tip separation using only the force generated during skin insertion. The axial pull test showed that both types of IRMN successfully induced separation of the tips with very little force (less than 0.05 N). Altogether, our results indicate that the tips were separated from the base during the insertion process and remained embedded within the skin when the base was removed by pulling.For the successful transition of this technology into practical applications, the concept of IRMNs should be further validated through in vivo experiments. As IRMNs could provide rapid vaccine delivery, animal vaccination studies will be performed to evaluate the feasibility and effectiveness of this microneedle system. From a manufacturing viewpoint, a suitable sterilization method, such as ethylene oxide gas and gamma irradiation processes, should be incorporated into the fabrication process, and the influence of sterilization on the device performance should be tested. Although IRMNs-SWS showed reliable skin insertion under well-controlled conditions, a countermeasure for secure needle insertion in practical situations should be considered. For example, the use of a properly designed applicator would minimize user-dependent variations in needle insertion. It would also be desirable to incorporate biocompatible dye into the tips of IRMNs, enabling the users to visually check the insertion sites. Furthermore, a strategy for safe distribution and disposal of the device needs to be considered. Firstly, the storage stability should be evaluated in terms of temperature, humidity, and period, and proper packaging should be determined. Packaging should also be designed to prevent tip-separation during transportation by effectively absorbing the shock and vibration. Constructing the product from materials that can be safely disposed after use is highly desirable, and will be particularly important for use in resource-poor settings.
In conclusion, this new microneedle system could significantly reduce or eliminate the problems associated with applying microneedle skin patches, such as skin erythema and itching. Furthermore, the new system could greatly enhance user convenience by allowing easy and instantaneous drug delivery.
Conflicts of interest
Hyesun Jun, Myun-Hwan Ahn, In-Jeong Choi, and Seung-Ki Baek are employees of QuadMedicine, which holds patents related to this study. Jung-Hwan Park and Seong-O Choi declare no conflicts of interest.Acknowledgements
This work was supported by the TIPS Program (S2497134) funded by the Ministry of SMEs and Startups (MSS, South Korea).References
- S. Henry, D. V. McAllister, M. G. Allen and M. R. Prausnitz, J. Pharm. Sci., 1998, 87, 922–925 CrossRef PubMed.
- W. Martanto, S. P. Davis, N. R. Holiday, J. Wang, H. S. Gill and M. R. Prausnitz, Pharm. Res., 2004, 21, 947–952 CrossRef.
- J. A. Mikszta, J. B. Alarcon, J. M. Brittingham, D. E. Sutter, R. J. Pettis and N. G. Harvey, Nat. Med., 2002, 8, 415–419 CrossRef PubMed.
- B. Al-Qallaf and D. B. Das, Ann. N. Y. Acad. Sci., 2009, 1161, 83–94 CrossRef PubMed.
- C. Baek, M. Han, J. Min, M. R. Prausnitz, J. H. Park and J. H. Park, J. Controlled Release, 2011, 154, 138–147 CrossRef PubMed.
- H. S. Gill and M. R. Prausnitz, J. Controlled Release, 2007, 117, 227–237 CrossRef PubMed.
- H. S. Lee, H. R. Ryu, J. Y. Roh and J. H. Park, Pharm. Res., 2017, 34, 101–112 CrossRef PubMed.
- J. A. Matriano, M. Cormier, J. Johnson, W. A. Young, M. Buttery, K. Nyam and P. E. Daddona, Pharm. Res., 2002, 19, 63–70 CrossRef.
- G. J. Fernando, X. Chen, T. W. Prow, M. L. Crichton, E. J. Fairmaid, M. S. Roberts, I. H. Frazer, L. E. Brown and M. A. Kendall, PLoS One, 2010, 5, e10266 Search PubMed.
- Y. H. Mohammed, M. Yamada, L. L. Lin, J. E. Grice, M. S. Roberts, A. P. Raphael, H. A. Benson and T. W. Prow, PLoS One, 2014, 9, e101956 Search PubMed.
- M. Pearton, V. Saller, S. A. Coulman, C. Gateley, A. V. Anstey, V. Zarnitsyn and J. C. Birchall, J. Controlled Release, 2012, 160, 561–569 CrossRef PubMed.
- J. W. Lee, J. H. Park and M. R. Prausnitz, Biomaterials, 2008, 29, 2113–2124 CrossRef PubMed.
- J. H. Park, M. G. Allen and M. R. Prausnitz, J. Controlled Release, 2005, 104, 51–66 CrossRef PubMed.
- J. Y. Kim, M. R. Han, Y. H. Kim, S. W. Shin, S. Y. Nam and J. H. Park, Eur. J. Pharm. Biopharm., 2016, 105, 148–155 CrossRef PubMed.
- S. P. Sullivan, D. G. Koutsonanos, M. Del Pilar Martin, J. W. Lee, V. Zarnitsyn, S. O. Choi, N. Murthy, R. W. Compans, I. Skountzou and M. R. Prausnitz, Nat. Med., 2010, 16, 915–920 CrossRef PubMed.
- K. Matsuo, S. Hirobe, Y. Yokota, Y. Ayabe, M. Seto, Y. S. Quan, F. Kamiyama, T. Tougan, T. Horii, Y. Mukai, N. Okada and S. Nakagawa, J. Controlled Release, 2012, 160, 495–501 CrossRef PubMed.
- J. W. Lee, S. O. Choi, E. I. Felner and M. R. Prausnitz, Small, 2011, 7, 531–539 CrossRef PubMed.
- M. H. Ling and M. C. Chen, Acta Biomater., 2013, 9, 8952–8961 CrossRef PubMed.
- C. Dillon, H. Hughes, N. J. O'Reilly and P. McLoughlin, Int. J. Pharm., 2017, 526, 125–136 CrossRef PubMed.
- Y. Ito, J. Ohta, K. Imada, S. Akamatsu, N. Tsuchida, G. Inoue, N. Inoue and K. Takada, J. Drug Targeting, 2013, 21, 770–775 CrossRef PubMed.
- G. Cole, J. McCaffrey, A. A. Ali, J. W. McBride, C. M. McCrudden, E. M. Vincente-Perez, R. F. Donnelly and H. O. McCarthy, Hum. Vaccines Immunother., 2017, 13, 50–62 CrossRef PubMed.
- N. G. Rouphael, M. Paine, R. Mosley, S. Henry, D. V. McAllister, H. Kalluri, W. Pewin, P. M. Frew, T. Yu, N. J. Thornburg, S. Kabbani, L. Lai, E. V. Vassilieva, I. Skountzou, R. W. Compans, M. J. Mulligan, M. R. Prausnitz and T.-M. S. Group, Lancet, 2017, 390, 649–658 CrossRef.
- D. D. Zhu, Q. L. Wang, X. B. Liu and X. D. Guo, Acta Biomater., 2016, 41, 312–319 CrossRef PubMed.
- L. Y. Chu and M. R. Prausnitz, J. Controlled Release, 2011, 149, 242–249 CrossRef PubMed.
- M. C. Chen, S. F. Huang, K. Y. Lai and M. H. Ling, Biomaterials, 2013, 34, 3077–3086 CrossRef PubMed.
- B. Bediz, E. Korkmaz, R. Khilwani, C. Donahue, G. Erdos, L. D. Falo Jr. and O. B. Ozdoganlar, Pharm. Res., 2014, 31, 117–135 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |