Open Access Article
Open Access ArticleSynthesis of gold nanorod/neodymium oxide yolk/shell composite with plasmon-enhanced near-infrared luminescence†
Yafang Zhang*ab,
Jiahong Wang *bcd,
Fan Nanbe and
Qu-Quan Wang
*bcd,
Fan Nanbe and
Qu-Quan Wang *b
*b
aSchool of Physics and Technology, University of Jinan, Jinan, 250022, P. R. China. E-mail: yfzhang_opt@126.com
bSchool of Physics and Technology, Wuhan University, Wuhan, 430072, P. R. China. E-mail: qqwang@whu.edu.cn
cShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, P. R. China. E-mail: jh.wang1@siat.ac.cn
dDepartment of Physics, City University of Hong Kong, Kowloon, Hong Kong, P. R. China
eDepartment of Chemical and Biomolecular Engineering, Clarkson University, Potsdam, NY 13699, USA
First published on 4th June 2018
Abstract
A yolk/shell composite consisting of an AuNR core and an Nd2O3 shell with a 19 nm gap is synthesized by a multi-step over-growth method. The near-infrared luminescence of AuNR@Nd2O3 is up to 4.6 times higher than that of Nd2O3 hollow nanoparticles. The underlying mechanism of plasmon-induced luminescence enhancement is further investigated.
Rare earth (RE)-based nanostructures have attracted a lot of attention for their promising applications ranging from photonics to biomedicines.1–4 The RE-based nanostructures have shown many advantages over the conventional luminescent materials such as semiconductor quantum dots (QDs) and organic dyes, as the luminescence of RE shows high purity, large stock-shifts and excellent stability.5–7 On the other hand, the RE material is also bio-compatible, which suggests that it has great potential in bio-imaging and therapy.8–10 Among the lanthanide elements, neodymium (Nd) has drawn a lot of interest for its potentials in sub-tissue imaging and bio-sensing as its luminescence is in the first biological window.4,11,12 However, the absorption cross-section of Nd is smaller than those of semiconductors or dyes, which seriously affects its fluorescence efficiency, and this prevents its practical applications.13,14
Great efforts are being made to improve the fluorescence of RE,15–17 in particular the combination between plasmonic noble metal structures and RE is an efficient approach.18–20 When the light excites noble metal nanostructures, the electron gas collectively oscillates and generates plasmons near the metal surface.21,22 The large absorption cross-section and strong local electromagnetic field dramatically improve the fluorescence efficiency of the nearby emitters.23–25 Thus, various hybrids composed of RE nanoparticles and metal nanostructures are designed.26–31 For instance, J. R. Lakowicz et al. encapsulated lanthanides with silver nanoshells, and the emissions were significantly enhanced by about 10 times.26 For the case of Ag@SiO2@Y2O3:Er synthesized by F. Zhang et al., the up-conversion luminescence (UCL) of Y2O3:Er was enhanced 4 times by the inner Ag nanoparticles.27 A. Priyam et al.28 found that the fluorescence of NaYF4:Yb, Er NPs can be improved by a gold-shell. The metal- and particle-size-dependent enhancements are both investigated.29,30 In addition, various 3D metamaterials and photonic crystals have been designed to adjust or enhance the emissions of RE.31
The luminescence of RE can be improved by the coupled plasmons, because the plasmons provide strong electromagnetic field to enhance the excitation/emission process; also, there may be energy transfer between the plasmons and emitters.32,33 Gold nanorod (AuNR) is a typical plasmonic structure used to enhance the fluorescence of emitters, and it has tunable longitudinal surface plasmon resonance (LSPR) ranging from visible to near-infrared.34–36 Since the excitation and emission frequencies of Nd are both in the near-infrared region, it is possible to design a resonance structure between the AuNR and Nd structures to achieve luminescence enhancement of Nd3+.37 For obtaining luminescence enhancement, isolation between the plasmonic structure and the emitters is very important; silica, alumina, polymers, or DNAs have been applied to adjust the distance to obtain the largest enhancement.27,38,39 However, there are a few reports on the structure consisting of a plasmonic core and an RE shell with a natural isolation layer; the plasmon-induced RE down-conversion luminescence enhancement is also not a popular topic.40–42 In this study, we developed a facile method to prepare AuNR@Nd2O3 yolk/shell composites containing a 19 nm gap between an AuNR core and an Nd2O3 shell. The effects of AuNRs on the down-conversion luminescence (DCL) properties of Nd2O3 shells were studied by comparing the luminescence intensities of the AuNR@Nd2O3 yolk/shell composites and the corresponding Nd2O3 hollow nanoparticles. It was found that the 873 nm emission of Nd3+ was enhanced by AuNRs up to 4.6 times. The LSPR-dependent enhancement was also investigated further.
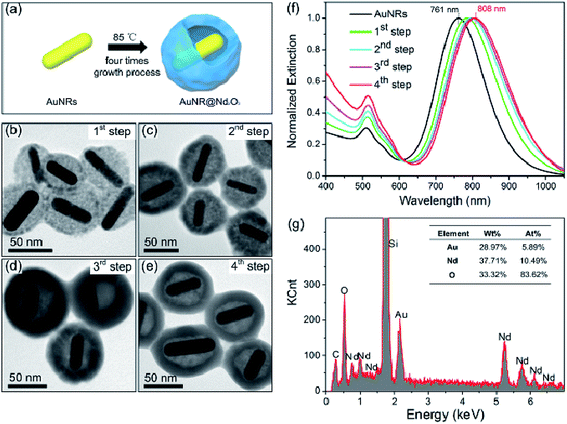
Cetyl-trimethyl ammonium bromide (CTAB)-capped AuNRs were first synthesized using the seed-mediated growth method.43,44 Then, CTAB was replaced by oleate with a ligand exchange approach.42 Au@Nd2O3 yolk/shell composites were prepared by an oleate-assisted hydrothermal method. In brief, for the 1st step of the growth procedure, 5 mL aqueous solution of oleate-AuNRs was diluted with 14 mL of ultrapure water. Nd(NO3)3 and HMT solutions were injected with stirring to form a well-dispersed solution; the mixture was incubated at 85 °C for 3 h, in which Nd(NO3)3 and HMT served as the cation and anion reagents, respectively. Then, the resultant solution was centrifuged; the precipitate was re-dispersed in ultrapure water, and it was used as seeds in the next step. This process was repeated three times to achieve the final yolk/shell composites (Fig. 1(a)). The transmission electron microscopy (TEM) images in Fig. 1(b–e) indicate the morphology evolution in the whole growth process. The length and diameter of the original AuNRs were about 60 nm and 15 nm, respectively, thus suggesting an aspect ratio of 4 (Fig. ESI-1a†). After the 3 hour 1st step growth, the Nd2O3 nanoparticles loosely surrounded AuNRs (Fig. 1(b)). In the 2nd growth step, the outer Nd2O3 shell became thicker and more compact (Fig. 1(c)). A gap formed between the AuNR core and the Nd2O3 shell, and the thickness of the Nd2O3 shell decreased from 28 nm to 16 nm after the 3rd growth step (Fig. 1(d)), which was probably due to the Ostwald ripening.45,46 When the 4th growth step was completed, the final products were collected. Fig. 1(e) and Fig. ESI-1b† demonstrate that the as-prepared hybrids were monodispersed hollow quasi-spheres consisting of AuNR cores and Nd2O3 shells. Interestingly, AuNRs were completely separated from Nd2O3, and the gap was about 19 nm. During the growth process, LSPR of AuNRs gradually red-shifted from 761 nm to 808 nm as the surrounding Nd2O3 increased the refractive index (Fig. 1(f)).47 The energy-dispersive X-ray (EDX) spectrum in Fig. 1(g) displays the relative element contents of the final AuNR@Nd2O3 composites; the Au/Nd ratio was about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.
6.
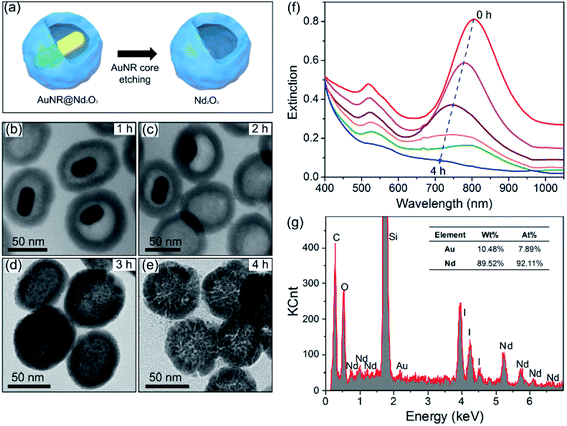
To reveal the plasmonic effect on the luminescence of Nd2O3, iodide/triiodide redox couple is used to corrode the inner AuNRs and to achieve the Nd2O3 hollow nanoparticles (Fig. 2(a)). Fig. 2(b–e) show the morphology evolution against the etching time. After etching for 1 h, AuNRs decrease in size and after 2 h, they transform into quasi-spheres about 10 nm in diameter. Then, AuNRs transform into 2–3 nm spheres after 3 h and finally disappear after 4 h. The completely etched Nd2O3 nanoparticles are uniform hollow spheres with inner cavities (Fig. 2(e) and Fig. ESI-1c†). With respect to size distributions (insets of Fig. ESI-1b and c†), the average diameters of AuNR@Nd2O3 composites and Nd2O3 hollow nanoparticles are similar, thus indicating that the iodide/triiodide electrolyte has not destroyed the Nd2O3 structure.48 The extinction spectra obtained at different etching times are displayed in Fig. 2(f). The LSPR location is blue-shifted, and the intensity is systematically decreased. When AuNRs are completely etched, the absorption of AuNRs completely disappears. As shown in Fig. 2(g), the corresponding EDX spectrum also demonstrates that AuNRs have been etched thoroughly.
The plasmon-enhanced near-infrared luminescence of AuNR@Nd2O3 is further studied by comparing the composites' emission spectra before and after etching (Fig. 3(a)). For the measurement, a 730 nm continuous wave laser is used for excitation; the luminescence spectra are recorded by a spectrometer with a liquid nitrogen-cooled CCD. The emission bands for the 4F3/2–4I9/2 transition of Nd3+ are located at 873 nm. As AuNRs are corroded, the emission peak position and shape remain unchanged. To calculate the enhancement factors of 873 nm emission against the etching time, the emission spectra are decomposed by Gaussian fitting (Fig. ESI-2†); the emission intensities at 873 nm are displayed in Fig. 3(b). The corresponding enhancement factors are also calculated in Fig. 3(b); the largest enhancement of about 3.5 is obtained at the beginning. As the AuNRs are corroded, the emission intensity is decreased. In another words, the results suggest that as the Au content increases, the luminescence becomes brighter.
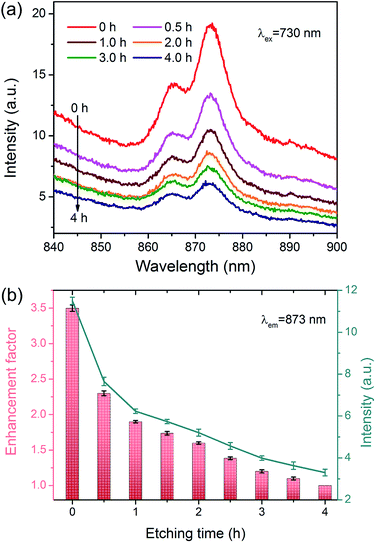 | ||
| Fig. 3 (a) Emission spectra of AuNR@Nd2O3 composites during the etching process. (b) Emission enhancement factors and the emission intensities of 873 nm against the etching time. | ||
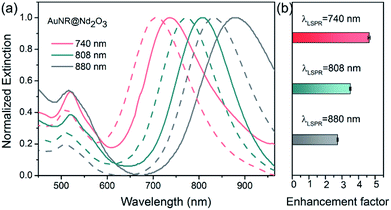
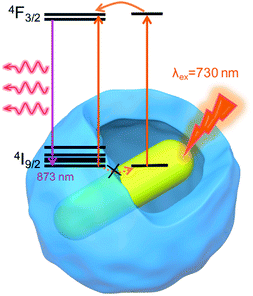
To further reveal the relationship between plasmon wavelength and luminescence enhancement, we prepared three kinds of AuNR@Nd2O3 with LSPRs at 740 nm (pink), 808 nm (green) and 880 nm (gray) (Fig. 4(a)). The emission spectra of the AuNR@Nd2O3 composites before and after etching were measured, and the enhancement factors at 873 nm emission are calculated in Fig. 4(b). The enhancement factors of Nd3+ at 873 nm were 4.6, 3.5 and 2.7 for the AuNR@Nd2O3 samples with LSPRs at 740 nm, 808 nm and 880 nm, respectively. When the LSPRs wavelength of AuNR@Nd2O3 is closer to the excitation wavelength, the enhancement factor was larger. The schematic of the plasmon-enhanced luminescence is shown in Fig. 5. Due to the gap between AuNR and Nd2O3, the non-radiative energy transfer from Nd2O3 to AuNR was negligible (dashed arrows).49 As the incident light excited AuNR and Nd2O3, the strong local electromagnetic field around AuNR enhanced the excitation and the emission processes of the Nd2O3 shells. There may be a resonance energy transfer between the AuNR and the Nd2O3, which was favorable for the improvement in Nd3+ excitation efficiency. As the LSPRs of AuNR@Nd2O3 were tuned from 740 nm to 880 nm, the enhancement factors decreased. All the results suggested that plasmons influenced the excitation process more efficiently than the emission process.
In summary, AuNR@Nd2O3 yolk/shell composites have been synthesized using a simple hydrothermal method. The reference samples (Nd2O3 hollow nanoparticles) are prepared by etching Au cores. The plasmon enhancement factor is also proved to be highly LSPR-dependent; the highest enhancement factor up to 4.6 is obtained when LSPR is resonant with the excitation. These findings provide a general pathway to modulate DCL of RE materials. The composites can be applied in photonics, bio-imaging, energy conversion and other fields.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (51702352), China Postdoctoral Science Foundation (2017M612762), Shenzhen Science and Technology Research Funding (JCYJ20170307100227392).Notes and references
- H. Chen, Y. Lang, Y. Zhang, D. Zhao, G. Qin, C. Wu, K. Zheng and W. Qin, J. Mater. Chem. C, 2015, 3, 6314–6321 RSC.
- G. Tian, Z. Gu, X. Liu, L. Zhou, W. Yin, L. Yan, S. Jin, W. Ren, G. Xing, S. Li and Y. Zhao, J. Phys. Chem. C, 2011, 115, 23790–23796 Search PubMed.
- T. Liu, Y. Wang, H. Qin, X. Bai, B. Dong, L. Sun and H. Song, Mater. Res. Bull., 2011, 46, 2296–2303 CrossRef.
- X. Yu, L. Chen, M. Li, M. Xie, L. Zhou, Y. Li and Q. Wang, Adv. Mater., 2008, 20, 4118–4123 CrossRef.
- G. S. Maciel and N. Rakov, J. Mater. Chem. C, 2013, 1, 3563–3568 RSC.
- M. Balestrieri, S. Colis, M. Gallart, G. Schmerber, M. Ziegler, P. Gilliot and A. Dinia, J. Mater. Chem. C, 2015, 3, 7014–7021 RSC.
- F. Liu, G. Aldea and J. Nunzi, J. Lumin., 2010, 130, 56–59 CrossRef.
- X. Li, R. Wang, F. Zhang, L. Zhou, D. Shen, C. Yao and D. Zhao, Sci. Rep., 2013, 3, 3536 CrossRef PubMed.
- T. Liu, Y. Wang, H. Qin, X. Bai, B. Dong, L. Sun and H. Song, Mater. Res. Bull., 2011, 46, 2296–2303 CrossRef.
- C. Bouzigues, T. Gacoin and A. Alexandrou, ACS Nano, 2011, 5, 8488–8505 CrossRef PubMed.
- Z. A. Ansari, S. Khalid, A. A. Khan, H. Fouad and S. G. Ansari, Sens. Lett., 2014, 12, 1495–1501 CrossRef.
- U. Rocha, K. U. Kumar, C. Jacinto, I. Villa, F. Sanz-Rodríguez, M. C. I. Cruz, A. Juarranz, E. Carrasco, F. C. J. M. Veggel, E. Bovero, J. G. Solé and D. Jaque, Small, 2014, 10, 1141–1154 CrossRef PubMed.
- C. Liu, Y. Hou and M. Gao, Adv. Mater., 2014, 26, 6922–6932 CrossRef PubMed.
- Y. Li, P. Qiu, H. Duan, J. Chen, G. J. Snyder, X. Shi, B. B. Iversen and L. Chen, J. Mater. Chem. C, 2016, 4, 4374–4379 RSC.
- G. Jia, C. Zhang, S. Ding, L. Wang, L. Li and H. You, CrystEngComm, 2012, 14, 573–578 RSC.
- D. Yang, G. Li, X. Kang, Z. Cheng, P. a. Ma, C. Peng, H. Lian, C. Li and J. Lin, Nanoscale, 2012, 4, 3450–3459 RSC.
- H.-X. Mai, Y.-W. Zhang, L.-D. Sun and C.-H. Yan, J. Phys. Chem. C, 2007, 111, 13721–13729 Search PubMed.
- H. Nabika and S. Deki, J. Phys. Chem. B, 2003, 107, 9161–9164 CrossRef.
- D. W. Lu, C. C. Mao, S. K. Cho, S. Ahn and W. Park, Sci. Rep., 2016, 6, 18894 CrossRef PubMed.
- M. Fujii, T. Nakano, K. Imakita and S. Hayashi, J. Phys. Chem. C, 2013, 117, 1113–1120 Search PubMed.
- Y. N. Xia and N. Halas, MRS Bull., 2005, 30, 338–348 CrossRef.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Y. Li, C. H. Moran, Q. Zhang, D. Qin and Y. N. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef PubMed.
- N. Li, H. Wang, M. Xue, C. Chang, Z. Chen, L. Zhou and B. Tang, Chem. Commun., 2012, 48, 2507–2509 RSC.
- Z. Buch, V. Kumar, H. Mamgain and S. Chawla, Chem. Commun., 2013, 49, 9485–9487 RSC.
- G. Kaur, R. K. Verma, D. K. Rai and S. B. Rai, J. Lumin., 2012, 132, 1683–1687 CrossRef.
- J. Zhang, Y. Fu and J. R. Lakowicz, J. Phys. Chem. C, 2009, 113, 19404–19410 Search PubMed.
- F. Zhang, G. B. Braun, Y. F. Shi, Y. C. Zhang, X. H. Sun, N. O. Reich, D. Y. Zhao and G. Stucky, J. Am. Chem. Soc., 2010, 132, 2850–2851 CrossRef PubMed.
- A. Priyam, N. M. Idris and Y. Zhang, J. Mater. Chem., 2012, 22, 960–965 RSC.
- M. Saboktakin, X. C. Ye, S. J. Oh, S. H. Hong, A. T. Fafarman, U. K. Chettiar, N. Engheta, C. B. Murray and C. R. Kagan, ACS Nano, 2012, 6, 8758–8766 CrossRef PubMed.
- P. Yuan, Y. H. Lee, M. K. Gnanasammandhan, Z. Guan, Y. Zhang and Q. Xu, Nanoscale, 2012, 4, 5132–5137 RSC.
- S. Lai, Z. Yang, J. Li, B. Shao, J. Yang, Y. Wang, J. Qiu and Z. Song, J. Mater. Chem. C, 2015, 3, 7699–7708 RSC.
- Y. Chen, K. Munechika and D. S. Ginger, Nano Lett., 2007, 7, 690–696 CrossRef PubMed.
- Y. Bernhard, B. Collin and R. A. Decréau, Sci. Rep., 2017, 7, 45063 CrossRef PubMed.
- H. Chen, L. Shao, Q. Li and J. Wang, Chem. Soc. Rev., 2013, 42, 2679–2724 RSC.
- B. Reischl, A. L. Rohl, A. Kuronen and K. Nordlund, Sci. Rep., 2017, 7, 16257 CrossRef PubMed.
- J. Shi, H. Wu, J. Liu, S. Li and X. He, Sci. Rep., 2015, 5, 11964 CrossRef PubMed.
- J. Lu, M. Prabhu, J. Song, C. Li, J. Xu, K. Ueda, A. A. Kaminskii, H. Yagi and T. Yanagitani, Appl. Phys. B, 2000, 71, 469–473 CrossRef.
- B.-C. Ye and B.-C. Yin, Angew. Chem., Int. Ed., 2008, 47, 1–6 CrossRef PubMed.
- R. Chhabra, J. Sharma, H. Wang, S. Zou, S. Lin, H. Yao, S. Lindsay and Y. Liu, Nanotechnology, 2009, 20, 485201 CrossRef PubMed.
- M. Chen, J. Wang, Z. Luo, Z. Cheng, Y. Zhang, X. Yu, L. Zhou and Q. Wang, RSC Adv., 2016, 6, 6912–6918 Search PubMed.
- T. Liu, X. Bai, C. Miao, Q. Dai, W. Xu, Y. Yu, Q. Chen and H. Song, J. Phys. Chem. C, 2014, 118, 3258–3265 Search PubMed.
- J. Wang, H. Huang, D. Zhang, M. Chen, Y. Zhang, X. Yu, L. Zhou and Q. Wang, Nano Res., 2015, 8, 2548–2561 CrossRef.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389 CrossRef.
- J. J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 485–491 CrossRef.
- N. Zhang, X. Fu and Y. Xu, J. Mater. Chem., 2011, 21, 8152–8158 RSC.
- N. G. Bastús, J. Comenge and V. Puntes, Langmuir, 2011, 27, 11098–11105 CrossRef PubMed.
- C. H. Fang, H. L. Jia, S. Chang, Q. F. Ruan, P. Wang, T. Chen and J. F. Wang, Energy Environ. Sci., 2014, 7, 3431–3438 Search PubMed.
- C. D. Geddes and J. R. Lakowicz, J. Fluoresc., 2002, 12, 121–129 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of gold nanorod/neodymium oxide yolk/shell composite with plasmon enhanced near-infrared luminescence. See DOI: 10.1039/c8ra01342j |
| This journal is © The Royal Society of Chemistry 2018 |