DOI:
10.1039/C8RA00440D
(Review Article)
RSC Adv., 2018,
8, 19739-19753
Strategies to synthesize various nanostructures of silver and their applications – a review
Received
15th January 2018
, Accepted 30th April 2018
First published on 29th May 2018
Abstract
Due to their various beneficial application-based properties, such as behavior, structure, and size, the synthesis of silver nanoparticles (Ag-NPs) with different structures has become an interesting yet common task for researchers to produce nanostructures for applications in various fields. This is because silver nanoparticles have interesting and unique properties, such as optical and catalytic, resulting from their different structures and sizes. These properties extend the use of nanostructures in various fields of research, especially in medicine, pharmacy, electronics, etc. Also, variations in their parameters affect the structures and sizes of Ag-NPs. This review provides an overview/brief presentation of various methodologies used to synthesize different application-based silver nanoparticles and lists areas where these nanoparticles are suitable for use according to their specific structures and sizes.
 Umme Thahira Khatoon | Ms. Umme Thahira Khatoon is a research scholar in the department of Metallurgical and Materials Engineering at the National Institute of Technology, Warangal, India. Her area of PhD research is the synthesis of metal nanoparticles and their antimicrobial/anticancer properties. She received her B. Tech (Biotechnology) and M. Tech (Nanotechnology) degrees in 2008 and 2011, respectively, from Jawaharlal Nehru Technological University, Hyderabad, India. During her studies, she attended and participated in international conferences, with paper and poster presentations in and outside India. Her research interests include the synthesis of nanomaterials and their biomedical applications as aspects of nanotechnology. |
 G. V. S. Nageswara Rao | Prof. Dr. G. V. S. Nageswara Rao is a professor in the Department of Metallurgical and Materials Engineering at the National Institute of Technology, Warangal, India. His research interests are surface engineering, powder metallurgy, materials testing and characterization, thermomechanical processing, and nanomaterials. He has several research publications, books, DST/AICTE/MHRD and other sponsored projects to his name. Prof. G. V. S. Nageswara Rao has research specialization as: Surface Engineering, Powder Metallurgy, Materials Testing and Characterization, Thermo-mechanical Processing, Nanomaterials. |
 Mantravadi Krishna Mohan | Dr. M. K. Mohan is a Professor at the National Institute of Technology, Warangal (India). He received his PhD from the Institute of Technology, Banaras Hindu University (India) in 1989. He visited The University of Alabama, Tuscaloosa, Alabama (USA) as a Post-Doctoral Fellow in 1998. He is Professor in charge at the Center for Advanced Materials. His research interests are corrosion engineering, extractive metallurgy and chemical synthesis of nanomaterials. |
 Yasemin Oztekin | Dr. Yasemin Oztekin is an associate professor at Selcuk University, Konya, Turkey. She completed her PhD at Selcuk University, Turkey and a post doctorate at Vilnius University, Lithuania. She has more than 11 years of academics and research experience at Selcuk University. Also, she has successfully supervised 3 doctoral students. Her research interests are electrochemistry, nanomaterials and nanostructures. To her credit, she has published more than 42 research articles in standard journals. Also, she served as the Selcuk University-Montana State University International Associate Degree Program Bio Biochemistry Division Coordinator, Selcuk University Chemistry Department ERASMUS Program Coordinator and Selcuk University Chemistry Department Mevlana Program Coordinator. |
1. Introduction
Nanotechnology is an advanced and modern field with novel research involving the synthesis and design of structures sized from 1 to 100 nm. Nanostructures have a broad range of applications.1 Nanostructures play roles in areas such as health care, cosmetics, food and feed, environmental health, mechanics, optics, and biomedical sciences. They are also important in the fields of chemical industries, electronics, space industries, drug–gene delivery, energy science, optoelectronics, catalysis, single electron transistors, light emitters, nonlinear optical devices, and photoelectrochemical applications.1–6 Researchers have synthesized nanoparticles with various interesting shapes, such as ellipsoidal, spherical, flowers, rods, stars, triangles, and tubes. There are different methods to synthesize metal nanostructures containing silver. These nanostructures can be obtained by physical, chemical and biological methods. Physical and chemical methods are used by many researchers.7,8 Biological methods are eco-friendly and non-toxic to the environment; these approaches include using metabolites as stabilizing and capping agents.9–11
Among the three methods, biological methods have advantages over conventional methods, which involve chemical agents associated with environmental toxicity. Currently, there is a growing need to develop eco-friendly processes which do not use toxic chemicals in synthesis methodologies. Selection of the solvent medium and of non-toxic reducing and stabilizing agents is the most important issue to be considered in the synthesis of nanoparticles. Ag-NPs are in demand among other metal nanoparticles due to their unique properties, which can be incorporated into antimicrobial applications.12,13 These include biosensor materials,14,15 composite fibers,16–18 cryogenic superconducting materials,19,20 cosmetic products,21,22 and electronic components.23,24 Several physical and chemical methods have been used for synthesizing and stabilizing silver nanostructures.25,26 The various sub methods of chemical methods include chemical reduction, electrochemical techniques, physical/chemical reduction and radiolysis. In comparison, the chemical reduction method has greater potential to synthesize a variety of nanostructures while changing several parameters involved in the synthesis of the nanostructures. Most of these methods are in the development stage and involve crystal growth, morphology, and size distribution and stability. Moreover, purification and extraction of nanoparticles for further applications are still significant concerns.27–30
This review presents an overview of the synthesis methods of silver nanostructures with different shapes for different applications.
2. Methods of synthesis
Various methods are involved in synthetic chemical procedures for the synthesis of nanostructures. Researchers have reported that various factors, such as temperature and concentration of metal precursors or chemicals, are used to optimize the growth and morphological behavior of the particles.31
2.1 Lee Miesel method
The Lee Miesel method is a common method to synthesize suspensions of Ag-NPs. This method uses many metal sources rather than only AgNO3, unlike the Turkevich method.32 Also, the Lee Miesel method produces a broad distribution of particle sizes. There are many examples where Ag salts and reducing agents were used.33–37 However, the most common method to synthesize Ag-NPs is reduction of AgNO3 with NaBH4.38
Yu Wan et al. described a robust method for the stable synthesis of quasi-spherical Ag-NPs. The method is based on a combination of seed-mediated growth and the Lee Miesel method. This synthesis includes the reduction of AgNO3 with citrate. Ag-NPs with sizes from 4 nm to 80 nm were obtained. Crucial factors such as pH, temperature, time, and concentration were also discussed during the synthesis. Fig. 1 and 2 present the growth of Ag-NPs obtained using the Lee Miesel reduction system and SEM images of Ag-NPs.39 Alexander Pyatenko et al. described a combination of the seed technique with laser treatment to synthesize Ag-NPs with diameters of 10 to 80 nm using a citrate reduction of AgNO3. Particles larger than 60 nm were produced using a multistep synthesis with laser treatment. The most important factor was soft laser treatment, which helped the heating and melting of the particles. However, measures were also taken to avoid evaporation of the nanoparticles. Furthermore, soft laser fluence had no effect on the size of the particles.40
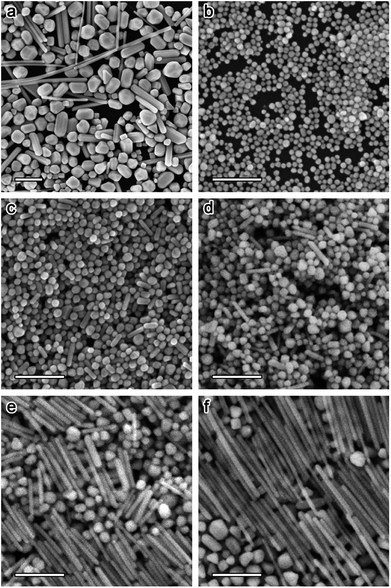 |
| | Fig. 1 (a) SEM image of Ag-NPs obtained by the Lee–Meisel method. (b–f): SEM images of Ag-NPs obtained by a combination of the Lee–Meisel method with seeded growth.39 | |
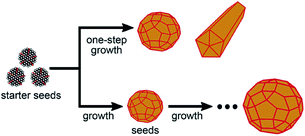 |
| | Fig. 2 Schematic of one-step and stepwise seeded growth of Ag-NPs in the Lee–Meisel reaction system.39 | |
2.2 Chemical reduction method
Ratan Das et al. presented the optical properties of nanocubes and nanocrystals which were prepared by chemical reduction methods. They confirmed that the nanocubes were stable for two months by SPR red shift.41 After two months, the particles started to agglomerate, indicating an aging process; the optical properties were defined by photoluminescence spectra after two months, which showed color changes and red-shifted SPR. Ratan Das et al. stated that temperature is the key factor during synthesis for a narrow distribution of nanocubes; the ideal temperature was determined to be 170 °C with 500 rpm stirring speed.41,42 The average particle size obtained using the Debye Scherrer formula43–45 was in agreement with the ICSD file.46–49 They confirmed that the size of the noble metal nanostructures increased beyond 20 nm; the bandwidth of SPR also increased due to the extrinsic size effect.41 Absorption and emission spectra, which determine the optical properties, were obtained in the visible range with 100 nm size distribution. Sang Hyuk Im et al. synthesized nanocubes with dimensions of 1.2*12 nm 21 nm; 130 nm wide using HNO3, NaCl and Cl−/O2 as etchants in a chemical process.50 Using this particular process, they were able to synthesize 0.25 gm of material. This indicates that the above process is robust and can be used to produce large-scale nanostructures. They also stated that the presence of protons slowed the reduction reaction and facilitated the formation of crystal seeds.50 Yun-Min Chang et al. synthesized Ag nanocubes with a heat treatment process using glucose,51,52 which is also known to be a mild reducing agent. The chemicals were autoclaved at 120 °C for 8 h to catalyze the process. The chemical reaction of the Ag nanocubes is shown in eqn (1).| | |
CH2OH–(CHOH)4–CHO + 2[Ag(NH3)2]+ + 2OH− → CH2OH–(CHOH)4–COOH + 2Ag + H2O + 2NH3
| (1) |
It is also evident from other studies that cetyl trimethyl ammonium bromide (CTAB) absorbed on the surface of nanoparticles provides steric hindrance, which prevents the coalescence of aggregates during the formation stage in solution due to weak van der Waals forces. When these particles are dried, they tend to retain a uniform size distribution.53 A change in morphology when the molar ratio of glucose/silver ions was changed was also described; the yields of the silver nanocubes were estimated to be greater than 95%. Qiang Zhang et al. provided a method to synthesize nanocubes with 30 to 70 nm edge lengths; this synthesis could be scaled up to 0.19 g per batch for 70 nm of Ag cubes.54 Many methods have been utilized to synthesize Ag nanocubes using different metal precursors, such as CF3COOAg, instead of AgNO3.55–61 This has three advantages: using the reaction time to control the size of the Ag nanocubes; lack of sensitivity to ethylene glycol, thus improving the strength/robustness and reproducibility of the polyol synthesis; and straightforward and large-scale production with high quality. Also, TEM images presented for different reaction times prove that using NaSH and HCl is necessary for the production of nanoparticles of uniform structure and size. It has also been mentioned that in this method, NaSH plays a role in the formation of single crystal seeds and Cl− ions function as a ligand for oxidative etching to eliminate twinned particles. Hence, this method is not sensible to ethylene glycol because it is sensitive towards polyol synthesis.
2.3 Polyol process
The polyol process is a chemical method where metals are synthesized from metal-containing compounds in poly(ethylene glycols). Ethylene glycol acts as both the solvent and reducing agent.62 Seog-Jin Jeon et al. synthesized Ag-Nps using HNO3 to initiate the process with minimal glycol aldehyde along with silver nitrate.63 The formation of nanocubes and nanowires was due to etching. Fig. 3 presents the principle of deoxygenation behind the production of nanocubes.
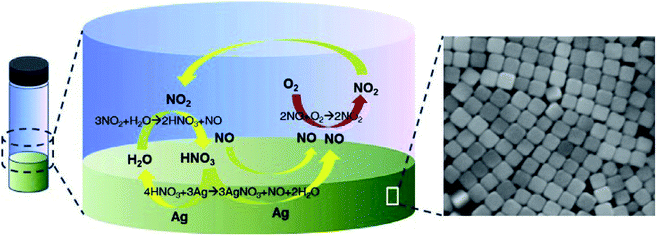 |
| | Fig. 3 Working hypothesis for the deoxygenation process.63 | |
Sang Hyuk Im et al. have put forward a protocol to synthesize nanocubes on a grand large scale by introducing HCl to a simple polyol process where the HCl played a major role in etching and dissolving twinned Ag-NPs.50 Eqn (2)–(4) explain the formation of the nanoparticles.
| | |
2HOCH2CH2OH → 2CH3CHO + 2H2O
| (2) |
| | |
2Ag+ + 2CH3CHO → CH3CHO–OHCCH3 + 2Ag + 2H+
| (3) |
| | |
4HNO3 + 3Ag → 3AgNO3 + NO + 2H2O
| (4) |
Sang Hyuk Im et al. synthesized nanocubes with different edge lengths of 30 nm, 45 nm, and 60 nm, as shown in Fig. 4.50,64 Sara E. Skrabalak et al. described a protocol for the preparation of Ag nanocubes in a rapid reaction time of 15 min using a polyol method, where the color of the sample changed from purple/black to yellow-orange to brown-opaque-red to green-ochre.65 Sara E. Skrabalak et al. are among the few researchers who have described the phenomenon of Ag-NPs formation as Ag atoms reach supersaturation.65 They agglomerate to form seeds and then grow into Ag nanostructures, which is the core formation process of any nanoparticle.66 Yugang Sun et al. attempted a large-scale synthesis of Ag nanowires with diameters of 30 to 40 nm and lengths of 50 μm; they presented the properties of the nanowires, describing them as electrically continuous with a conductivity (−0.8 × 105 S cn−1) approaching that of bulk silver.67 Table 1 presents the procedures used to obtain the product. Yugang Sun et al. presented an interesting method of a Pt-seeded polyol process to prepare silver nanowires. UV-vis SPR showed a peak at 530 nm, indicating the formation of rod-like structures, whereas the UV-vis spectrum showed a peak at 410 nm that red-shifted to 510 nm, showing a change of the structures from spheres to rods. Fig. 5 and 6 show the different mechanisms involved in synthesizing nanowires.69,72,73 Nanowires have been prepared by many methods; the mechanisms corresponding to the different methods are shown in Fig. 7 and 8.74,75 The researchers also stated that surface roughness can be minimized by depositing thin and long Ag NWs on polymer matrices.68–71
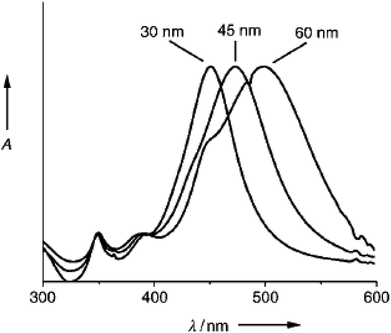 |
| | Fig. 4 UV/vis absorption spectra of aqueous solutions containing silver nanocubes with different edge lengths (30 nm, 45 nm, and 60 nm).64 | |
Table 1 Products obtained when the reactants were added using different proceduresa
| Sample no. |
Procedure |
Product |
| The concentrations of the AgNO3 and PVP solutions were 0.12 and 0.36 M, respectively. |
| AG04 |
2.5 mL AgNO3 and 5 mL PVP solutions were simultaneously injected using syringes within a period of 6 min (the standard procedure described in the Experimental section) |
A mixture of silver nanowires (70%) and colloidal particles (30%) |
| AG12 |
1 mL PVP solution was injected within 10 s before the addition of AgNO3, and the AgNO3 (2.5 mL) and PVP (4 mL) solutions were simultaneously injected within a period of 6 min |
A mixture of silver nanorods (10%) and particles (90%) |
| AG35 |
5 mL PVP solution was injected within 10 s, and 2.5 mL AgNO3 solution was then injected within a period of 10 s |
Silver nanoparticles only |
| AG36 |
5 mL PVP solution was injected within 10 s, and 2.5 mL AgNO3 solution was then injected within a period of 6 min |
Silver nanoparticles only |
| AG38 |
2.5 mL AgNO3 solution was injected in 10 s, and 5 mL PVP solution was then injected within a period of 6 min |
A mixture of silver nanowires (70%) and colloidal particles (30%) |
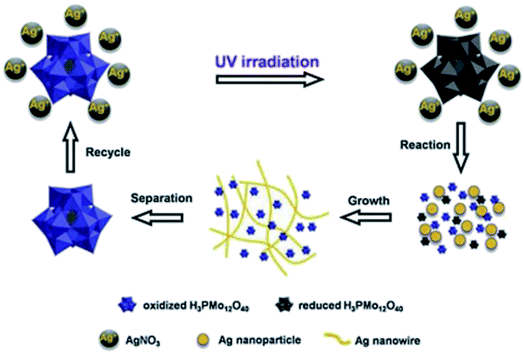 |
| | Fig. 5 The processes involved in the preparation of Ag-NWs.69 | |
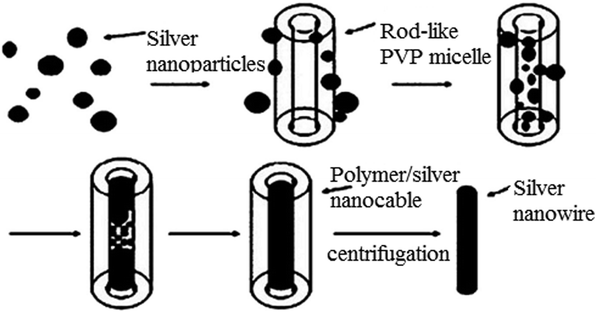 |
| | Fig. 6 Formation process of Ag-NWs.69 | |
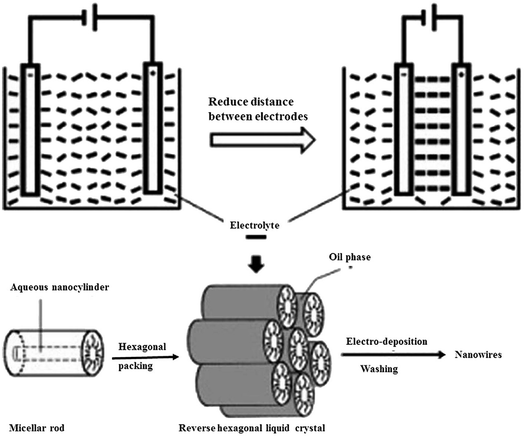 |
| | Fig. 7 Process of the enhanced alignment of the reverse hexagonal liquid-crystalline phase during nanowire electro-deposition.74 | |
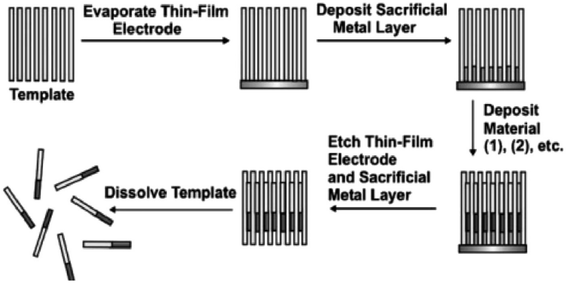 |
| | Fig. 8 General scheme for the synthesis of one-dimensional nanostructures (nanowires and nanorods) by the deposition of materials into nanoporous templates.75 | |
2.4 Electrochemical method
Cai Hong Liu et al. presented the application of silver nanowires (NW) as a transparent flexible and conductive thin film. The NWs were also applied as electrodes, where silver nanowires were preserved in isopropyl alcohol using polyethylene terephthalate [PET] as a substrate to prepare the electrodes.76 Fig. 9 presents nanowires with different sizes.77 Rodriguez et al. described an electrochemical procedure to synthesize Ag-NPs ranging from 2 to 7 nm in size by dissolving a metallic anode in an aprotic solvent. Ag-NPs were obtained by reduction of silver ions in acetonitrile containing tetrabutylammonium salts (TBA bromide or TBA acetate). It has been noted that current density plays an important role in maintaining particle size as well as the efficiency of the process.77
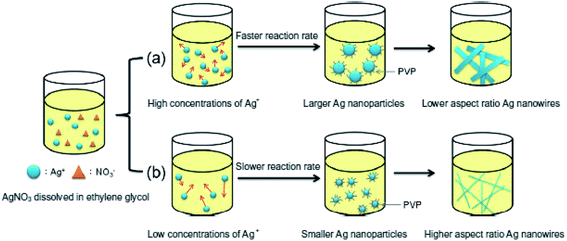 |
| | Fig. 9 Schematic of the use of different concentrations of silver nitrate to change the diameters of silver nanowires.77 | |
2.5 Electrodeposition method
Nasir M Abbasi et al. presented different synthetic techniques for the preparation of Ag-NWs, indicating the effects of various factors, such as temperature, time, rate, materials, and inert conditions.78 They also proved that the Ag-NWs have high electrical conductivity, a high surface area, and a high aspect ratio, which are essential for the preparation of Ag-NWs/conducting polymer nanocomposites. Useful applications include touch screens, liquid crystal displays, and solar cells.78
2.6 Biological method
Cheng Yang et al. attempted to produce nanowires (NWs) by green methods rather than polyol, chemical reduction or other chemical-based methods. To synthesize nanowires, they used water and glycerol as reducing agents and solvents at a high temperature (210 °C) in an optimal ratio of 0.25%. They also observed that a small amount of water increased the yield of the nanowires.79 When NaCl and KCl were used as controlling agents, they observed the formation of 60 to 70 nm nanowires, respectively. They also observed this method to be simple and reproducible compared to other methods.79 In another investigation, glycerol was used as a reducing agent instead of EG, in contrast to nanostructures syntheses reported by other researchers.80 An optimum ratio of water/glycerol (0.25%) was necessary to produce nanowires; otherwise, the presence of other shapes was observed. Xiao lian Jing et al. synthesized nanoflowers via a biosynthesis method using Flos-Magnolia E. officinalis extract and compared this method with a green synthesis involving reducing and stabilizing agents. Polyphenols played the main role in the reduction and synthesis of nanoflowers. They also reported that in the green synthesis, no stabilizing agent was used; different shapes of Ag nanoproducts, such as spheres, ellipsoids, and rods, could be prepared by varying the amounts of trisodium citrate (1 M) and gallic acid. For example, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.5
1 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of the metal precursor and reducing agent were used. The TEM micrographs obtained by these investigators are shown in Fig. 10.81 Fig. 11 shows a schematic of the formation of Ag nanoflowers. Bikash Kumar et al. applied bio-inspired techniques to synthesize branched flowerlike Ag nanostructures which could be implemented in surface enhanced Raman scattering (SERS) and antibacterial activity.81,82 The shape, size and surrounding medium of the particles were studied by UV-vis optical spectra with surface plasmon resonance (SPR) wavelengths (438 nm, 600 nm).83,84 Also, the spherical NPs were formed by reducing Ag+ with rutin; with time, the structure of the NPs changed to short branches. After that, further nucleation and assembly took place, and the crystals finally grew into flower-shaped nanostructures. Also, with increasing concentration of rutin, the shape of particles changed.
1 ratios of the metal precursor and reducing agent were used. The TEM micrographs obtained by these investigators are shown in Fig. 10.81 Fig. 11 shows a schematic of the formation of Ag nanoflowers. Bikash Kumar et al. applied bio-inspired techniques to synthesize branched flowerlike Ag nanostructures which could be implemented in surface enhanced Raman scattering (SERS) and antibacterial activity.81,82 The shape, size and surrounding medium of the particles were studied by UV-vis optical spectra with surface plasmon resonance (SPR) wavelengths (438 nm, 600 nm).83,84 Also, the spherical NPs were formed by reducing Ag+ with rutin; with time, the structure of the NPs changed to short branches. After that, further nucleation and assembly took place, and the crystals finally grew into flower-shaped nanostructures. Also, with increasing concentration of rutin, the shape of particles changed.
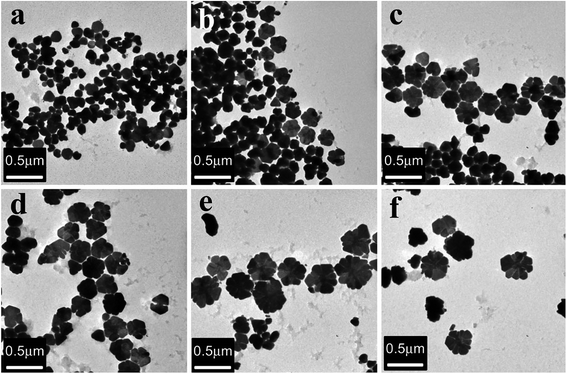 |
| | Fig. 10 TEM images of silver nanoproducts synthesized from the extract of Flos Magnoliae officinalis: (a) 2.5 g L−1; (b) 5 g L−1; (c) 10 g L−1; (d) 20 g L−1; (e) 30 g L−1; (f) 40 g L−1.81 | |
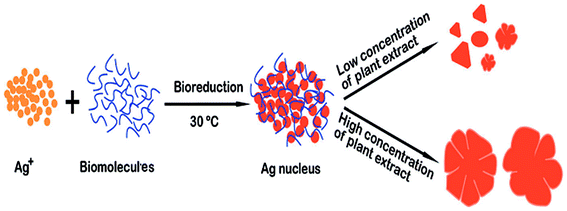 |
| | Fig. 11 Schematic of the morphology of Ag structures obtained using Flos Magnoliae officinalis.81 | |
Ma Xinfu et al. followed an eco-friendly route to synthesize nanoflowers. This biosynthesis route used L-cysteine as a reducing agent and capping agent in alkaline solution in a 70 °C water bath.85 This biological approach is simple, convenient and significant for preparing stable metal nanostructures in an aqueous medium.
3. Different shapes of silver nanostructures
3.1 Silver nanocubes
Xia et al. synthesized Ag nanocubes with edge lengths of 115 ± 9 and 95 ± 7 nm using ethylene glycol to reduce AgNO3.86,87 Dabin Yu et al. reported another approach to produce silver nanocubes with sizes of 55 ± 5 nm using a silver mirror reaction approach.86,88 The silver mirror reaction is an old chemical route to generate reflective mirrors on solid supports.89
Ag nanocubes were prepared at high temperatures above 80 °C. Similarly, there are reports on the synthesis of Ag nanocubes which were used as templates for gold nanoboxes and iron nanocubes, which were then used as building blocks of magnetic superlattices.87,90 XRD analysis of the Ag nanocubes indicated that the product stretches in a preferential100 orientation, as the [200] intensity was three times greater than that of.100 This observation indicates the ability of the Ag nanocubes to assemble into a 2D array on a solid surface.91 Dabin Yu et al. were able to produce uniform nanocubes, as shown in Fig. 12.92
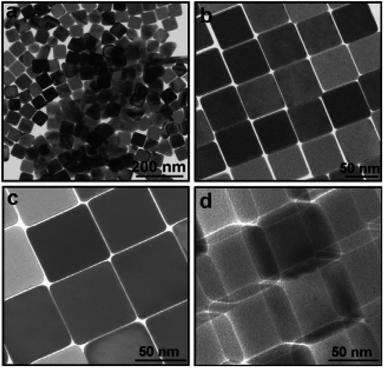 |
| | Fig. 12 TEM images of Ag nanocubes: (a) with low magnifications; (b–d) with higher magnifications. Images c and d were recorded from almost the same cubes; however, image d was recorded after rotating the cubes by an angle of 30°.92 | |
Also, they mentioned that increasing the molar ratio of HTAB/[Ag(H3)2]+ led to an obvious shape evolution of the Ag nanostructures from spheres to cubes due to adsorption of the surfactant on the surface of the silver crystal faces; these adsorption characteristics depended on the molar ratio. The minimum molar ratio to form nanocubes is 2.5 M, where the sample color changed from orange to yellow in agreement with the UV-vis spectra peak at 420 nm; this may lead to general and mechanical applications of these nanocubes. E. V. Panfilova et al. stated that the abovementioned method was not efficient for controlling the size of cubic particles; changing the reagent concentration93 and reaction temperature changed the yield of the nanocubes. Furthermore, Shengli Qi et al. stated that the basic punch action is annealing. By altering the annealing time at high temperature, the size of the nanocubes could be changed from 90 to 160 nm.94 Jiejun Zhu et al. attempted to produce nanocubes which were obtained at different reaction times of 40 minutes (70 nm) and 70 min (230 nm) at a maximum temperature of 150 °C. XRD and TEM studies showed the presence of sharp edges on the cubes; they were also able to produce/observe nanorods by increasing the reaction time. Also, with increasing time, the edge length of the Ag nanocubes increased. Interestingly, the UV-vis spectra showed SPR peaks at 543 nm and 665 nm with increasing reaction time from 40 to 70 min and SPR peaks at 417 nm to 461 nm, respectively, at low wavelengths.95 Qiyu Wang et al. demonstrated the structure effects on the oxygen reduction reaction (ORR) on similarly sized Ag dodecahedra and Ag nanocubes as the working anode; they used a lamp to create the nanocubes.96 Qiyu Wang et al. synthesized Ag dodecahedra and Ag nanocubes via an electrochemical method; they also stated that the Ag nanodecahedra had more active sites for catalytic activity than the Ag nanocubes.97 Yi Wang et al. followed a robust method for the facile synthesis of small Ag nanocubes in the range of 18 to 32 nm;98 they successfully used diethylene glycol rather than ethylene glycol to produce Ag nanocubes based on their previous protocol. Ag nanocubes can be synthesized in different nanosize ranges from 10 to 32 nm depending on the time scale ranging from 30 minutes to 180 minutes, using ethylene glycol.98 Andrew R. Siekkinen et al. described the formation of nanocubes using a series of concentrations of NaS at different temperatures ranging from 145 °C to 160 °C; during the UV-vis analysis, the SPR peak blue-shifted as the cube length decreased and became closer to spherical.99–101
3.2 Silver nanowires
Jian Yang Lin et al. clearly proved that the growth of Ag nanowires (NWs) is affected by the synthesis temperature, the concentration of silver nitrate, and the rate at which silver nitrate is added. It has been shown that with changing concentration of AgNO3, the diameter of the Ag nanowires also decreases and the aspect ratio increases. These nanowires are used for conductive films. Fig. 13 shows the NWs fabricated with different concentrations of AgNO3.102 Fig. 14 and 15 show the different morphologies of Ag NWs at various annealing temperatures.102
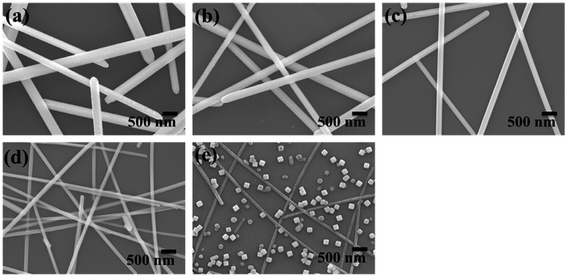 |
| | Fig. 13 The synthesis of silver nanowires using different concentrations of silver nitrate; (a)–(e) PVP/silver nitrate molar ratios of 2, 4, 8, 16, and 32, respectively.102 | |
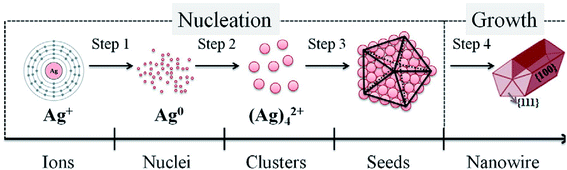 |
| | Fig. 14 A simple graph of the mechanism for generating silver nanowires.102 | |
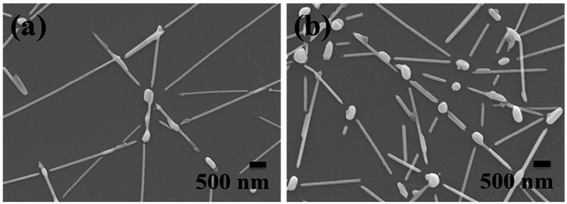 |
| | Fig. 15 The surface morphologies of silver nanowires with different annealing temperatures: (a) 300 °C and (b) 400 °C.102 | |
3.3 Silver nanotriangles
Chun fang Wu et al. demonstrated a simple, reproducible controllable route to synthesize nanotriangles using olive seeds and capping agents; the size could be tuned between 50 and 260 nm by adjusting the number of seeds or the mole ratio of polyvinyl pyrrolidone [PVP] and AgNO3.103 By changing the dropping speed and using a suitable concentration of trisodium citrate, Ag nanotriangles with sharper corners and narrow size distributions were synthesized. Fig. 16 shows the presence of nanotriangles. Yi He et al. synthesized five silver equilateral triangle plates with thicknesses of 10 nm and side lengths of 50, 100, 150, 200 and 250 nm, respectively. Ag nanotriangles were synthesized using the discrete dipole approximation (DDA) method.104 Similarly, Andrew et al. experimented with synthesizing silver nanoparticles with variable sizes and investigated their optical properties.105
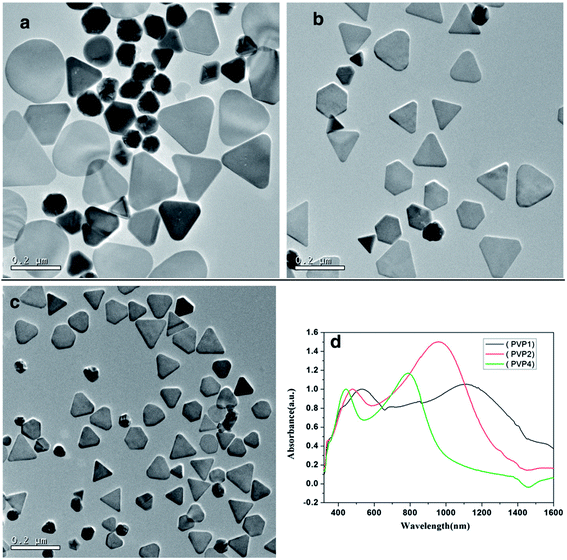 |
| | Fig. 16 (a–c) TEM images of as-prepared silver nanotriangles with different molar ratios of PVP and AgNO3; (d) vis-NIR absorption spectra of the corresponding samples.103 | |
4. Applications
Metal NPs, especially Ag NPs, are interesting because of their superior characteristics, such as magnetic, electrical, and morphology controlled optical activities. These can be exploited in electronic instruments, anti-microbial preparations, cosmetics consumables, biosensor components, cryogenic superconducting parts and composite threads.106 Additionally, these nanostructures have numerous uses in various areas, such as curative imaging, nano-hybrids, filters, medicinal transportation and hyperthermia of cancerous cells.107,108 Nanostructures of metallic Ag have aroused the interest of researchers because of their substantial associated activities in various fields, such as integrated circuits,109 bio-tagging, sensors and filters,110 anti-microbe and deodorant threads,111 cell electrodes,112 Ag nanowires, economical paper batteries113 and anti-microbes.114 Metallic Ag NPs have been utilized extensively as anti-microbial components in consumable preservation, cloth plating, health concerns and numerous environmental applications.
4.1 Applications in catalysis
Nanocatalysis is an emerging area which employs nanostructures as catalysts. It is recognized that elements such as silver, platinum and gold in ionic form can catalyze oxygen formation by degradation of hydrogen peroxide.114,115 Guo et al. also reported that silver is an important catalyst for the reduction of formaldehyde to methanol and of ethylene oxide to ethylene. Silver nanostructures inactivated on silica spheres were studied for their capability to catalyze the oxidation of dyes by NaBH4 (sodium borohydride). In the absence of silver nanoparticles, there was no motion and no reaction of the dyes.116
4.2 Electrochemical applications
Additionally, the electrochemical characteristics of silver NPs in nanorange sensors offer rapid feedback times and lower detection ranges. For example, electrodeposited silver nanostructures on Al2O3 plates with gold microstructured electrodes manifested very high sensitivity to H2O2.117 Singaravelan et al. synthesized Ag-NPs via an electrochemical route without using special reagents. The Ag-NPs were characterized by TEM, UV-vis spectroscopy, and FTIR. The optical band gap energy of Ag-NPs (3.39 eV) is a potential candidate for optical and optoelectronic devices. Furthermore, antibacterial activity studies showed acceptable ZoI of Ag-NPs against Gram +ve and Gram −ve bacteria. This property suggests that Ag-NPs can also be used as a bactericidal agent in biomedical applications.118
4.3 Magnetic beads
Susanne Pahlow et al. prepared silver nanoflowers on magnetic beads, as shown in Fig. 17.119 This method is promising because it can be used to easily and inexpensively produce silver nanoflowers-coated magnetic beads which can also be used for surface enhanced Raman scattering (SERS) diagnostics. Hongyan Liang et al. demonstrated the use of Ag nanoflowers as susceptible substrates of SERS (surface enhanced Raman scattering). Researchers have also stated that Ag nanoflowers can serve as highly sensitive and reproducible SERS substrates.120 Ag nanoflowers have many advantages, such as highly sensitive SERS substrates, because these particles are stable and can be reused several times.120 These particles can be readily synthesized on a large scale, and no byproducts are observed; as a result, the environment will not be polluted. Hence, these nanoflowers are a promising candidate for SERS applications.
 |
| | Fig. 17 Scheme of the preparation of silver nanoflowers on magnetic beads.119 | |
4.4 Optical applications
The striking optical characteristics of NPs are responsible for their applications in chemosensors and optical biosensors. One of the objectives of investigation is the analysis of bio-binding communication between antigens and antibiotics utilizing triangular silver NPs.121 Sensitivity is enhanced in the presence of silver nanoparticles to detect zeptomoles. Utilizing the SPR effect, the Ag-NPs attain immense sensitivity, and quantitative analysis can be coordinated in real time. With the help of detectors containing silver nanoparticles, one can examine an organic substance inside a cell as well as observing the dynamical reactions that occur.122
4.5 Plasmonic applications
Based on the localized surface plasmon resonance (LSPR) of metallic nanoparticles, plasmonic nanosensors have proved to be a powerful tool for biosensing applications.123 Lehui Lu et al. synthesized Ag nanoplates with different sizes by reducing [Ag(NH3)2]+ with ascorbic acid. As a result, the flowerlike nanoplates have exhibited good surface-enhanced Raman scattering (SERS) compared to spherical silver nanoparticles. It has also been stated that flowerlike silver nanoplates show potential applications in biological sensing and labeling systems.124
Mehmet et al. presented a novel method of combining soft lithography and nanosphere lithography to fabricate flexible and tunable plasmonic nanostructures. It has also been observed that the surface plasmon absorption depends on the dimensions (diameter and depth) of the nanostructures. Also, the SERS measurement factor is dependent on the plasmonic absorption wavelength and laser wavelength.125
4.6 Antimicrobial activity
Fig. 18 shows the antibacterial activity of Ag nanoflowers which are capable of performing diverse biological/clinical applications as stated by Bikash Kumar Jena et al.126 The investigators also reported that Ag NPs processed utilizing extracts of leaves from Vitex negundo at a convergence of 2 ppm displayed distinct anti-microbial characteristics against clinically quarantined pathogenic bacteria. In this interesting research, Ag-NPs synthesised from extracts of Vitex negundo leaves had excellent antibacterial characteristics.127,128 The processed silver NPs showed strong bacteriostatic motion on Escherichia coli and Staphylococcus aureus; furthermore, they had stronger reactions with Gram negative bacteria than with Gram positive bacteria.129,130 The antimicrobial influence of the silver metal NPs showed more influential bacteriostatic effects on the microorganism S. aureus.131,132
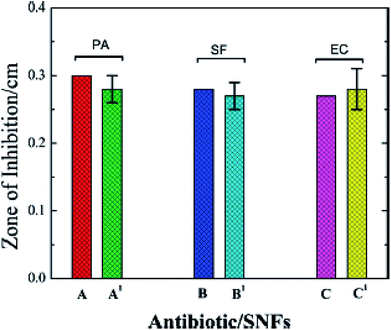 |
| | Fig. 18 Plots showing the antibacterial activity of Ag-NFs (SNFs) (A′–C′) with respect to the standard antibiotics gentamicin (A and C) and penicillin (B).126 | |
4.7 Antifungal activity
Silver NPs at 50 μL displayed advanced antifungal activity against Aspergillus nigeris, Candida albicans and Candida kefyr, while intermediary activity was visualized against A. fumigates, C. tropicalis, A. flavus and C. krusei.133,134 The assessment of their antimicrobial activities, such as antifungal and antibacterial, was compared with Ag-NPs. Researchers observed that the lowest prevention concentration of S. saprophyticus was 10.19 ± 0.08 mg mL−1, which is lower than that of the other investigated microbes. Uropathogenic Staphylococcus is a type of S. saprophyticus which is frequently collected from immature female outpatients with primary urinary zone infections. Silver NPs show dominant antifungal characteristics against A. nigeris, C. albicans, and C. kefyr. These investigators suggested that silver nanoparticles synthesized by a green method show high antifungal activity.133,134
4.8 Antibacterial applications
Dolgaev et al. claim that Ag-NPs possess indispensable activities in ecological areas, such as DNA sequencing and antimicrobial activity. Silver has been recognized to inhibit the spread of microbes by powerful toxicity (antibacterial activity).135 Technologists have acknowledged that Ag-NPs chemically combined with O2 (oxygen) are dangerous to microbes, especially bacteria.136 Ag-NPs have numerous applications, such as beauty products, socks, consumable containers, cleansers, sprays and an immense number of different commodities, to hinder the advancement of microbes.
4.9 Medical applications
Particular implementations of ionic silver and silver nanoparticles can be deployed in therapeutic applications for burn therapy, teeth parts, sunscreen cosmetics, etc.137,138
5. Conclusions
The synthesis of Ag nanostructures with different shapes represents a rapidly growing research area in the field of nanomaterials because of the strong dependence of their properties on their shapes. In this review, the formation and the use of stabilizing agents, where solvents play a major role in the formation of different silver nanostructures, has been presented; also, different shapes of nanoparticles and their applications were studied. Possible methods for the synthesis of various silver nanostructures have been elaborated. An attempt was made to select procedures for synthesizing various shapes of nanoparticles for applications in various fields. It has been observed that the growth of nanoparticles usually follows a trend of three steps, particularly in solution-based methods: these include nucleation, seeding, and growth. It has been observed that the environment of production, parameters during synthesis, thermodynamics, and kinetics at each stage of synthesis have great impacts on the physical and chemical behavior, properties, and control of nanostructures. Among the methods of synthesis of nanosilver, the polyol method is suitable to produce different-shaped nanostructures by changing the reaction conditions. As presented in this review, different structures of nanosilver synthesized by various assembly techniques have varied applications in many fields of science and technology, such as biomedical, electronics, and computers.
Conflicts of interest
The authors declare no conflict of interest.
References
- V. L. Colvin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 354 CrossRef.
- C. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115(19), 8706–8715 CrossRef.
- G. Schmid, Chem. Rev., 1992, 92, 1709–1727 CrossRef.
- A. J. Hoffman, G. Mills, H. Yee and M. R. Hoffmann, J. Phys. Chem., 1992, 96, 5546–5552 CrossRef.
- J. F. Hamilton and R. C. Baetzold, Science, 1979, 205, 1213–1220 Search PubMed.
- H. S. Mansur, F. Grieser, M. S. Marychurch, S. Biggs, R. S. Urquhart and D. N. Furlong, J. Chem. Soc., Faraday Trans., 1995, 91, 665–672 RSC.
- X. F. Zhang, Z. G. Liu, W. Shen and S. Gurunathan, Int. J. Mol. Sci., 2016, 17, 1534 CrossRef PubMed.
- A. Ali, M. Z. Hira Zafar, I. ul Haq, A. R. Phull, J. S. Ali and A. Hussain, Nanotechnol., Sci. Appl., 2016, 9, 49 CrossRef PubMed.
- M. Shah, D. Fawcett, S. Sharma, S. K. Tripathy and G. E. J. Poinern, Materials, 2015, 8, 7278–7308 CrossRef PubMed.
- M. Imran Din and A. Rani, Int. J. Anal. Chem., 2016, 2016, 1–14 CrossRef PubMed.
- K. Selvam, C. Sudhakar, M. Govarthanan, P. Thiyagarajan, A. Sengottaiyan, B. Senthilkumar and T. Selvankumar, J. Radiat. Res. Appl. Sci., 2017, 10, 6–12 CrossRef.
- S. Prabhu and E. K. Poulose, Int. Nano Lett., 2012, 2, 32 CrossRef.
- P. Singh, Y. J. Kim, H. Singh, C. Wang, K. H. Hwang, M. E. A. Farh and D. C. Yang, Int. J. Nanomed., 2015, 10, 2567 Search PubMed.
- X. Luo, A. Morrin, A. J. Killard and M. R. Smyth, Electroanalysis, 2006, 18, 319–326 CrossRef.
- G. Doria, J. Conde, B. Veigas, L. Giestas, C. Almeida, M. Assunção and P. V. Baptista, Sensors, 2012, 12, 1657–1687 CrossRef PubMed.
- S. Zhang, Y. Tang and B. Vlahovic, Nanoscale Res. Lett., 2016, 11, 80 CrossRef PubMed.
- P. Dubey, B. Bhushan, A. Sachdev, I. Matai, S. Uday Kumar and P. Gopinath, J. Appl. Polym. Sci., 2015, 132 Search PubMed.
- J. M. Patrascu, I. A. Nedelcu, M. Sonmez, D. Ficai, A. Ficai, B. S. Vasile and L. C. Rusu, J. Nanomater., 2015, 2015, 3 Search PubMed.
- T. M. D. Dang, T. T. T. Le, E. Fribourg-Blanc and M. C. Dang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2012, 3, 035004 CrossRef.
- T. Panigrahi, Doctoral dissertation, in Proceedings of the Jangjeon Mathematical Society, ed. T. Panigrahi and G. Murugusundaramoorthy, 2013, vol. 16, ch. 1, pp. 91–100 Search PubMed.
- S. Gajbhiye and S. Sakharwade, J. Cosmet., Dermatol. Sci. Appl., 2016, 6, 48 Search PubMed.
- A. Lohani, A. Verma, H. Joshi, N. Yadav and N. Karki, ISRN Dermatol., 2014, 2014, 1–14 CrossRef PubMed.
- M. J. Firdhouse and P. Lalitha, J. Nanotechnol., 2015, 2015, 1–18 CrossRef.
- E. Abbasi, M. Milani, S. Fekri Aval, M. Kouhi, A. Akbarzadeh, H. Tayefi Nasrabadi and M. Samiei, Crit. Rev. Microbiol., 2016, 42, 173–180 Search PubMed.
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Res. Pharm. Sci., 2014, 9, 385 Search PubMed.
- T. Klaus-Joerger, R. Joerger, E. Olsson and C. G. Granqvist, Trends Biotechnol., 2001, 19, 15–20 CrossRef PubMed.
- M. Sastry, A. Ahmad, M. I. Khan and R. Kumar, Curr. Sci., 2003, 85, 162–170 Search PubMed.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- H. Korbekandi, S. Iravani and S. Abbasi, Crit. Rev. Biotechnol., 2009, 29, 279–306 CrossRef PubMed.
- F. E. Kruis, H. Fissan and B. Rellinghaus, J. Mater. Sci. Eng. B, 2000, 69, 329–334 CrossRef.
- Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14, 4736–4745 CrossRef.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef.
- R. W. Scott, H. Ye, R. R. Henriquez and R. M. Crooks, Chem. Mater., 2003, 15, 3873–3878 CrossRef.
- I. Sondi, D. V. Goia and E. Matijević, J. Colloid Interface Sci., 2003, 260, 75–81 CrossRef PubMed.
- J. P. Cason, K. Khambaswadkar and C. B. Roberts, Ind. Eng. Chem. Res., 2000, 39, 4749–4755 CrossRef.
- X. Li, J. Zhang, W. Xu, H. Jia, X. Wang, B. Yang and Y. Ozaki, Langmuir, 2003, 19, 4285–4290 CrossRef.
- N. Shirtcliffe, U. Nickel and S. Schneider, J. Colloid Interface Sci., 1999, 211, 122–129 CrossRef PubMed.
- S. He, J. Yao, P. Jiang, D. Shi, H. Zhang, S. Xie and H. Gao, Langmuir, 2001, 17, 1571–1575 CrossRef.
- Y. Wan, Z. Guo, X. Jiang, K. Fang, X. Lu, Y. Zhang and N. Gu, J. Colloid Interface Sci., 2013, 394, 263–268 CrossRef PubMed.
- A. Pyatenko, M. Yamaguchi and M. Suzuki, J. Phys. Chem. C, 2007, 111, 7910–7917 Search PubMed.
- R. Das and S. Sarkar, Opt. Mater., 2015, 48, 203–208 CrossRef.
- S. Biswas, A. K. Kole, R. Sarkar and P. Kumbhakar, Mater. Res. Express, 2014, 1, 045043 CrossRef.
- R. Das, S. S. Nath and R. Bhattacharjee, Phys. E, 2010, 43, 224–227 CrossRef.
- K. D. Rogers and P. Daniels, Biomaterials, 2002, 23, 2577–2585 CrossRef PubMed.
- R. Das, S. S. Nath and R. Bhattacharjee, J. Lumin., 2011, 131, 2703–2706 CrossRef.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410–8426 CrossRef.
- S. Link and M. A. El-Sayed, Int. Rev. Phys. Chem., 2000, 19, 409–453 CrossRef.
- S. Berciaud, L. Cognet, P. Tamarat and B. Lounis, Nano Lett., 2005, 5, 515–518 CrossRef PubMed.
- S. Hashimoto, D. Werner and T. Uwada, J. Photochem. Photobiol., C, 2012, 13, 28–54 CrossRef.
- S. H. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., 2005, 117, 2192–2195 CrossRef.
- Y. M. Chang, I. T. Lu, C. Y. Chen, Y. C. Hsieh and P. W. Wu, J. Alloys Compd., 2014, 586, 507–511 CrossRef.
- Y. Wang, Y. Zheng, C. Z. Huang and Y. Xia, J. Am. Chem. Soc., 2013, 135, 1941–1951 CrossRef PubMed.
- I. Capek, Nanocomposite structures and dispersions, Elsevier, 2006, p. 23 Search PubMed.
- Q. Zhang, W. Li, L. P. Wen, J. Chen and Y. Xia, Chem.–Eur. J., 2010, 16, 10234–10239 CrossRef PubMed.
- Q. Zhang, N. Li, J. Goebl, Z. Lu and Y. Yin, J. Am. Chem. Soc., 2011, 133, 18931–18939 CrossRef PubMed.
- V. Germain, J. Li, D. Ingert, Z. L. Wang and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 8717–8720 CrossRef.
- B. Rodríguez-González, I. Pastoriza-Santos and L. M. Liz-Marzán, J. Phys. Chem. B, 2006, 110, 11796–11799 CrossRef PubMed.
- R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901–1903 CrossRef PubMed.
- L. M. Liz-Marzán, Langmuir, 2006, 22, 32–41 CrossRef PubMed.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Nano Lett., 2002, 2, 903–905 CrossRef.
- Y. Sun, B. Mayers and Y. Xia, Nano Lett., 2003, 3, 675–679 CrossRef.
- F. Fiévet and R. Brayner, The polyol process. Nanomaterials: A Danger or a Promise?, Springer, London, 2013, pp. 1–25 Search PubMed.
- S. J. Jeon, J. H. Lee and E. L. Thomas, J. Colloid Interface Sci., 2014, 435, 105–111 CrossRef PubMed.
- B. Wiley, T. Herricks, Y. Sun and Y. Xia, Nano Lett., 2004, 4, 1733–1739 CrossRef.
- L. Au, S. E. Skrabalak, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182 CrossRef PubMed.
- S. Omobayo Adio, C. Basheer, K. Zafarullah, A. Alsharaa and Z. Siddiqui, Int. J. Environ. Anal. Chem., 2016, 96, 776–788 CrossRef.
- Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14, 4736–4745 CrossRef.
- Q. N. Luu, J. M. Doorn, M. T. Berry, C. Jiang, C. Lin and P. S. May, J. Colloid Interface Sci., 2011, 356, 151–158 CrossRef PubMed.
- Y. Zhou, S. H. Yu, X. P. Cui, C. Y. Wang and Z. Y. Chen, Chem. Mater., 1999, 11, 545–546 CrossRef.
- L. M. Huang, H. T. Wang, Z. B. Wang, A. Mitra, K. N. Bozhilov and Y. S. Yan, Adv. Mater., 2002, 14, 61 CrossRef.
- J. H. Song, Y. Wu, B. Messer, H. Kind and P. Yang, J. Am. Chem. Soc., 2001, 123, 10397–10398 CrossRef PubMed.
- H. Jiang, J. Jiang, J. Cheng, W. Dou, X. Tang, L. Yang and D. Bai, New J. Chem., 2014, 38, 109–114 RSC.
- L. Liu, C. He, J. Li, J. Guo, D. Yang and J. Wei, New J. Chem., 2013, 37, 2179–2185 RSC.
- L. M. Huang, H. T. Wang, Z. B. Wang, A. Mitra, K. N. Bozhilov and Y. S. Yan, Adv. Mater., 2002, 14, 61 CrossRef.
- S. J. Hurst, E. K. Payne, L. Qin and C. A. Mirkin, Angew. Chem., Int. Ed., 2006, 45, 2672–2692 CrossRef PubMed.
- Liu and Yu, Nanoscale Res. Lett., 2011, 6, 75 CrossRef PubMed.
- L. Rodriguez-Sanchez, M. C. Blanco and M. A. Lopez-Quintela, J. Phys. Chem. B, 2000, 104, 9683–9688 CrossRef.
- N. M. Abbasi, H. Yu, L. Wang, W. A. Amer, M. Akram, H. Khalid and J. Shan, Mater. Chem. Phys., 2015, 166, 1–15 CrossRef.
- C. Yang, Y. Tang, Z. Su, Z. Zhang and C. Fang, J. Mater. Sci. Technol., 2015, 31, 16–22 Search PubMed.
- S. Hemmati, D. P. Barkey, N. Gupta and R. Banfield, ECS J. Solid State Sci. Technol., 2015, 4(4), P3075–P3079 CrossRef.
- X. Jing, J. Huang, L. Wu, D. Sun and Q. Li, J. Nanopart. Res., 2014, 16, 2327 CrossRef.
- B. K. Jena, B. K. Mishra and S. Bohidar, J. Phys. Chem. C, 2009, 113, 14753–14758 Search PubMed.
- D. F. Zhang, L. Y. Niu, L. Jiang, P. G. Yin, L. D. Sun, H. Zhang and C. H. Yan, J. Phys. Chem. C, 2008, 112, 16011–16016 Search PubMed.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261–3266 CrossRef PubMed.
- X. Ma, Q. Guo, Y. Xie and H. Ma, Chem. Phys. Lett., 2016, 652, 148–151 CrossRef.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261–3266 CrossRef PubMed.
- Y. Wang, Y. Zheng, C. Z. Huang and Y. Xia, J. Am. Chem. Soc., 2013, 135(5), 1941–1951 CrossRef PubMed.
- D. Yu and V. W. W. Yam, J. Am. Chem. Soc., 2004, 126, 13200–1320189 CrossRef PubMed.
- D. Yu and V. W. W. Yam, J. Phys. Chem. B, 2005, 109(12), 5497–5503 CrossRef PubMed.
- B. Jiang, M. Li, F. Bai, H. Yu, T. Mwenya, Y. Li and D. Song, AIP Adv., 2013, 3, 032119 CrossRef.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef PubMed.
- D. Yu and V. W. W. Yam, J. Phys. Chem. B, 2005, 109(12), 5497–5503 CrossRef PubMed.
- E. V. Panfilova, B. N. Khlebtsov, A. M. Burov and N. G. Khlebtsov, Colloid J., 2012, 74, 99–109 CrossRef.
- S. Qi, X. Shen, Z. Lin, G. Tian, D. Wu and R. Jin, Nanoscale, 2013, 5, 12132–12135 RSC.
- J. J. Zhu, C. X. Kan, J. G. Wan, M. Han and G. H. Wang, J. Nanomater., 2011, 2011, 40 Search PubMed.
- Q. Wang and Q. Lu, Nat. Commun., 2017, 8, 709 CrossRef PubMed.
- Q. Wang, C. U. I. Xiaoqiang, W. Guan, L. Zhang, X. Fan, Z. Shi and W. Zheng, J. Power Sources, 2014, 269, 152–157 CrossRef.
- Y. Wang and N. Herron, J. Phys. Chem., 1991, 95, 525–532 CrossRef.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef PubMed.
- A. R. Siekkinen, J. M. McLellan, J. Chen and Y. Xia, Chem. Phys. Lett., 2006, 432, 491–496 CrossRef PubMed.
- B. Wiley, T. Herricks, Y. Sun and Y. Xia, Nano Lett., 2004, 4, 1733–1739 CrossRef.
- J. Y. Lin, Y. L. Hsueh, J. J. Huang and J. R. Wu, Thin Solid Films, 2015, 584, 243–247 CrossRef.
- C. Wu, X. Zhou and J. Wei, Nanoscale Res. Lett., 2015, 10, 354 CrossRef PubMed.
- Y. He and G. Shi, J. Phys. Chem. B, 2005, 109, 17503–17511 CrossRef PubMed.
- A. J. Frank, N. Cathcart, K. E. Maly and V. Kitaev, J. Chem. Educ., 2010, 87, 1098–1101 CrossRef.
- S. Hemmati, D. P. Barkey, N. Gupta and R. Banfield, ECS J. Solid State Sci. Technol., 2015, 4, P3075–P3079 CrossRef.
- M. A. Albrecht, C. W. Evans and C. L. Raston, Green Chem., 2006, 8, 417–432 RSC.
- D. Pissuwan, S. M.Valenzuela and M. B. Cortie, Trends Biotechnol., 2006, 24, 62–67 CrossRef PubMed.
- M. Tan, G. Wang, Z. Ye and J. Yuan, J. Lumin., 2006, 117, 20–28 CrossRef.
- S. Kotthaus, B. H. Gunther, R. Hang and H. Schafer, IEEE Trans. Compon., Packag., Manuf. Technol., Part A, 1997, 20, 15–20 CrossRef.
- G. Cao, Nanostructures & nanomaterials: synthesis, properties & applications, Imperial College Press, 2004 Search PubMed.
- W. Z. Zhang and G. Wang, New Chem. Mater., 2003, 31, 42–44 Search PubMed.
- L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. La Mantia, L. F. Cui and Y. Cui, Proc. Natl. Acad. Sci., 2009, 106, 21490–21494 CrossRef PubMed.
- K. H. Cho, J. E. Park, T. Osaka and S. G. Park, Electrochim. Acta, 2005, 51, 956–960 CrossRef.
- G. Merga, R. Wilson, G. Lynn, B. H. Milosavljevic and D. Meisel, J. Phys. Chem. C, 2007, 111, 12220–12226 Search PubMed.
- J. Z. Guo, H. Cui, W. Zhou and W. Wang, J. Photochem. Photobiol., A, 2008, 193, 89–96 CrossRef.
- Z. J. Jiang, C. Y. Liu and L. W. Sun, J. Phys. Chem. B, 2005, 109, 1730–1735 CrossRef PubMed.
- R. Singaravelan and S. B. Alwar, Appl. Nanosci., 2015, 5, 983–991 CrossRef.
- D. Cialla, K. Weber, J. Popp, S. Pahlow and A. März, Tagungsband, 2013, 345–350 Search PubMed.
- H. Liang, Z. Li, W. Wang, Y. Wu and H. Xu, Adv. Mater., 2009, 21(45), 4614–4618 CrossRef.
- X. Xu, Y. Ma, Y. Du, T. Jiang, J. Zhou and Z. Zhao, Mater. Res. Express, 2018, 5, 015013 CrossRef.
- S. Zhu, C. Du and Y. Fu, Opt. Mater., 2009, 31, 769–774 CrossRef.
- L. Guo, J. A. Jackman, H. H. Yang, P. Chen, N. J. Cho and D. H. Kim, Nano Today, 2015, 10, 213–239 CrossRef.
- L. Lu, A. Kobayashi, K. Tawa and Y. Ozaki, Chem. Mater., 2006, 18, 4894–4901 CrossRef.
- M. Kahraman, P. Daggumati, O. Kurtulus, E. Seker and S. Wachsmann-Hogiu, Sci. Rep., 2013, 3, 3396 CrossRef PubMed.
- B. K. Jena, B. K. Mishra and S. Bohidar, J. Phys. Chem. C, 2009, 113, 14753–14758 Search PubMed.
- M. C. Estevez, M. A. Otte, B. Sepulveda and L. M. Lechuga, Anal. Chim. Acta, 2014, 806, 55–73 CrossRef PubMed.
- M. Ahmad, N. Ghafoor and M. N. Aamir, Indian J. Pharm. Sci., 2012, 74, 465 CrossRef PubMed.
- Y. He, Z. Du, H. Lv, Q. Jia, Z. Tang, X. Zheng and F. Zhao, Int. J. Nanomed., 2013, 8, 1809 CrossRef PubMed.
- W. K. Jung, H. C. Koo, K. W. Kim, S. Shin, S. H. Kim and Y. H. Park, Appl. Environ. Microbiol., 2008, 74, 2171–2178 CrossRef PubMed.
- J. Suriya, S. B. Raja, V. Sekar and R. Rajasekaran, Afr. J. Biotechnol., 2012, 11, 12192–12198 Search PubMed.
- S. B. Almasaudi, A. A. Al-Nahari, M. El Sayed, E. Barbour, S. M. Al Muhayawi, S. Al-Jaouni and S. Harakeh, Saudi J. Biol. Sci., 2017, 24, 1255–1261 CrossRef PubMed.
- Y. Rout, S. Behera, A. K. Ojha and P. L. Nayak, J. Microbiol. Antimicrob., 2012, 4, 103–109 CrossRef.
- A. Lalitha, R. Subbaiya and P. Ponmurugan, Int. J. Curr. Microbiol. Appl. Sci., 2013, 2, 228–235 Search PubMed.
- S. I. Dolgaev, A. V. Simakin, V. V. Voronov, G. A. Shafeev and F. Bozon-Verduraz, Appl. Surf. Sci., 2002, 186, 546–551 CrossRef.
- S. Agnihotri, S. Mukherji and S. Mukherji, RSC Adv., 2014, 4, 3974–3983 RSC.
- A. Schmidt-Ott, J. Aerosol Sci., 1988, 19, 553559–557563 Search PubMed.
- N. Durán, P. D. Marcato, G. I. De Souza, O. L. Alves and E. Esposito, J. Biomed. Nanotechnol., 2007, 3, 203–208 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
G. V. S. Nageswara Rao
a,
Krishna Mohan Mantravadi
a and
Yasemin Oztekin
b
*a,
G. V. S. Nageswara Rao
a,
Krishna Mohan Mantravadi
a and
Yasemin Oztekin
b









![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.5
1 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of the metal precursor and reducing agent were used. The TEM micrographs obtained by these investigators are shown in Fig. 10.81 Fig. 11 shows a schematic of the formation of Ag nanoflowers. Bikash Kumar et al. applied bio-inspired techniques to synthesize branched flowerlike Ag nanostructures which could be implemented in surface enhanced Raman scattering (SERS) and antibacterial activity.81,82 The shape, size and surrounding medium of the particles were studied by UV-vis optical spectra with surface plasmon resonance (SPR) wavelengths (438 nm, 600 nm).83,84 Also, the spherical NPs were formed by reducing Ag+ with rutin; with time, the structure of the NPs changed to short branches. After that, further nucleation and assembly took place, and the crystals finally grew into flower-shaped nanostructures. Also, with increasing concentration of rutin, the shape of particles changed.
1 ratios of the metal precursor and reducing agent were used. The TEM micrographs obtained by these investigators are shown in Fig. 10.81 Fig. 11 shows a schematic of the formation of Ag nanoflowers. Bikash Kumar et al. applied bio-inspired techniques to synthesize branched flowerlike Ag nanostructures which could be implemented in surface enhanced Raman scattering (SERS) and antibacterial activity.81,82 The shape, size and surrounding medium of the particles were studied by UV-vis optical spectra with surface plasmon resonance (SPR) wavelengths (438 nm, 600 nm).83,84 Also, the spherical NPs were formed by reducing Ag+ with rutin; with time, the structure of the NPs changed to short branches. After that, further nucleation and assembly took place, and the crystals finally grew into flower-shaped nanostructures. Also, with increasing concentration of rutin, the shape of particles changed.













