Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved performance and stability of perovskite solar cells with bilayer electron-transporting layers
Tingting Jianga and
Weifei Fu *b
*b
aThe State Key Laboratory of Refractories and Metallurgy, College of Materials and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, P. R. China
bState Key Laboratory of Silicon Materials, MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: zjufwf@zju.edu.cn
First published on 6th February 2018
Abstract
Zinc oxide nanoparticles (NPs) are very promising in replacing the phenyl-C61-butyric acid methyl ester (PC61BM) as electron-transporting materials due to the high carrier mobilities, superior stability, low cost and solution processability at low temperatures. The perovskite/ZnO NPs heterojunction has also demonstrated much better stability than perovskite/PC61BM, however it shows lower power conversion efficiency (PCE) compared to the state-of-art devices based on perovskite/PCBM heterojunction. Here, we demonstrated that the insufficient charge transfer from methylammonium lead iodide (MAPbI3) to ZnO NPs and significant interface trap-states lead to the poor performance and severe hysteresis of PSC with MAPbI3/ZnO NPs heterojunction. When PC61BM/ZnO NPs bilayer electron transporting layers (ETLs) were used with a device structure of ITO/poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) (PTAA)/MAPbI3/PC61BM/ZnO NPs/Al, which can combine the advantages of efficient charge transfer from MAPbI3 to PC61BM and excellent blocking ability of ZnO NPs against oxygen, water and electrodes, highly efficient PSCs with PCE as high as 17.2% can be achieved with decent stability.
Organic–inorganic hybrid perovskite solar cells (PSCs) have recently attracted tremendous attention because of their excellent photovoltaic efficiencies.1–4 Since the initial results published in 2009 with efficiencies about 4% using a typical dye-sensitized solar cell structure with liquid electrolyte,5 significant progress has been made in device performance through developing high quality film processing methods,6–10 tuning the perovskite composition,11–15 optimizing the device architectures16,17 and synthesizing new hole/electron transport materials.18–21 Recently, a certified record power conversion efficiency (PCE) of 22.7% was achieved.22 Despite of the success in obtaining dramatically improved PCE, there are certain concerns about the stability and cost towards commercialization. For the state-of-the-art PSCs, perovskites are susceptible to degradation in moisture and air, thus the charge transport materials should prevent the perovskite from exposure to such environments.20,23–25 One the other hand, PSCs also suffer from the high cost of widely used organic charge transport materials such as 2,2,7,7-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (spiro-OMeTAD), phenyl-C61/71-butyric acid methyl ester (PC61/71BM).3,18,26 As alternatives, inorganic materials such as CuSCN,27 CuI,28 CuGaO2,20 and NiOx
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29,30 which can be acted as hole transport materials and ZnO,31,32 SnO2
29,30 which can be acted as hole transport materials and ZnO,31,32 SnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12,33,34 and TiO2
12,33,34 and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10,35 which can be acted as electron transport materials are widely studied. Among them, metal oxide nanoparticles (NPs) are very promising in replacing the organic counterparts due to the high carrier mobilities, superior stability, low cost and solution processability at low temperatures.16,31,33
10,35 which can be acted as electron transport materials are widely studied. Among them, metal oxide nanoparticles (NPs) are very promising in replacing the organic counterparts due to the high carrier mobilities, superior stability, low cost and solution processability at low temperatures.16,31,33
The perovskite/ZnO NPs heterojunction has been demonstrated much better stability than perovskite/PCBM,23 however it shows lower PCE compared to the state-of-art devices based on perovskite/PCBM heterojunction.36–38 Thus in this paper, we systematically studied the charge transfer and recombination at CH3NH3PbI3 (MAPbI3) and ZnO NPs or PC61BM interfaces and tried to fabricate devices with high PCE and super stability simultaneously. We demonstrated that insufficient charge transfer from MAPbI3 to ZnO NPs and significant interface trap-states lead to the poor performance and severe hysteresis of PSCs based on MAPbI3/ZnO NPs heterojunction, while the devices based on MAPbI3/PC61BM show high PCE and negligible hysteresis due to the efficient charge transfer from MAPbI3 to PC61BM and less recombination at the interface. On the other hand, the MAPbI3/ZnO NPs devices show excellent stability in air because of the excellent capping ability of ZnO NPs while the stability of MAPbI3/PC61BM devices is very poor. Thus, we fabricated the PSCs with bilayer electron-transporting layers (ETLs) with the device structure of ITO/poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) (PTAA)/MAPbI3/PC61BM/ZnO NPs/Al, trying to combine the advantages of efficient charge extraction ability of PC61BM and excellent blocking ability of ZnO NPs against oxygen, water and electrode, and finally device with PCE as high as 17.2% was achieved with decent stability.
1 Experimental
1.1 Materials
Zinc acetate dehydrate, tetramethylammonium hydroxide (TMAH), lead iodide, DMSO, DMF, chlorobenzene and toluene were purchased from Sigma-Aldrich and used as received. [6,6]-Phenyl-C61-butyric acid methyl ester (PC61BM) was purchased from American Dyes Source, Inc. CH3NH3I (MAI) was purchased from Shanghai Materwin New Materials Co. Ltd. Poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) (PTAA) was purchased from Xi'an Polymer Light Technology Corporation. ZnO nanoparticles were synthesized by a sol–gel process using Zn acetate dehydrate and TMAH, and dispersed in anhydrous isopropanol with a concentration of 20 mg mL−1.391.2 Device fabrication and testing
Prior to fabrication, the substrates were cleaned by sonication using detergent, deionized water, acetone, and isopropanol sequentially for every 15 min followed by 15 min of ultraviolet ozone (UV-ozone) treatment. The substrates were transferred to a glovebox. PTAA film was fabricated by spin-coating a toluene solution with a concentration of 5 mg mL−1 on the ITO substrates in glove-box.PbI2 (1 M) and DMSO (1 M) were dissolved in DMF under stirring at 70 °C. The solution was then spin coated on the PTAA film at 3000 rpm for 60 s. Then a solution of MAI in 2-propanol (IPA) (50 mg mL−1) was dropped and spin-coated at 3000 rpm for 60 s. Afterwards, the as prepared films were heated at 90 °C for 15 min. After cooling down, a layer of PC61BM (20 mg mL−1 in chlorobenzene) was spin-coated at 2000 rpm for 45 s for MAPbI3/PCBM junction solar cells. While for MAPbI3/ZnO junction solar cells ZnO nanoparticles in isopropanol was spin-coated at 4000 rpm for 30 s. Subsequently, samples were loaded into a vacuum deposition chamber (background pressure ≈ 5 × 10−4 Pa) to deposit a 100 nm thick Al cathode with a shadow mask. To specify the illuminated area, we used an aperture with an area of 0.06 cm2, whereas the total device area defined by the overlap of the electrodes was approximately 0.12 cm2.
The J–V characteristics were measured with Keithley 2400 measurement source units with the devices maintained at room temperature in glove-box. The photovoltaic response was measured under a calibrated solar simulator (Enli Technology) at 100 mW cm−2, and the light intensity was calibrated with a standard photovoltaic reference cell. The devices were stored in glove-box in dark overnight before measurement. The forward J–V scans were measured from −0.1 V to 1.2 V with a scan rate of 0.05 V s−1 and a voltage step of 0.01 V while the reverse J–V scans were measured from 1.2 V to −0.1 V with a scan rate of 0.05 V s−1 and a voltage step of 0.01 V. The EQE spectrum was measured using a QE-R Model of Enli Technology.
2 Results and discussion
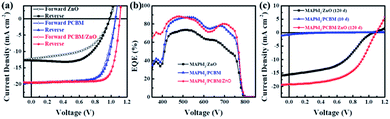
Inverted perovskite solar cells with the device architecture of ITO/PTAA/MAPbI3/PC61BM or ZnO NPs/Al were fabricated, which were shown in Fig. 1a. Fig. 1b shows the corresponding energy level diagram of the devices. The reported valance band of ZnO NPs and PC61BM are similar with a value of 4.2 eV, which is 0.3 eV lower the valance band of MAPbI3, and thus the electrons in perovskite film can transfer to both ETLs and be collected by electrodes. Fig. 2a shows the current–voltage (J–V) characteristics of PSCs based on ZnO NPs and PC61BM as ETLs under 100 mW cm−2 AM1.5G solar illumination with reverse and forward scans. The corresponding photovoltaic parameters are summarized in Table 1. The device employing ZnO as ETL exhibits an open-circuit voltage (VOC) of 0.98 V, a short-circuit current density (JSC) of 12.5 mA cm−2, and a fill factor (FF) of 0.64, yielding a PCE of 8.0% at reverse scan, and suffers severe hysteresis with a much lower PCE of 6.3% (VOC = 0.99 V, JSC = 12.0 mA cm−2 and FF = 0.52) at forward scan, respectively. While the device using PC61BM as ETL shows a significant improvement in PCE up to 15.0% at reverse scan with a VOC of 1.06 V, a JSC of 19.1 mA cm−2 and a FF of 0.72, and more importantly, with negligible hysteresis (14.70% PCE at forward scan with a VOC of 1.05 V, a JSC of 19.2 mA cm−2 and a FF of 0.71). It exhibits improvement on all three parameters simultaneously compared to those of ZnO NPs based devices. The much enhanced JSC was also demonstrated by the external quantum efficiency (EQE) spectra shown in Fig. 2b.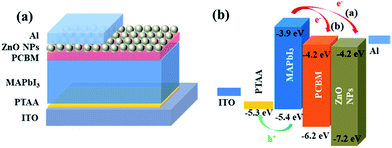 | ||
| Fig. 1 Schematic illustration of the perovskite solar cell configuration with PC61BM/ZnO NPs as ETL (a). (b) The corresponding energy level diagram of the devices. | ||
| ETL | Scan direction | VOC [V] | JSC [mA cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|
| ZnO NPs | Forward | 0.99 | 12.0 | 0.52 | 6.3 |
| Reverse | 0.98 | 12.5 | 0.64 | 8.0 | |
| PCBM | Forward | 1.05 | 19.2 | 0.71 | 14.7 |
| Reverse | 1.06 | 19.1 | 0.72 | 15.0 | |
| PCBM/ZnO | Forward | 1.11 | 19.5 | 0.78 | 16.9 |
| Reverse | 1.11 | 19.6 | 0.79 | 17.2 |
We also tested the stability of devices which were stored in air under dark with a humidity of 20% for 120 days. The corresponding J–V curves were shown in Fig. 2c. We found that the device with ZnO NPs as ETL retained 95% of the initial PCE, with a VOC of 1.06 V, a JSC of 15.6 mA cm−2, a FF of 0.45 and a PCE of 7.6% after 120 days storage, while the device with only PC61BM as ETL almost died only after 10 days, showing a VOC of 0.56 V, a JSC of 0.77 mA cm−2, a FF of 0.13 and a PCE of 0.06%. The much worse stability was attributed to the poor blocking ability of PC61BM against oxygen, water and the electrode.23
In order to obtain both high PCE and excellent stability, we also fabricated device with bilayer ETLs in a structure of ITO/PTAA/MAPbI3/PC61BM/ZnO NPs/Al shown in Fig. 1a. The device shows highest VOC, JSC, FF and PCE which ups to 17.2% at reverse scan with a VOC of 1.11 V, a JSC of 19.6 mA cm−2 and a FF of 0.79, and also shows negligible hysteresis with a VOC of 1.11 V, a JSC of 19.5 mA cm−2, a FF of 0.78 and a PCE of 16.9% at forward scan. After stored in air 120 days, the device also shows a decent PCE of 11.4%, with a VOC of 1.09 V, a JSC of 19.2 mA cm−2, a FF of 0.54, which is 66% of the initial value. Thus we demonstrated that this type of device show the advantages of the MAPbI3/PC61BM device with high PCE and the MAPbI3/ZnO NPs device with good stability.
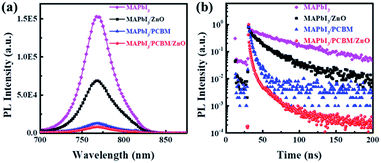
The effects of different ETLs on the charge extraction and recombination process at perovskite/ETL interface were investigated in details to find out the reasons of different behaviours of corresponding devices. Steady-state photoluminescence (PL) was performed to compare the electron transfer efficiency from perovskite to ETLs. As shown in Fig. 3a, only 54% of the PL intensity was quenched by depositing ZnO NPs on top, while more than 90% PL intensity was quenched when PC61BM or PC61BM/ZnO NPs were deposited on top of the perovskite layer. This means insufficient charge transfer from perovskite to ZnO NPs, while electrons in perovskite film can be efficiently transferred to PC61BM layer. This was further confirmed by time-resolved PL (TRPL) (Fig. 3b). The TRPL curve was fitted to a biexponential equation: Y = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) and the detailed data are shown in Table 2. In the absence of ETL quencher, the pristine perovskite film showed a relatively long PL lifetime of 56.8 ns, while it decreased to 25.6, 5.4 and 5.3 ns for the ZnO NPs, PC61BM and PC61BM/ZnO NPs-based films, respectively. This implies that faster and more efficient electron extraction was achieved at the perovskite/PC61BM interface. The insufficient charge transfer from perovskite to the ZnO NPs layer compared to PC61BM could due to the shallower conduction band and lower electron mobility of ZnO NPs, or the interfacial traps at the perovskite/ZnO NPs interface,23 and the worse contact at perovskite/ZnO NPs interface. These could cause charge accumulation at the perovskite/ZnO NPs interface and thus leads to poor performance and severe hysteresis in the corresponding device.23,33,40
exp(−t/τ2) and the detailed data are shown in Table 2. In the absence of ETL quencher, the pristine perovskite film showed a relatively long PL lifetime of 56.8 ns, while it decreased to 25.6, 5.4 and 5.3 ns for the ZnO NPs, PC61BM and PC61BM/ZnO NPs-based films, respectively. This implies that faster and more efficient electron extraction was achieved at the perovskite/PC61BM interface. The insufficient charge transfer from perovskite to the ZnO NPs layer compared to PC61BM could due to the shallower conduction band and lower electron mobility of ZnO NPs, or the interfacial traps at the perovskite/ZnO NPs interface,23 and the worse contact at perovskite/ZnO NPs interface. These could cause charge accumulation at the perovskite/ZnO NPs interface and thus leads to poor performance and severe hysteresis in the corresponding device.23,33,40
| Sample | τ1 [ns] | Frac. [%] | τ2 [ns] | Frac. [%] | Average [ns] |
|---|---|---|---|---|---|
| MAPbI3 | 10.0 | 4.2 | 58.9 | 95.8 | 56.8 |
| MAPbI3/ZnO | 9.3 | 34.0 | 34.0 | 66.0 | 25.6 |
| MAPbI3/PCBM | 2.3 | 61.5 | 10.4 | 38.5 | 5.4 |
| MAPbI3/PCBM/ZnO | 1.6 | 68.5 | 13.4 | 31.5 | 5.3 |
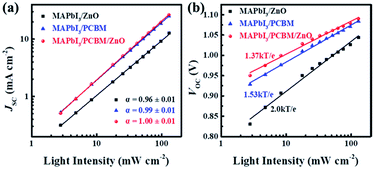
The recombination kinetics were also studied carefully by measuring JSC and VOC at various light intensities (I) from 130 to 2.8 mW cm−2 (Fig. 4a and b). A power law dependence of JSC upon illumination intensity is generally expressed as JSC ∝ Iα, where I is the light intensity and α is the exponential factor. At short circuit condition, the bimolecular recombination should be minimum (α ≈ 1) for maximum carrier sweep out. Any deviation from α ≈ 1 implies bimolecular recombination.41–43 Fig. 4a shows that JSC ∝ Iα, where α = 0.96 ± 0.01 for the device using ZnO NPs as ETL while α ≈ 1 for both devices using PC61BM or PC61BM/ZnO NPs as ETLs, indicating weak bimolecular recombination at short-circuit condition in the latter two types of devices.43 For the MAPbI3/ZnO NPs device, the lower α could be attributed to the bimolecular recombination during sweep-out.41,42 At open-circuit conditions, the current is zero, all carriers recombine within the cell. Thus, recombination studies near open circuit are particularly sensitive to the details of the recombination mechanism.43 Fig. 4b shows VOC varies logarithmically (ln(I)) with light intensity. The slopes for device using ZnO NPs, PC61BM and PC61BM/ZnO NPs as ETLs are 2.0kT/e, 1.53kT/e and 1.37kT/e respectively, where k is the Boltzmann constant, T is absolute temperature, and e is elementary charge. In principle, the slope of VOC versus light intensity will be equal to kT/e if device without trapping of charge carriers or governed by bimolecular recombination, which refers to the recombination of free electrons and holes in the photoactive layer.20,41,43 The slope of ZnO NPs based device is 2.0kT/e, implying that the monomolecular recombination (Shockley–Read–Hall) recombination through trap states or recombination centers is dominant even at open circuit, which leading to the reduced VOC and severe hysteresis.36,44 In the MAPbI3/PC61BM and MAPbI3/PC61BM/ZnO NPs devices, the slopes indicate that recombination at open circuit is a combination of monomolecular and bimolecular process in both cases and the smaller slopes imply reduced SRH recombination, which contribute to the negligible hysteresis and high device performance. The smallest slope with PC61BM/ZnO NPs bilayer ETLs was also attributed the better contact at ZnO/Al interface except for MAPbI3/PCBM interface because the robust ZnO NPs film can prevent the Al diffusing into perovskites.23 The reduced recombination at both interfaces renders the highest VOC and performance of this type device.
3 Conclusions
We demonstrated that the insufficient charge transfer from MAPbI3 to ZnO NPs and significant interface trap-states lead to the poor performance and severe hysteresis of PSC with MAPbI3/ZnO NPs heterojunction, but the device shows super stability in air. While the device based on MAPbI3/PC61BM heterojunction shows high PCE and negligible hysteresis due to the efficient charge transfer from MAPbI3 to PC61BM and less recombination at the interface, however the device show very poor stability in air. When PC61BM/ZnO NPs bilayer ETLs were used with a device structure of ITO/PTAA/MAPbI3/PC61BM/ZnO NPs/Al, high efficient PSCs with PCE as high as 17.2% can be achieved with decent stability. Our study also showed the possibility of obtaining highly efficient perovskite/metal oxide NPs heterojunction solar cells by interface engineering without high cost PC61BM.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51602234) and the National Science Foundation for Post-doctoral Scientists of China (2015M580512).References
- W. Zhang, G. E. Eperon and H. J. Snaith, Nat. Energy, 2016, 1, 16048 CrossRef CAS.
- C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2016, 28, 5778–5793 CrossRef CAS PubMed.
- N.-G. Park, M. Grätzel, T. Miyasaka, K. Zhu and K. Emery, Nat. Energy, 2016, 1, 16152 CrossRef CAS.
- B. G. Zhao, L. Zhu, Y. L. Zhao, Y. Yang, J. Song, X. Q. Gu, Z. Xing and Y. H. Qiang, J. Mater. Sci.: Mater. Electron., 2016, 27, 10869–10876 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- F. Z. Huang, Y. Dkhissi, W. C. Huang, M. D. Xiao, I. Benesperi, S. Rubanov, Y. Zhu, X. F. Lin, L. C. Jiang, Y. C. Zhou, A. Gray-Weale, J. Etheridge, C. R. McNeill, R. A. Caruso, U. Bach, L. Spiccia and Y. B. Cheng, Nano Energy, 2014, 10, 10–18 CrossRef CAS.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- M. Xiao, F. Huang, W. Huang, Y. Dkhissi, Y. Zhu, J. Etheridge, A. Gray-Weale, U. Bach, Y. B. Cheng and L. Spiccia, Angew. Chem., Int. Ed., 2014, 53, 9898–9903 CrossRef CAS PubMed.
- X. Li, D. Bi, C. Yi, J. D. Decoppet, J. Luo, S. M. Zakeeruddin, A. Hagfeldt and M. Gratzel, Science, 2016, 353, 58–62 CrossRef CAS PubMed.
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Science, 2016, 354, 206–209 CrossRef CAS PubMed.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Hörantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, Science, 2016, 351, 151–155 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- T. Jesper Jacobsson, J.-P. Correa-Baena, M. Pazoki, M. Saliba, K. Schenk, M. Gratzel and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 1706–1724 CAS.
- T. Ye, S.-L. Lim, X. Li, M. Petrović, X. Wang, C. Jiang, W.-P. Goh, C. Vijila and S. Ramakrishna, Sol. Energy Mater. Sol. Cells, 2018, 175, 111–117 CrossRef CAS.
- H. J. Snaith, J. Phys. Chem. Lett., 2013, 4, 3623–3630 CrossRef CAS.
- S. Gamliel and L. Etgar, RSC Adv., 2014, 4, 29012–29021 RSC.
- M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J. P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi, K. H. Dahmen, F. De Angelis, A. Abate, A. Hagfeldt, G. Pozzi, M. Graetzel and M. K. Nazeeruddin, Nat. Energy, 2016, 1, 15017 CrossRef CAS.
- Z. Zhu, J. Q. Xu, C. C. Chueh, H. Liu, Z. Li, X. Li, H. Chen and A. K. Jen, Adv. Mater., 2016, 28, 10786–10793 CrossRef CAS PubMed.
- H. Zhang, H. Wang, W. Chen and A. K. Jen, Adv. Mater., 2017, 29, 1604984 CrossRef PubMed.
- W. Chen, Y. Wu, Y. Yue, J. Liu, W. Zhang, X. Yang, H. Chen, E. Bi, I. Ashraful, M. Gratzel and L. Han, Science, 2015, 350, 944–948 CrossRef CAS PubMed.
- https://www.nrel.gov/pv/assets/images/efficiency-chart.png.
- J. You, L. Meng, T. B. Song, T. F. Guo, Y. M. Yang, W. H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. De Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75–81 CrossRef CAS PubMed.
- D. Wang, M. Wright, N. K. Elumalai and A. Uddin, Sol. Energy Mater. Sol. Cells, 2016, 147, 255–275 CrossRef CAS.
- D. Li, P. Liao, X. Shai, W. Huang, S. Liu, H. Li, Y. Shen and M. Wang, RSC Adv., 2016, 6, 89356–89366 RSC.
- H. Choi, S. Park, M. S. Kang and J. Ko, Chem. Commun., 2015, 51, 15506–15509 RSC.
- S. Ye, W. Sun, Y. Li, W. Yan, H. Peng, Z. Bian, Z. Liu and C. Huang, Nano Lett., 2015, 15, 3723–3728 CrossRef CAS PubMed.
- J. A. Christians, R. C. M. Fung and P. V. Kamat, J. Am. Chem. Soc., 2014, 136, 758–764 CrossRef CAS PubMed.
- J. W. Jung, C.-C. Chueh and A. K. Y. Jen, Adv. Mater., 2015, 27, 7874–7880 CrossRef CAS PubMed.
- S. Weber, T. Rath, J. Mangalam, B. Kunert, A. M. Coclite, M. Bauch, T. Dimopoulos and G. Trimmel, J. Mater. Sci.: Mater. Electron., 2018, 29, 1847–1855 CrossRef CAS.
- D. Liu and T. L. Kelly, Nat. Photonics, 2014, 8, 133–138 CrossRef CAS.
- J. Duan, Q. Xiong, H. Wang, J. Zhang and J. Hu, J. Mater. Sci.: Mater. Electron., 2017, 28, 60–66 CrossRef CAS.
- Q. Jiang, L. Zhang, H. Wang, X. Yang, J. Meng, H. Liu, Z. Yin, J. Wu, X. Zhang and J. You, Nat. Energy, 2016, 2, 16177 CrossRef.
- Y. Li, J. Zhu, Y. Huang, F. Liu, M. Lv, S. Chen, L. Hu, J. Tang, J. Yao and S. Dai, RSC Adv., 2015, 5, 28424–28429 RSC.
- D. Bi, C. Yi, J. Luo, J.-D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Nat. Energy, 2016, 1, 16142 CrossRef CAS.
- C. Bi, Q. Wang, Y. Shao, Y. Yuan, Z. Xiao and J. Huang, Nat. Commun., 2015, 6, 7747 CrossRef CAS PubMed.
- Y. Shao, Y. Yuan and J. Huang, Nat. Energy, 2016, 1, 15001 CrossRef CAS.
- W. Fu, J. Yan, Z. Zhang, T. Ye, Y. Liu, J. Wu, J. Yao, C.-Z. Li, H. Li and H. Chen, Sol. Energy Mater. Sol. Cells, 2016, 155, 331–340 CrossRef CAS.
- L. Qian, J. Yang, R. Zhou, A. Tang, Y. Zheng, T.-K. Tseng, D. Bera, J. Xue and P. H. Holloway, J. Mater. Chem., 2011, 21, 3814–3817 RSC.
- H. Li, W. Shi, W. Huang, E. P. Yao, J. Han, Z. Chen, S. Liu, Y. Shen, M. Wang and Y. Yang, Nano Lett., 2017, 17, 2328–2335 CrossRef CAS PubMed.
- A. K. Kyaw, D. H. Wang, D. Wynands, J. Zhang, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Nano Lett., 2013, 13, 3796–3801 CrossRef CAS PubMed.
- V. Gupta, A. K. Kyaw, D. H. Wang, S. Chand, G. C. Bazan and A. J. Heeger, Sci. Rep., 2013, 3, 1965 CrossRef PubMed.
- S. R. Cowan, A. Roy and A. J. Heeger, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 245207 CrossRef.
- Y. Shao, Z. Xiao, C. Bi, Y. Yuan and J. Huang, Nat. Commun., 2014, 5, 5784 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |