DOI:
10.1039/C7RA13207G
(Paper)
RSC Adv., 2018,
8, 5702-5713
Green synthesis of new pyrrolidine-fused spirooxindoles via three-component domino reaction in EtOH/H2O†
Received
10th December 2017
, Accepted 19th January 2018
First published on 2nd February 2018
Abstract
An efficient, green and sustainable approach for the synthesis of novel polycyclic pyrrolidine-fused spirooxindole compounds was developed. The synthesis included a one-pot, three-component, domino reaction of (E)-3-(2-nitrovinyl)-indoles, isatins and chiral polycyclic α-amino acids under catalyst-free conditions at room temperature in EtOH–H2O. The salient features of this methodology are eco-friendliness, high yields and the ease of obtaining target compounds without the involvement of toxic solvents and column chromatography. These novel polycyclic pyrrolidine-fused spirooxindoles provide a collection of structurally diverse compounds that show promise for future bioassays and medical treatments.
Introduction
To adhere to the principles of “green chemistry” and “benign by design”,1 there is an immediate need to develop efficient chemical synthetic strategies that are environmentally friendly, sustainable, and atom-economical. To maximize reaction sustainability and safety,2 development of novel synthetic strategies that involve greener reaction media have resulted in sustainable pathways for chemical synthesis.3 An alcohol–water solution, as a nontoxic, inexpensive and widely available solvent, has been shown to not only accelerate the rate of organic reactions, even for water-insoluble reactants, but can also simplify purification operations to standard filtration or recrystallization.4 Recently, multicomponent reactions (MCRs)5 have emerged as efficient tools for synthesizing functionally and chemically diverse novel heterocyclic compounds in organic and medicinal chemistry. Of immediate need is the exploration of new MCRs in alcohol–water solution for the organic synthesis of novel compounds for drug discovery and the promotion of green chemistry.6
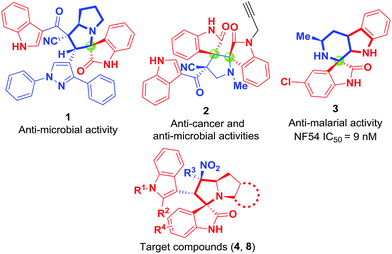
Spirooxindole ring systems are frequently encountered in natural alkaloids7 and are often considered as attractive templates for drug discovery.8 Natural and synthetic alkaloids containing an indole moiety exhibit a wide spectrum of biological activities including anti-tumor,9 anti-microbial,10 anti-malarial,11 anti-diabetic,12 anti-tubercular,13 anti-HIV,14 anti-oxidant15 and other biological activities.16 Their remarkable pharmacological activity and unique molecular architecture have made spirooxindoles, and their derivatives, attractive synthetic targets.17 In addition, functionalized polycyclic N-fused-pyrrolidines have also been shown to have a wide spectrum of biological activities that include anti-tumor, anti-HIV and other anti-viral disease activities.18 Spiro-fused cyclic frameworks are known to be the central skeletons of numerous alkaloids and pharmacologically important compounds with various types of bioactivities.19 The two organic frameworks are regarded as templates for drug discovery and scaffolds for combinatorial libraries, respectively,20 the presence of two or more different heterocyclic moieties in a single molecule could remarkably enhance biological activity.21 To date, some representative molecules (1–3) have been designed and synthesized, which exhibit outstanding pharmacological activities22 (Fig. 1). Here we speculate that the integration of spirooxindole, polycyclic N-fused-pyrrolidines and spiro-fused cyclic frameworks into a molecule may result in the discovery of new drug candidates. Polycyclic pyrrolidine-fused spirooxindoles may exhibit a wide range of useful pharmacological properties and biological activities in combination with the pharmacological activity of spirooxindole, N-fused-pyrrolidines and spiro-fused cyclic frameworks.
 |
| | Fig. 1 Selected representative biologically active heterocycles and target compounds that exhibit pharmacological activities. | |
Despite recent attention on the synthesis of novel spirocyclic oxindoles, the development of green and sustainable methods to access spirooxindoles remains challenging. Only a limited number of methodologies have been reported for the synthesis of the above-mentioned compounds,23 and most require toxic organic solvents, harsh reaction conditions, expensive catalysts or involve complex separation processes. The development of a milder, more eco-friendly and efficient method for synthesizing combined novel spirooxindoles under aqueous solvent conditions is necessary. Here, we described a relatively easy and green method for the synthesis of novel polycyclic pyrrolidine-fused spirooxindole derivatives (4, 8) (Fig. 1) using a catalyst-free, one-pot three-component reaction of (E)-3-(2-nitrovinyl)-indoles (5), isatins (6) and polycyclic chiral α-amino acids (7) in alcohol–water solution at room temperature. To the best of our knowledge, this is the first green synthesis of a heterocyclic compound 4.
Results and discussion
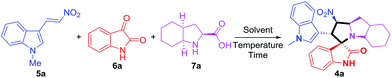
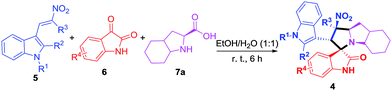
In this study, we report a green method for the synthesis of novel polycyclic pyrrolidine-fused spirooxindole derivatives (4) via one-pot three-component domino reaction of (E)-3-(2-nitrovinyl)-1H-indoles (5), isatins (6) and polycyclic chiral α-amino acids (7) using an alcohol–water solution as a green medium at room temperature.
To establish the feasibility of this strategy as well as to optimize the reaction conditions, the three-component reaction of (E)-1-methyl-3-(2-nitrovinyl)-1H-indole (5a, 1.0 mmol), isatin (6a, 1.1 mmol) and (2S,3aS,7aS)-octahydro-1H-indole-2-carboxylic acid (7a, 1.2 mmol) was selected as the model reaction. Initially, the model reaction was performed in a range of organic solvents at room temperature for 6 h of stirring (Table 1, entries 1–14). The model reaction barely proceeded when acetone, acetonitrile, toluene and diethyl ether were used as reaction solvents, respectively (Table 1, entries 1–4). Conversely, the model reaction proceeded faster with moderate (38–81%) to high (91–92%) yields when other (non-)low-polar solvents (CH2Cl2, CHCl3) and polar solvents (THF, 1,4-dioxane, DMSO, DMF, iso-propanol, glycerol, MeOH and EtOH) were screened (Table 1, entries 5–14): the highest yield was achieved when EtOH was used as a solvent (Table 1, entry 14, 92% yield). Interestingly, the desired product was also obtained in water medium (Table 1, entry 15) although the yield was only 21%. The low yield in water medium could be attributed to the poor dissolution of raw materials since almost all the unreacted starting material could been recycled and reused.
Table 1 Optimization of the reaction conditions for the model reactiona
Based on a comprehensive assessment of these results, we chose an ethanol–water solution as the optimal reaction solvent, which we then optimized to further explore a more eco-friendly synthetic condition (Table 1, entries 16 and 17). Among the different proportions of EtOH/H2O solvents surveyed, EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be the most suitable solvent for the model reaction (94% yield, Table 1, entry 16). To maximize the product yield, we then tested the best reaction temperature (Table 1, entries 16, 18–19). Yields were not significantly different when the reaction temperature was increased from room temperature to 50 °C or to reflux (94% vs. 95% vs. 95% yields, respectively, Table 1, entries 16, 18–19): room temperature was the ideal reaction temperature for the synthesis of polycyclic pyrrolidine-fused spirooxindole derivative 4a (94% yield). There was no significant increase in yield when the reaction time was increased from 6 h to 12 h (95% vs. 94% yields, respectively, entries 16, 20). It is worth mentioning that the highly purified target compound could be obtained by filtering the reaction precipitates and then washing with EtOH/H2O (1
1) was found to be the most suitable solvent for the model reaction (94% yield, Table 1, entry 16). To maximize the product yield, we then tested the best reaction temperature (Table 1, entries 16, 18–19). Yields were not significantly different when the reaction temperature was increased from room temperature to 50 °C or to reflux (94% vs. 95% vs. 95% yields, respectively, Table 1, entries 16, 18–19): room temperature was the ideal reaction temperature for the synthesis of polycyclic pyrrolidine-fused spirooxindole derivative 4a (94% yield). There was no significant increase in yield when the reaction time was increased from 6 h to 12 h (95% vs. 94% yields, respectively, entries 16, 20). It is worth mentioning that the highly purified target compound could be obtained by filtering the reaction precipitates and then washing with EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 2–3 times in the absence of traditional purification techniques such as column chromatography or recrystallization. Overall, the best reaction conditions for synthesizing polycyclic pyrrolidine-fused spirooxindole compound 4a are achieved by employing (E)-1-methyl-3-(2-nitrovinyl)-1H-indole (5a, 1.0 mmol), isatin (6a, 1.1 mmol) and (2S,3aS,7aS)-octahydroindole-2-carboxylic acid (7a, 1.2 mmol) in EtOH/H2O (1
1) for 2–3 times in the absence of traditional purification techniques such as column chromatography or recrystallization. Overall, the best reaction conditions for synthesizing polycyclic pyrrolidine-fused spirooxindole compound 4a are achieved by employing (E)-1-methyl-3-(2-nitrovinyl)-1H-indole (5a, 1.0 mmol), isatin (6a, 1.1 mmol) and (2S,3aS,7aS)-octahydroindole-2-carboxylic acid (7a, 1.2 mmol) in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent at room temperature for 6 h.
1) solvent at room temperature for 6 h.
To explore the scope of the model reaction, we substituted various (E)-3-(2-nitrovinyl)-indoles (5) (Fig. 2) and isatin derivatives (6) (Fig. 2) as well as (2S,3aS,7aS)-octahydro-1H-indole-2-carboxylic acid (7a) under optimal reaction conditions. Results indicated that the desired polycyclic pyrrolidine-fused spirooxindole compounds could be obtained in relatively high yields (Table 2) from a diverse set of substrates. As shown in Table 2, (E)-3-(2-nitrovinyl)-indoles (5) bearing diverse functional groups, such as H, CH3 or Ph group were suitable for the reaction, respectively. For the isatin derivatives (6), the aromatic ring bearing either electron-donating (CH3, OCH3) or electron-withdrawing functional groups (F, Cl, Br), and substitution patterns (5-substitution, 6-substitution and 7-substitution) could form the target products 4 (4a–4w) with high yields (86–95%).
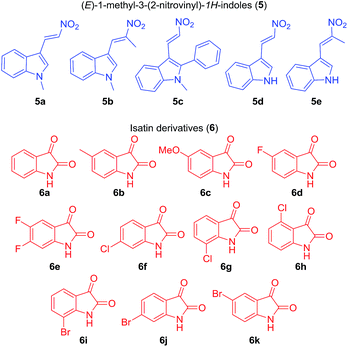 |
| | Fig. 2 The diversity of reagents (5 and 6). | |
Table 2 The synthesis of polycyclic pyrrolidine-fused spirooxindole derivatives 4a
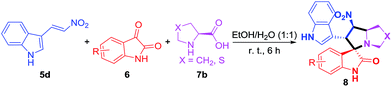
To further explore the scope of the procedure reported in Table 2, the methodology was evaluated by using (E)-3-(2-nitrovinyl)-1H-indole (5d), isatin derivatives (6) and chiral α-amino acids (7b) under similar conditions. The reactions proceeded smoothly and the desired polycyclic pyrrolidine-fused spirooxindole compounds (8) from a diverse set of substrates were obtained (Table 3). Isatins with electron-withdrawing groups as well as electron-donating substituents underwent this one-pot conversion to give the corresponding spirooxindoles with high yields (88–96%).
Table 3 The synthesis of polycyclic pyrrolidine-fused spirooxindole derivatives 8a
A plausible reaction mechanism was proposed (Scheme 1) based on our previous work and previous reports.24 The pyrrolidine functionality activates the carbonyl of the isatins through the formation of an enamine intermediate. Accompanying the loss of one H2O and CO2 molecule, a carbanion is produced (transition state I). Subsequently, two nucleophilic carbons then add to the corresponding electron deficient carbons of the dipolarophile during the cycloaddition via a 1,3-cycloaddition reaction (transition state II), which leads to the formation of compounds 4 and 8. From a regioselectivity perspective, the steric bulk of compounds 5 and the stability of the transition state II may explain the configuration of compounds 4 and 8. Some other typical literatures25 could be taken as a support of the regioselectivity of compounds 4 and 8. The structure deduced from NMR data and the reaction mechanism was further confirmed by X-ray analysis of a single crystal of 4c (Fig. 3, CCDC: 1817780†). Of note, in Fig. 3 we only presented one molecule of compound 4c while in the single crystal structure the minimum asymmetric units contain two identical molecules of compound 4c.
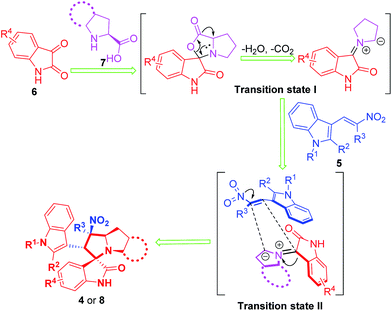 |
| | Scheme 1 Proposed reaction mechanism for the synthesis of 4 and 8. | |
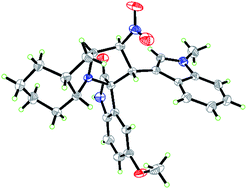 |
| | Fig. 3 Single crystal X-ray diffraction study of compound 4c. | |
Conclusions
In this study, we developed an efficient, green and sustainable approach for the construction of multiple new C–C bonds, C–N bonds and polycyclic pyrrolidine-fused structure, which resulted in the synthesis of 35 novel polycyclic pyrrolidine-fused spirooxindole compounds. This synthetic method involves a one-pot, three-component domino reaction of (E)-3-(2-nitrovinyl)-indoles, isatins and polycyclic chiral α-amino acids under a catalyst-free condition at room temperature in an EtOH–H2O solution. The salient features of this methodology are environmental sustainability, high yields, ease of product purification, and the lack of toxic solvents or column chromatography during synthesis. These novel polycyclic pyrrolidine-fused spirooxindoles provide a collection of promising compounds with structural diversity for future bioassays and medical treatments. In addition, the nitro group can be easily transformed into amines, amides, sulfamides, nitrile oxides and various useful functional groups for the optimization and enhancement of pharmacological activities.
Experimental
General information
Reagents and materials were of the highest commercial grade and were used without further purification. NMR spectra were recorded on a Bruker DRX 400 (1H: 400 MHz, 13C: 100 MHz), DRX 500 (1H: 500 MHz, 13C: 125 MHz) or DRX 600 (1H: 600 MHz, 13C: 150 MHz) with TMS as the internal standard. Chemical shifts (δ) were expressed in ppm, J values were given in Hz, and deuterated DMSO-D6 was used as a solvent. IR spectra were recorded on a FT-IR Thermo Nicolet Avatar 360 using KBr pellet. The mass spectroscopic data were obtained from an Agilent 1100 LC/MSD Trap LC-mass spectrometer. Melting points were determined with an XT-4A melting-point apparatus. The reactions were monitored by thin layer chromatography (TLC) with silica gel GF254, and all compounds were visualized by UV and sprayed with H2SO4 (10%) in ethanol, followed by heating. Suitable single crystal was selected. Data collections were performed by graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). Data reduction, cell refinement and experimental absorption correction were performed with the software package of Agilent Gemini Ultra CrysAlisPro (Ver 1.171.35.11). The structures were solved by direct methods and refined against F2 by full-matrix least-squares. All non-hydrogen atoms were refined anisotropically. All calculations were carried out in SHELXTL ver 6.2 and Olex2 ver 1.2.9.
General procedure for the synthesis of compounds 526
Benzene (18 μL, 0.2 mmol) was titrated into a stirred solution of indole-3-carboxaldehyde (5 mmol) and AcONH4 (385 mg, 5 mmol) in nitromethane (15 mL). The mixture was stirred at reflux for 1–4 h. After the starting aldehyde was completely consumed (monitored by TLC), the reaction mixture was cooled to 0 °C. The reaction was quenched with water and extracted with ethyl acetate (3 × 80 mL). The combined organic layers were washed with saturated brine solution (50 mL), followed by drying with Na2SO4 and evaporating in vacuo. The crude product was purified by recrystallization to give the pure corresponding (E)-3-(2-nitrovinyl)-indoles (5).
(E)-1-Methyl-3-(2-nitrovinyl)-1H-indole (5a). Yellow solid; 95% yield; mp 165–167 °C; IR (KBr) 746, 791, 951, 1084, 1252, 1308, 1491, 1618, 2374 cm−1; HRMS (EI) calcd for C11H11N2O2 [M + H]+ 203.2205, found 203.2208. 1H NMR (600 MHz, DMSO-D6): δ 8.35 (d, J = 13.8 Hz, 1H, CH), 8.20 (s, 1H, ArH), 8.00 (s, 1H, ArH), 7.97 (d, J = 7.2 Hz, 1H, ArH), 7.58 (d, J = 8.4 Hz, 1H, CH), 7.36–7.33 (m, 1H, ArH), 7.29 (t, J = 15.0 Hz, 1H, ArH), 3.86 (s, 3H, NCH3); 13C NMR (150 MHz, DMSO): δ 139.8, 138.7, 134.5, 131.5, 125.5, 123.8, 122.6, 121.0, 111.7, 107.6, 33.8.
(E)-1-Methyl-3-(2-nitroprop-1-en-1-yl)-1H-indole (5b). Yellow solid; 96% yield; mp 136–138 °C; IR (KBr) 754, 916, 972, 1126, 1236, 1283, 1470, 1531, 1634, 3124, 3297 cm−1; HRMS (EI) calcd for C12H13N2O2 [M + H]+ 217.0972, found 217.0977. 1H NMR (600 MHz, DMSO-D6): δ 8.41 (s, 1H, ArH), 8.01 (s, 1H, CH), 7.82 (d, J = 7.8 Hz, 1H, ArH), 7.54 (d, J = 7.8 Hz, 1H, ArH), 7.53–7.30 (m, 1H, ArH), 7.25–7.23 (m, 1H, ArH), 3.89 (s, 3H, NCH3), 2.45 (s, 3H, CH3); 13C NMR (150 MHz, DMSO): δ 141.2, 137.2, 134.1, 128.5, 126.5, 123.5, 121.8, 118.8, 111.2, 107.7, 33.7, 15.0.
(E)-1-Methyl-3-(2-nitrovinyl)-2-phenyl-1H-indole (5c). Yellow solid; 96% yield; mp 156–158 °C; IR (KBr) 750, 806, 982, 1070, 1263, 1304, 1466, 1601 cm−1; HRMS (EI) calcd for C17H15N2O2 [M + H]+ 279.1128, found 279.1124. 1H NMR (600 MHz, DMSO-D6): δ 8.04 (d, J = 7.8 Hz, 1H, ArH), 8.01 (d, J = 13.2 Hz, 1H, CH), 7.91 (d, J = 13.8 Hz, 1H, CH), 7.70 (s, 1H, ArH), 7.69–7.66 (m, 3H, ArH), 7.58–7.57 (m, 2H, ArH), 7.43 (t, J = 15.0 Hz, 1H, ArH), 7.36 (t, J = 15.0 Hz, 1H, ArH), 3.69 (s, 3H, NCH3);13C NMR (150 MHz, DMSO): δ 150.1, 138.5, 134.2, 131.7, 131.3, 131.3, 130.5, 129.4, 129.4, 128.9, 124.8, 124.4, 123.3, 121.3, 112.0, 106.5, 32.1.
(E)-3-(2-Nitrovinyl)-1H-indole (5d). Yellow solid; 95% yield; mp 171–173 °C; IR (KBr) 642, 754, 797, 974, 1103, 1319, 1425, 1470, 1516, 1618, 2367, 3402, 3746 cm−1; HRMS (EI) calcd for C10H8NaN2O2 [M + Na]+ 211.0478, found 211.0481. 1H NMR (600 MHz, DMSO-D6): δ 12.25 (s, 1H, NH), 8.42 (d, J = 13.2 Hz, 1H, CH), 8.25 (s, 1H, ArH), 8.02 (d, J = 13.8 Hz, 1H, CH), 7.96 (d, J = 7.8 Hz, 1H, ArH), 7.54 (d, J = 3.9 Hz, 1H, ArH), 7.30–7.27 (m, 1H, ArH), 7.26–7.23 (m, 1H, ArH); 13C NMR (150 MHz, DMSO): δ 138.2, 136.7, 135.2, 131.6, 125.1, 123.8, 122.4, 120.9, 113.3, 108.7.
(E)-3-(2-Nitroprop-1-en-1-yl)-1H-indole (5e). Yellow solid; 94% yield; mp 195–197 °C; IR (KBr) 750, 972, 1105, 1224, 1267, 1420, 1630, 3428 cm−1; HRMS (EI) calcd for C11H10NaN2O2 [M + Na]+ 225.0634, found 225.0630. 1H NMR (600 MHz, DMSO-D6): δ 12.19 (s, 1H, NH), 8.47 (s, 1H, ArH), 8.00 (s, 1H, CH), 7.83 (d, J = 7.8 Hz, 1H, ArH), 7.52 (d, J = 7.8 Hz, 1H, ArH), 7.27–7.24 (m, 1H, ArH), 7.21–7.19 (m, 1H, ArH), 2.49 (s, 3H, CH3); 13C NMR (150 MHz, DMSO): δ 141.5, 136.7, 130.5, 128.0, 127.1, 123.4, 121.5, 118.7, 112.8, 108.7, 15.1.
General procedure for the synthesis of compounds 4 and 8
A mixture of (E)-3-(2-nitrovinyl)-indoles (5) (1 mmol), isatins (6) (1.1 mmol) and chiral compounds 7 (1.2 mmol) in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mL) was stirred at reflux for 6 h at room temperature. The resulting precipitate was collected by filtration and washed with cold EtOH/H2O (v/v = 1
1 v/v, 10 mL) was stirred at reflux for 6 h at room temperature. The resulting precipitate was collected by filtration and washed with cold EtOH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3–5 mL) for 2–3 times to yield pure products (4 and 8).
1, 3–5 mL) for 2–3 times to yield pure products (4 and 8).
2′-(1-Methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4a). Yellow solid; 94% yield; mp 220–222 °C; IR (KBr) 745, 1188, 1335, 1472, 1547, 1620, 1721, 2918, 3399 cm−1; HRMS (EI) calcd for C27H28NaN4O3 [M + Na]+ 479.2054, 479.2057. 1H NMR (400 MHz, DMSO-D6): δ 9.95 (s, Hz, 1H, NH), 7.75 (d, J = 8.0 Hz, 1H, ArH), 7.41 (d, J = 8.0 Hz, 1H, ArH), 6.98 (d, J = 4.8 Hz, 2H, ArH), 6.85–6.76 (m, 2H, ArH), 6.67 (t, J = 14.8 Hz, 2H, ArH), 6.32 (t, J = 7.6 Hz, 1H, ArH), 6.15 (t, J = 21.2 Hz, 1H, CH), 4.61 (d, J = 11.6 Hz, 1H, CH), 4.48–4.41 (m, 1H, CH), 3.64 (s, 1H, CH), 3.39 (s, 3H, CH3), 1.89 (t, J = 10.4 Hz, 1H, CH), 1.56–1.49 (m, 1H, CH2), 1.33–1.27 (m, 3H, CH2), 1.05–0.99 (m, 2H, CH2), 0.95–0.81 (m, 2H, CH2), 0.76–0.79 (m, 1H, CH2), 0.29–0.32 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 179.5, 143.6, 136.4, 130.0, 128.2, 127.9, 127.3, 125.2, 121.6, 121.6, 119.3, 119.1, 109.9, 109.8, 107.1, 91.7, 73.8, 63.2, 59.0, 43.0, 38.3, 34.2, 32.9, 29.2, 27.3, 24.8, 19.6.
5-Methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4b). Yellow solid; 92% yield; mp 221–223 °C; IR (KBr) 741, 812, 1177, 1211, 1333, 1491, 1537, 1707, 2934, 3482 cm−1; HRMS (EI) calcd for C28H30NaN4O3 [M + Na]+ 493.2210, found 493.2215. 1H NMR (400 MHz, DMSO-D6): δ 10.06 (s, 1H, NH), 7.84 (s, 1H, ArH), 7.63 (d, J = 8.0 Hz, 1H, ArH), 7.23 (d, J = 6.8 Hz, 2H, ArH), 7.03 (t, J = 14.8 Hz, 1H, ArH), 6.93–6.86 (m, 2H, ArH), 6.42 (t, J = 13.2 Hz, 1H, ArH), 6.37 (d, J = 9.6 Hz, 1H, CH), 4.82–4.79 (m, 1H, CH), 4.71–4.65 (m, 1H, CH), 3.88 (s, 1H, CH2), 3.64 (s, 3H, NCH3), 2.30 (s, 3H, CH3), 2.24–2.13 (m, 1H, CH2), 1.79–1.73 (m, 1H, CH2), 1.57–1.53 (m, 3H, CH2), 1.29–1.20 (m, 2H, CH2), 1.17–1.06 (m, 2H, CH2), 0.89–0.94 (m, 1H, CH2), 0.56–0.61 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 179.4, 141.1, 136.4, 130.5, 130.0, 128.1, 128.0, 127.9, 125.2, 121.6, 119.3, 118.9, 110.0, 109.4, 107.1, 91.5, 73.8, 63.2, 59.0, 43.2, 38.3, 34.2, 32.9, 29.2, 27.3, 24.8, 21.1, 19.6.
5-Methoxy-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4c). Yellow solid; 91% yield; mp 219–221 °C; IR (KBr) 745, 814, 1032, 1206, 1333, 1489, 1543, 1714, 2926, 3234, 3406 cm−1; HRMS (EI) calcd for C28H31N4O4 [M + H]+ 487.2340, found 487.2336. 1H NMR (600 MHz, DMSO-D6): δ 9.99 (s, 1H, NH), 7.78–7.74 (m, 1H, ArH), 7.25 (s, 1H, ArH), 7.22 (d, J = 9.0 Hz, 1H, ArH), 7.05–7.02 (m, 1H, ArH), 6.94 (t, J = 7.8 Hz, 1H ArH), 6.92 (d, J = 0.6 Hz, 1H, ArH), 6.65–6.63 (m, 1H, ArH), 6.46 (d, J = 8.4 Hz, 1H, ArH), 6.41–6.37 (m, 1H, CH), 4.89 (d, J = 11.4 Hz, 1H, CH), 4.68–4.66 (m, 1H, CH), 3.94 (d, J = 1.8 Hz, 1H, CH), 3.75 (s, 3H, NCH3), 3.65 (s, 3H, OCH3), 2.15 (d, J = 6.0 Hz, 1H, CH), 1.79 (t, J = 10.2 Hz, 1H, CH2), 1.55–1.52 (m, 3H, CH2), 1.26 (t, J = 13.2 Hz, 1H, CH2), 1.24–1.17 (m, 1H, CH2), 1.07–1.01 (m, 2H, CH2), 0.95 (d, J = 3.0 Hz, 1H, CH2), 0.55 (d, J = 13.8 Hz, 1H, CH2); 13C NMR (150 MHz, DMSO): δ 179.5, 155.1, 136.9, 136.4, 128.3, 127.8, 126.4, 121.6, 119.4, 119.0, 115.5, 114.2, 110.0, 109.9, 107.2, 91.7, 74.2, 63.2, 58.8, 56.4, 42.9, 38.3, 34.2, 32.9, 29.2, 27.3, 24.8, 19.7.
5-Fluoro-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4d). Yellow solid; 95% yield; mp 243–245 °C; IR (KBr) 743, 820, 1177, 1329, 1375, 1489, 1547, 1720, 2938, 3410, 3424 cm−1; HRMS (EI) calcd for C27H27NaFN4O3 [M + Na]+ 497.1959, found 497.1957. 1H NMR (600 MHz, DMSO-D6): δ 10.21 (s, 1H, NH), 8.09 (d, J = 7.8 Hz, 1H, ArH), 7.74 (d, J = 7.8 Hz, 1H, ArH), 7.24 (d, J = 9.0 Hz, 2H, ArH), 7.04 (d, J = 7.2 Hz, 1H, ArH), 6.96–6.92 (m, 2H, ArH), 6.55 (s, 1H, ArH), 6.40 (t, J = 20.4 Hz, 1H, CH), 4.91 (d, J = 11.4 Hz, 1H, CH), 4.71 (d, J = 6.6 Hz, 1H, CH), 3.93 (s, 1H, CH), 3.64 (s, 3H, NCH3), 2.16 (d, J = 3.6 Hz, 1H, CH), 1.81 (d, J = 4.8 Hz, 1H, CH2), 1.55 (s, 3H, CH3), 1.27–1.19 (m, 2H, CH2), 1.09–0.99 (m, 3H, CH2), 0.55 (d, J = 12.6 Hz, 1H, CH2); 13C NMR (150 MHz, DMSO): δ 179.5, 159.0, 157.5, 140.0, 136.4, 128.2, 127.9, 121.7, 119.3, 119.1, 116.3, 116.2, 115.5, 110.4, 110.4, 106.9, 91.3, 74.1, 63.3, 58.8, 43.2, 38.3, 34.2, 32.9, 29.2, 27.3, 24.8, 19.6.
6-Chloro-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4e). Yellow solid; 95% yield; mp 182–184 °C; IR (KBr) 739, 1072, 1126, 1333, 1449, 1543, 1609, 1707, 2936, 3248, 3416, 3503, 3624 cm−1; HRMS (EI) calcd for C27H27NaClN4O3 [M + Na]+ 513.1664, found 513.1668. 1H NMR (600 MHz, DMSO-D6): δ 10.36 (s, 1H, NH), 8.08 (d, J = 7.8 Hz, 1H, CH2), 7.66 (d, J = 7.2 Hz, 1H, CH2), 7.25 (d, J = 12.0 Hz, 2H, CH2), 7.06 (t, J = 14.4 Hz, 1H, CH2), 6.98 (d, J = 7.2 Hz, 1H, CH2), 6.93 (t, J = 14.4 Hz, 1H, CH2), 6.59 (s, 1H, CH2), 6.38 (t, J = 21.0 Hz, 1H, CH), 4.88 (d, J = 11.4 Hz, 1H, CH), 4.69 (d, J = 7.2 Hz, 1H, CH), 3.87 (s, 1H, CH), 3.65 (s, 1H, NCH3), 2.16 (d, J = 5.4 Hz, 1H, CH), 1.80–1.76 (m, 1H, CH2), 1.57–1.52 (m, 3H, CH2), 1.28–1.15 (m, 2H, CH2), 1.09–0.94 (m, 3H, CH2), 0.57–0.54 (m, 1H, CH2); 13C NMR (150 MHz, DMSO): δ 179.4, 145.1, 136.4, 134.4, 128.9, 128.1, 128.0, 124.2, 121.7, 121.3, 119.2, 119.1, 110.0, 110.0, 106.7, 91.3, 73.5, 63.3, 59.0, 49.1, 38.3, 34.2, 32.9, 29.3, 27.2, 24.7, 19.6.
6-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4f). Yellow solid; 96% yield; mp 223–225 °C; IR (KBr) 739, 1067, 1128, 1333, 1447, 1481, 1543, 1607, 1107, 2934, 3258, 3416, 3501, 3622 cm−1; HRMS (EI) calcd for C27H27NaBrN4O3 [M + Na]+ 557.1159, found 557.1164. 1H NMR (600 MHz, DMSO-D6): δ 10.30 (s, 1H, NH), 8.01 (d, J = 6.0 Hz, 1H, ArH), 7.66 (d, J = 7.8 Hz, 1H, ArH), 7.25 (t, J = 19.2 Hz, 2H, ArH), 7.12 (t, J = 8.4 Hz, 1H, ArH), 7.05 (t, J = 15.0 Hz, 1H, ArH), 6.93 (t, J = 14.4 Hz, 1H, ArH), 6.72 (d, J = 1.8 Hz, 1H, ArH), 6.39–6.35 (m, 1H, CH), 4.93 (d, J = 10.8 Hz, 1H, CH), 4.68 (d, J = 6.6 Hz, 1H, CH), 3.92 (s, 1H, CH), 3.65 (s, 3H, CH3), 3.19 (d, J = 4.8 Hz, 1H, CH), 2.16–2.14 (m, 1H, CH2), 1.79–1.76 (m, 1H, CH2), 1.57–1.52 (m, 3H, CH2), 1.25–1.16 (m, 2H, CH2), 1.10–0.98 (m, 3H, CH2); 13C NMR (150 MHz, DMSO): δ 179.3, 145.3, 136.4, 129.2, 128.1, 128.0, 124.6, 124.2, 122.9, 121.7, 119.2, 112.7, 110.0, 106.7, 91.4, 73.6, 63.3, 59.0, 49.1, 43.1, 38.3, 34.2, 32.9, 29.3, 27.2, 24.7, 19.6.
5-Bromo-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4g). Yellow solid; 94% yield; mp 223–225 °C; IR (KBr) 739, 816, 1186, 1331, 1477, 1545, 1616, 1717, 2363, 2930, 3395, 3426, 3624 cm−1; HRMS (EI) calcd for C27H27NaBrN4O3 [M + Na]+ 557.1159, found 557.1157. 1H NMR (600 MHz, DMSO-D6): δ 10.30 (s, 1H, NH), 8.37 (s, 1H, ArH), 7.69 (d, J = 7.2 Hz, 1H, ArH), 7.24 (d, J = 7.2 Hz, 3H, ArH), 7.05 (t, J = 13.2 Hz, 1H, ArH), 6.94 (d, J = 6.6 Hz, 1H, ArH), 6.51 (d, J = 7.8 Hz, 1H, ArH), 6.38 (t, J = 20.4 Hz, 1H, CH), 4.93 (d, J = 10.8 Hz, 1H, CH), 4.68 (d, J = 6.6 Hz, 1H, CH), 3.92 (s, 1H, CH), 3.65 (s, 3H, CH3), 2.16 (d, J = 4.2 Hz, 1H, CH), 1.83 (d, J = 4.8 Hz, 1H, CH2), 1.54 (d, J = 6.0 Hz, 3H, CH2), 1.25–1.16 (m, 2H, CH2), 1.10–0.98 (m, 3H, CH2), 0.56 (d, J = 13.2 Hz, 1H, CH2); 13C NMR (150 MHz, DMSO): δ 179.1, 142.9, 136.5, 132.6, 130.4, 128.1, 127.8, 127.7, 121.7, 119.4, 119.0, 113.7, 111.6, 110.0, 106.8, 91.0, 74.0, 63.4, 58.8, 43.2, 38.3, 34.2, 32.9, 29.3, 27.2, 24.8, 19.6.
5-Methoxy-1′-methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4h). Yellow solid; 87% yield; mp 215–217 °C; IR (KBr) 741, 1042, 1120, 1489, 1543, 1732, 2365, 2930, 3304 cm−1; HRMS (EI) calcd for C29H33N4O4 [M + H]+ 501.2496, found 501.2499. 1H NMR (500 MHz, DMSO-D6): δ 10.30 (s, 1H, NH), 7.84 (d, J = 7.5 Hz, 1H, ArH), 7.53 (s, 1H, ArH), 7.39 (d, J = 8.5 Hz, 1H, ArH), 7.17–7.11 (m, 1H, ArH), 7.09 (d, J = 7.5 Hz, 2H, ArH), 6.89–6.86 (m, 1H, ArH), 6.74 (d, J = 8.5 Hz, 1H, ArH), 4.52 (d, J = 11.0 Hz, 1H, CH), 4.26–4.25 (m, 1H, CH), 3.80 (s, 3H, NCH3), 3.78 (s, 3H, OCH3), 3.17 (d, J = 3.5 Hz, 1H, CH), 2.09–2.06 (m, 1H, CH2), 1.89 (s, 3H, CH3), 1.76–1.75 (m, 1H, CH2), 1.56–1.52 (m, 2H, CH2), 1.46–1.36 (m, 3H, CH2), 1.19–1.12 (m, 2H, CH2), 1.03–0.96 (m, 2H, CH2); 13C NMR (125 MHz, DMSO): δ 177.5, 154.4, 136.8, 136.3, 130.0, 128.6, 125.4, 121.5, 120.1, 119.3, 115.1, 114.3, 110.6, 110.1, 106.8, 101.4, 74.7, 66.7, 58.0, 56.1, 48.6, 40.4, 37.4, 33.0, 27.8, 27.6, 24.7, 20.2, 19.7.
5-Fluoro-1′-methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4i). Yellow solid; 92% yield; mp 170–172 °C; IR (KBr) 741, 818, 1182, 1333, 1487, 1537, 1717, 2930, 3393, 3426 cm−1; HRMS (EI) calcd for C28H28NaFN4O3 [M + Na]+ 511.2116, found 511.2120. 1H NMR (500 MHz, DMSO-D6): δ 10.31 (s, 1H, NH), 7.76–7.73 (m, 1H, ArH), 7.67 (d, J = 8.0 Hz, 1H, ArH), 7.27 (d, J = 8.0 Hz, 1H, ArH), 7.11 (s, 1H, ArH), 7.08–7.01 (m, 1H, ArH), 6.99–6.91 (m, 1H, ArH), 6.90–6.88 (m, 1H, ArH), 6.60–6.57 (m, 1H, ArH), 4.44–4.41 (m, 1H, CH), 3.74 (d, J = 3.5 Hz, 1H, CH), 3.69 (s, 3H, NCH3), 2.13–2.09 (m, 1H, CH), 2.08 (s, 3H, CH3), 1.73–1.69 (m, 1H, CH2), 1.59–1.55 (m, 1H, CH2), 1.52–1.50 (m, 2H, CH2), 1.27–1.24 (m, 1H, CH2), 1.19–1.18 (m, 1H, CH2), 1.07–1.02 (m, 2H, CH2), 1.00–0.91 (m, 1H, CH2), 0.55–0.52 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 180.3, 159.1, 157.2, 139.6, 136.0, 129.2, 128.9, 128.4, 121.7, 119.4, 118.7, 116.4, 114.2, 110.4, 110.4, 104.9, 99.8, 74.4, 73.9, 58.1, 50.3, 38.9, 36.3, 33.0, 28.7, 27.5, 25.9, 24.5, 19.4.
5-Chloro-1′-methyl-2′-(1-methyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4j). Yellow solid; 92% yield; mp 183–185 °C; IR (KBr) 743, 820, 1194, 1474, 1543, 1614, 1738, 2859, 2928, 3308 cm−1; HRMS (EI) calcd for C28H29NaClN4O3 [M + Na]+ 527.1820, found 527.1825. 1H NMR (500 MHz, DMSO-D6): δ 10.64 (s, 1H, NH), 7.88 (d, J = 7.5 Hz, 1H, ArH), 7.73 (s, 1H, ArH), 7.53 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.40 (d, J = 8.0 Hz, 1H, ArH), 7.16 (t, J = 14.5 Hz, 1H, ArH), 7.10 (t, J = 14.5 Hz, 1H, ArH), 6.80 (d, J = 8.0 Hz, 1H, ArH), 4.52 (d, J = 10.5 Hz, 1H, CH), 4.32–4.27 (m, 1H, CH), 3.78 (s, 3H, NCH3), 3.16 (s, 1H, CH), 2.08 (d, J = 5.0 Hz, 1H, CH), 1.90 (d, J = 15.5 Hz, 3H, CH3), 1.54–1.51 (m, 3H, CH2), 1.42–1.35 (m, 3H, CH2), 1.15–1.13 (m, 1H, CH2), 1.03–0.92 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 177.3, 142.3, 136.8, 133.4, 130.2, 129.7, 128.5, 126.6, 121.6, 120.3, 119.3, 113.3, 112.4, 110.1, 106.6, 101.8, 74.5, 66.5, 58.2, 48.8, 41.0, 37.1, 33.0, 27.8, 27.6, 24.7, 20.3, 19.6.
5-Fluoro-2′-(1-methyl-2-phenyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4k). Yellow solid; 91% yield; mp 210–213 °C; IR (KBr) 743, 1182, 1364, 1487, 1551, 1724, 1734, 2857, 2928, 3345 cm−1; HRMS (EI) calcd for C33H31NaFN4O3 [M + Na]+ 573.2272, found 573.2269. 1H NMR (500 MHz, DMSO-D6): δ 10.67 (s, 1H, NH), 7.88 (d, J = 6.5 Hz, 1H, ArH), 7.63 (d, J = 7.5 Hz, 3H, ArH), 7.53 (d, J = 8.0 Hz, 3H, ArH), 7.28–7.25 (m, 1H, ArH), 7.22–7.20 (m, 1H, ArH), 7.15–7.11 (m, 1H, ArH), 6.86–6.81 (m, 2H, ArH), 5.98 (d, J = 10.0 Hz, 1H, CH), 4.33 (s, 1H, CH), 4.05–4.00 (m, 1H, CH), 3.51 (s, 3H, NCH3), 3.10 (s, 1H, CH), 2.04–2.02 (m, 1H, CH), 1.65–1.40 (m, 4H, CH2), 1.31–1.12 (m, 3H, CH2), 0.98–0.85 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 178.6, 158.6, 156.7, 139.6, 137.3, 131.3, 131.1, 129.5, 129.0, 123.5, 123.5, 122.2, 120.2, 118.9, 117.6, 117.4, 114.4, 114.3, 111.8, 111.8, 111.1, 105.9, 94.4, 72.0, 66.6, 57.9, 46.4, 41.0, 37.8, 31.0, 27.9, 27.4, 24.6, 19.6.
5-Bromo-2′-(1-methyl-2-phenyl-1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4l). Yellow solid; 92% yield; mp 185–188 °C; IR (KBr) 702, 743, 818, 1192, 1366, 1470, 1551, 1612, 1734, 2855, 2928, 3252, 3366 cm−1; HRMS (EI) calcd for C33H31NaBrN4O3 [M + Na]+ 633.1472, found 633.1474. 1H NMR (500 MHz, DMSO-D6): δ 10.80 (s, 1H, NH), 7.81 (t, J = 14.5 Hz, 1H, ArH), 7.62–7.56 (m, 3H, ArH), 7.53–7.50 (m, 2H, ArH), 7.47–7.42 (m, 2H, ArH), 7.26–7.17 (m, 2H, ArH), 7.02 (s, 1H, ArH), 6.83–6.79 (m, 1H, ArH), 5.98 (s, 1H, CH), 4.31 (s, 1H, CH), 4.00–3.98 (m, 1H, CH), 3.54 (s, 3H, NCH3), 3.03–3.02 (m, 1H, CH), 2.04–2.02 (m, 1H, CH), 1.62–1.48 (m, 3H, CH2), 1.39–1.26 (m, 2H, CH2), 1.19–1.12 (m, 2H, CH2), 0.94–0.84 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 178.1, 142.7, 140.3, 137.3, 133.9, 131.3, 131.1, 131.1, 129.5, 129.2, 129.0, 129.0, 125.3, 124.3, 122.3, 120.2, 118.9, 113.5, 113.0, 111.2, 105.7, 94.2, 71.7, 66.3, 58.0, 46.6, 40.9, 37.7, 31.1, 27.8, 27.4, 24.6, 19.6.
2′-(1H-Indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4m). Yellow solid; 94% yield; mp 214–216 °C; IR (KBr) 741, 1103, 1194, 1341, 1468, 1543, 1620, 1694, 1711, 2930, 3256, 3440 cm−1; HRMS (EI) calcd for C26H26NaN4O3 [M + Na]+ 465.1897, found 465.1894. 1H NMR (600 MHz, DMSO-D6): δ 10.97 (d, J = 2.4 Hz, 1H, NH), 10.16 (s, 1H, NH), 7.97–7.63 (m, 1H, ArH), 7.21 (t, J = 6.6 Hz, 1H, ArH), 7.09 (d, J = 1.2 Hz, 2H, ArH), 7.08–7.07 (m, 1H, ArH), 6.98–6.95 (m, 1H, ArH), 6.93–6.90 (m, 1H, ArH), 6.89–6.86 (m, 1H, ArH), 6.56 (d, J = 7.8 Hz, 1H, ArH), 6.43–6.39 (m, 1H, CH), 4.83 (d, J = 11.4 Hz, 1H, CH), 4.69 (d, J = 6.6 Hz, 1H, CH), 4.06–4.03 (m, 1H, CH), 2.16 (d, J = 5.4 Hz, 1H, CH), 1.78–1.77 (m, 1H, CH2), 1.58–1.53 (m, 3H, CH2), 1.20–1.17 (m, 3H, CH2), 1.05–0.91 (m, 3H, CH2); 13C NMR (150 MHz, DMSO): δ 179.6, 143.6, 136.0, 129.9, 127.8, 127.2, 125.3, 123.7, 121.6, 121.5, 119.0, 118.9, 111.7, 109.7, 107.7, 91.7, 73.8, 63.3, 59.1, 43.3, 38.4, 34.2, 29.2, 27.3, 24.8, 19.7.
2′-(1H-Indol-3-yl)-5-methyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4n). Yellow solid; 91% yield; mp 189–191 °C; IR (KBr) 739, 818, 1045, 1333, 1373, 1493, 1547, 1626, 1690, 1711, 2849, 2932, 3277 cm−1; HRMS (EI) calcd for C27H28NaN4O3 [M + Na]+ 479.2054, found 479.2051. 1H NMR (500 MHz, DMSO-D6): δ 10.93 (s, 1H, NH), 10.00 (s, 1H, NH), 7.79 (s, 1H, ArH), 7.61 (d, J = 8.0 Hz, 1H, ArH), 7.21–7.18 (m, 2H, ArH), 6.95 (t, J = 15.0 Hz, 1H, ArH), 6.86 (t, J = 14.5 Hz, 2H, ArH), 6.42–6.36 (m, 1H, ArH), 4.77 (d, J = 11.5 Hz, 1H, CH), 4.74–4.68 (m, 1H, CH), 4.08–4.01 (m, 1H, CH), 3.18 (d, J = 5.5 Hz, 1H, CH), 2.24 (s, 3H, CH3), 2.16–2.13 (m, 1H, CH), 1.75–1.73 (m, 1H, CH), 1.56–1.51 (m, 3H, CH2), 1.26–1.23 (m, 1H, CH2), 1.18–1.15 (m, 2H, CH2), 1.05–1.00 (m, 2H, CH2), 0.98–0.92 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 179.5, 141.1, 136.0, 130.5, 130.0, 127.9, 127.7, 125.3, 123.7, 121.4, 119.1, 118.8, 111.7, 109.4, 107.8, 91.6, 73.8, 63.3, 59.0, 43.6, 38.4, 34.2, 29.2, 27.3, 24.8, 21.2, 19.7.
2′-(1H-Indol-3-yl)-5-methoxy-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4o). Yellow solid; 95% yield; mp 178–180 °C; IR (KBr) 754, 1032, 1206, 1337, 1456, 1491, 1543, 1707, 2930, 3375 cm−1; HRMS (EI) calcd for C27H28NaN4O4 [M + Na]+ 495.2003, found 515, 495.2006. 1H NMR (400 MHz, DMSO-D6): δ 10.72 (d, J = 1.2 Hz 1H, NH), 9.74 (s, 1H, NH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.47 (d, J = 2.4 Hz, 1H, ArH), 6.96 (t, J = 10.4 Hz, 2H, ArH), 6.72 (t, J = 15.2 Hz, 1H, ArH), 6.64 (t, J = 14.8 Hz, 1H, ArH), 6.39 (t, J = 10.8 Hz, 1H, ArH), 6.19 (t, J = 14.0 Hz, 1H, ArH), 6.15 (t, J = 11.2 Hz, 1H, CH), 4.63 (d, J = 11.6 Hz, 1H, CH), 4.46–4.39 (m, 1H, CH), 3.49 (s, 3H, OCH3), 2.94 (d, J = 4.8 Hz, 1H, CH), 1.92–1.88 (m, 1H, CH), 1.59–1.52 (m, 1H, CH2), 1.31–1.27 (m, 3H, CH2), 1.04–0.92 (m, 2H, CH2), 0.83–0.71 (m, 4H, CH2); 13C NMR (100 MHz, DMSO): δ 179.6, 155.0, 136.9, 135.9, 127.9, 126.5, 123.6, 121.5, 119.2, 118.8, 115.4, 114.2, 111.7, 110.0, 107.9, 91.6, 74.2, 63.3, 58.7, 56.3, 43.0, 38.3, 34.2, 29.2, 27.3, 24.8, 19.7.
5-Fluoro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4p). Yellow solid; 92% yield; mp 151–153 °C; IR (KBr) 743, 816, 1180, 1337, 1487, 1545, 1717, 2855, 2930, 3414 cm−1; HRMS (EI) calcd for C26H25NaFN4O3 [M + Na]+ 483.1803, found 483.1807. 1H NMR (500 MHz, DMSO-D6): δ 10.97 (s, 1H, NH), 10.17 (s, 1H, NH), 8.03 (d, J = 8.0 Hz, 1H, ArH), 7.71 (d, J = 7.5 Hz, 1H, ArH), 7.23 (d, J = 7.0 Hz, 2H, ArH), 6.98 (t, J = 14.5 Hz, 1H, ArH), 6.92 (t, J = 14.5 Hz, 2H, ArH), 6.55–6.52 (m, 1H, ArH), 6.42–6.38 (m, 1H, CH), 4.88 (d, J = 11.0 Hz, 1H, CH), 4.72–4.67 (m, 1H, CH), 3.91 (s, 1H, CH), 2.17–2.16 (m, 1H, CH), 1.81–1.78 (m, 1H, CH2), 1.55–1.54 (m, 3H, CH2), 1.27–1.18 (m, 2H, CH2), 1.10–0.96 (m, 3H, CH2), 0.57–0.55 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 179.6, 159.2, 157.3, 139.8, 136.0, 127.8, 127.2, 123.7, 121.5, 119.0, 118.9, 116.3, 115.4, 111.8, 110.4, 107.5, 91.3, 74.2, 63.4, 58.8, 43.4, 38.3, 34.2, 29.2, 27.3, 24.8, 19.6.
7-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4q). Yellow solid; 93% yield; mp 195–197 °C; IR (KBr) 739, 1182, 1337, 1541, 1620, 1713, 2930, 3426, 3449 cm−1; HRMS (EI) calcd for C26H26ClN4O3 [M + H]+ 477.1688, found 477.1685. 1H NMR (500 MHz, DMSO-D6): δ 11.00 (s, 1H, NH), 10.63 (s, 1H, NH), 8.01 (d, J = 7.0 Hz, 1H, ArH), 7.63 (d, J = 8.0 Hz, 1H, ArH), 7.22 (t, J = 9.0 Hz, 2H, ArH), 7.16 (d, J = 8.0 Hz, 1H, ArH), 6.99–6.94 (m, 2H, ArH), 6.88 (t, J = 14.5 Hz, 1H, ArH), 6.42–6.38 (m, 1H, CH), 4.86 (d, J = 11.5 Hz, 1H, CH), 4.74–4.68 (m, 1H, CH), 3.90 (d, J = 5.4 Hz, 1H, CH), 2.18–2.14 (m, 1H, CH), 1.81–1.76 (m, 1H, CH2), 1.59–1.53 (m, 3H, CH2), 1.27–1.22 (m, 1H, CH2), 1.20–1.15 (m, 1H, CH2), 1.09–1.00 (m, 2H, CH2), 0.96–0.91 (m, 1H, CH2), 0.52–0.49 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 179.5, 141.3, 136.0, 130.1, 127.7, 127.2, 126.0, 123.8, 122.9, 121.6, 119.0, 118.9, 113.8, 111.8, 107.4, 91.4, 74.5, 63.4, 59.0, 43.6, 38.3, 34.2, 29.3, 27.3, 24.7, 19.7.
5-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4r). Yellow solid; 93% yield; mp 192–194 °C; IR (KBr) 739, 820, 1045, 1192, 1333, 1375, 1476, 1545, 1618, 1713, 2851, 2936, 3298, 3333 cm−1; HRMS (EI) calcd for C26H25NaBrN4O3 [M + Na]+ 543.1002, found 543.1006. 1H NMR (500 MHz, DMSO-D6): δ 10.95 (s, 1H, NH), 10.95 (s, 1H, NH), 8.31 (d, J = 1.5 Hz, 1H, ArH), 7.65 (d, J = 8.0 Hz, 1H, ArH), 7.25–7.19 (m, 3H, ArH), 6.98–6.95 (m, 1H, ArH), 6.89–6.86 (m, 1H, ArH), 6.48 (d, J = 8.5 Hz, 1H, ArH), 6.49–6.35 (m, 1H, CH), 4.90 (d, J = 11.5 Hz, 1H, CH), 4.67–4.65 (m, 1H, CH), 4.08–3.90 (m, 1H, CH), 3.18 (d, J = 5.0 Hz, 1H, CH), 2.15–2.14 (m, 1H, CH2), 1.82–1.81 (m, 1H, CH2), 1.54–1.50 (m, 2H, CH2), 1.22–1.16 (m, 3H, CH2), 1.09–0.97 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 179.2, 142.9, 136.0, 132.6, 130.3, 127.8, 127.7, 123.7, 121.5, 119.1, 118.8, 113.6, 111.8, 111.5, 107.5, 90.9, 74.0, 63.4, 58.8, 43.4, 38.3, 34.2, 29.3, 27.3, 24.8, 19.6.
2′-(1H-Indol-3-yl)-1′,5-dimethyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4s). Yellow solid; 89% yield; mp 193–195 °C; IR (KBr) 741, 814, 1186, 1207, 1493, 1541, 1620, 1728, 2860, 2924, 3292, 3441 cm−1; HRMS (EI) calcd for C28H30NaN4O3 [M + Na]+ 493.2210, found 493.2206. 1H NMR (500 MHz, DMSO-D6): δ 11.16 (s, 1H, NH), 10.33 (s, 1H, NH), 7.88 (d, J = 7.0 Hz, 1H, ArH), 7.53 (d, J = 2.0 Hz, 1H, ArH), 7.36 (d, J = 8.5 Hz, 2H, ArH), 7.10–7.05 (m, 3H, ArH), 6.71 (d, J = 8.0 Hz, 1H, ArH), 4.59 (d, J = 11.0 Hz, 1H, CH), 4.34–4.29 (m, 1H, CH), 3.17 (d, J = 3.0 Hz, 1H, CH), 2.38 (s, 3H, CH3), 2.09–2.05 (m, 1H, CH), 1.91 (d, J = 12.0 Hz, 3H, CH3), 1.81–1.75 (m, 1H, CH2), 1.57–1.53 (m, 2H, CH2), 1.46–1.41 (m, 3H, CH2), 1.39 (m, 1H, CH2), 1.15 (s, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 177.7, 140.5, 136.4, 130.7, 130.1, 128.3, 127.9, 125.7, 124.2, 121.4, 120.1, 119.1, 111.8, 110.2, 107.8, 101.5, 74.5, 66.7, 58.0, 48.7, 40.9, 37.4, 27.9, 27.6, 24.8, 21.4, 20.3, 19.7.
2′-(1H-Indol-3-yl)-5-methoxy-1′-methyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4t). Yellow solid; 86% yield; mp 161–163 °C; IR (KBr) 743, 1040, 1201, 1300, 1449, 1487, 1541, 1605, 1728, 2930, 3277, 3418, 3628 cm−1; HRMS (EI) calcd for C28H31N4O4 [M + H]+ 487.2340, found 487.2344. 1H NMR (500 MHz, DMSO-D6): δ 11.17 (s, 1H, NH), 10.29 (s, 1H, NH), 7.83 (d, J = 8.0 Hz, 1H, ArH), 7.53 (d, J = 2.0 Hz, 1H, ArH), 7.36 (d, J = 8.0 Hz, 1H, ArH), 7.10–7.04 (m, 3H, ArH), 6.89–6.87 (m, 1H, ArH), 6.75 (d, J = 8.5 Hz, 1H, ArH), 4.54 (d, J = 10.5 Hz, 1H, CH), 4.34–4.29 (m, 1H, CH), 4.06–4.02 (m, 1H, CH), 3.80 (s, 3H, OCH3), 3.18 (d, J = 4.0 Hz, 1H, CH), 2.10–2.06 (m, 1H, CH2), 1.90 (s, 3H, CH3), 1.79–1.73 (m, 1H, CH2), 1.56–1.53 (m, 2H, CH2), 1.46–1.38 (m, 2H, CH2), 1.17 (s, 2H, CH2), 1.89–1.94 (m, 2H, CH2); 13C NMR (125 MHz, DMSO): δ 177.5, 154.4, 136.4, 136.3, 128.2, 125.8, 125.5, 121.4, 120.0, 119.1, 115.1, 114.3, 111.9, 110.6, 107.6, 101.5, 74.7, 66.6, 58.1, 56.1, 48.8, 40.9, 37.5, 27.9, 27.6, 24.7, 20.3, 19.7.
5-Fluoro-2′-(1H-indol-3-yl)-1′-methyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4u). Yellow solid; 91% yield; mp 155–157 °C; IR (KBr) 743, 1184, 1456, 1543, 1730, 2930, 3402, 3437 cm−1; HRMS (EI) calcd for C27H27NaFN4O3 [M + Na]+ 497.1959, found 497.1955. 1H (NMR 500 MHz, DMSO-D6): δ 11.16 (d, J = 1.5 Hz, 1H, NH), 10.49 (s, 1H, NH), 7.92 (d, J = 7.5 Hz, 1H, ArH), 7.58–7.56 (m, 1H, ArH), 7.53 (d, J = 2.5 Hz, 1H, ArH), 7.35 (d, J = 7.0 Hz, 1H, ArH), 7.15–7.11 (m, 1H, ArH), 7.09–7.03 (m, 2H, ArH), 6.82–6.80 (m, 1H, ArH), 4.57 (d, J = 11.0 Hz, 1H, CH), 4.33–4.32 (m, 1H, CH), 3.17 (t, J = 8.0 Hz, 1H, CH), 2.50 (t, J = 3.5 Hz, 1H, CH), 1.89 (s, 3H, CH3), 1.57–1.50 (m, 2H, CH2), 1.49–1.37 (m, 3H, CH2), 1.38–1.15 (m, 2H, CH2), 1.04–0.97 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 177.8, 158.7, 156.8, 139.2, 136.4, 128.2, 125.8, 121.4, 120.4, 119.0, 117.0, 116.8, 115.4, 111.8, 111.1, 107.6, 101.7, 74.8, 66.6, 58.1, 48.6, 41.0, 37.1, 27.8, 27.6, 24.7, 20.3, 19.6.
7-Chloro-2′-(1H-indol-3-yl)-1′-methyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4v). Yellow solid; 89% yield; mp 161–163 °C; IR (KBr) 743, 1142, 1182, 1337, 1456, 1535, 1618, 1715, 2930, 3335, 3401 cm−1; HRMS (EI) calcd for C27H28ClN4O3 [M + H]+ 491.1844, found 491.1840. 1H NMR (500 MHz, DMSO-D6): δ 10.84 (d, J = 1.5 Hz, 1H, NH), 10.54 (s, 1H, NH), 7.45 (d, J = 7.5 Hz, 1H, ArH), 7.30 (d, J = 8.0 Hz, 1H, ArH), 7.02 (d, J = 8.0 Hz, 1H, ArH), 6.91–6.88 (m, 2H, ArH), 6.77–6.71 (m, 1H, ArH), 6.69–6.65 (m, 2H, ArH), 4.23–4.19 (m, 1H, CH), 3.86–3.46 (m, 1H, ArH), 3.12 (s, 1H, CH), 2.94 (d, J = 5.5 Hz, 1H, CH), 1.89–1.86 (m, 1H, CH2), 1.74 (s, 3H, CH3), 1.51–1.47 (m, 1H, CH2), 1.31–1.26 (m, 3H, CH2), 1.03–1.00 (m, 1H, CH2), 0.94–0.92 (m, 2H, CH2), 0.82–0.74 (m, 2H, CH2); 13C NMR (125 MHz, DMSO): δ 180.4, 141.1, 135.5, 130.1, 128.6, 128.4, 124.9, 124.7, 123.1, 121.7, 119.4, 118.3, 113.9, 111.9, 105.6, 100.1, 74.6, 74.2, 58.2, 51.0, 39.0, 36.0, 28.7, 27.6, 25.5, 24.5, 19.4.
5-Bromo-2′-(1H-indol-3-yl)-1′-methyl-1′-nitro-1′,2′,4a′,5′,6′,7′,8′,8a′,9′,9a′-decahydrospiro[indoline-3,3′-pyrrolo[1,2-a]indol]-2-one (4w). Yellow solid; 93% yield; mp 172–174 °C; IR (KBr) 741, 870, 1194, 1344, 1450, 1541, 1616, 1732, 2922, 3287, 3441 cm−1; HRMS (EI) calcd for C27H28BrN4O3 [M + H]+ 535.1339, found 535.1352. 1H NMR (500 MHz, DMSO-D6): δ 11.19 (s, 1H, NH), 10.64 (d, J = 6.0 Hz, 1H, NH), 7.87 (t, J = 12.5 Hz, 1H, ArH), 7.73 (d, J = 4.5 Hz, 1H, ArH), 7.54 (d, J = 4.0 Hz, 1H, ArH), 7.49–7.47 (m, 1H, ArH), 7.38–7.35 (m, 1H, ArH), 7.10–7.06 (m, 2H, ArH), 6.80 (d, J = 15.0 Hz, 1H, ArH), 4.55–4.51 (m, 1H, CH), 4.34 (s, 1H, CH), 3.17 (s, 1H, CH), 2.51 (d, J = 1.0 Hz, 1H, CH), 2.09 (t, J = 10.5 Hz, 1H, CH2), 1.88 (t, J = 19.0 Hz, 3H, CH3), 1.55–1.41 (m, 5H, CH2), 1.16–1.15 (m, 1H, CH2), 1.02–0.96 (m, 3H, CH2); 13C NMR (125 MHz, DMSO): δ 177.3, 142.3, 136.4, 133.4, 129.7, 128.1, 126.6, 125.9, 121.4, 120.1, 119.1, 113.3, 112.4, 111.9, 107.4, 101.9, 74.5, 66.5, 58.2, 48.9, 41.0, 37.1, 27.8, 27.6, 24.7, 20.3, 19.6.
2′-(1H-Indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro [indoline-3,3′-pyrrolizin]-2-one (8a). Yellow solid; 96% yield; mp 232–234 °C; IR (KBr) 754, 1196, 1344, 1545, 1620, 1719, 3352 cm−1; HRMS (EI) calcd for C22H20NaN4O3 [M + Na]+ 411.1428, found 411.1425. 1H NMR (500 MHz, DMSO-D6): δ 10.99 (s, 1H, NH), 10.23 (s, 1H, NH), 7.90 (d, J = 7.5 Hz, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.22 (t, J = 11.5 Hz, 2H, ArH), 6.95 (t, J = 15.5 Hz, 1H, ArH), 6.83 (t, J = 15.0 Hz, 2H, ArH), 6.56 (d, J = 8.0 Hz, 2H, ArH), 6.25 (t, J = 20.0 Hz, 1H, CH), 4.91 (d, J = 10.5 Hz, 1H, CH), 4.66–4.61 (m, 1H, CH), 3.43–3.34 (m, 1H, CH2), 2.64–2.61 (m, 1H, CH2), 2.04–1.99 (m, 1H, CH2), 1.96–1.92 (m, 1H, CH2), 1.69–1.63 (m, 1H, CH2), 1.45–1.39 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 178.6, 143.7, 136.0, 130.1, 127.6, 127.2, 126.1, 123.8, 121.7, 121.5, 119.0, 118.8, 111.8, 110.0, 107.6, 94.2, 74.7, 63.7, 51.0, 44.1, 27.9, 25.6.
2′-(1H-Indol-3-yl)-5-methyl-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8b). Yellow solid; 94% yield; mp 231–233 °C; IR (KBr) 743, 812, 1209, 1337, 1493, 1545, 1626, 1717, 2968, 3381 cm−1; HRMS (EI) calcd for C23H22NaN4O3 [M + Na]+ 425.1584, found 425.1587. 1H NMR (500 MHz, DMSO-D6): δ 11.00 (s, 1H, NH), 10.14 (s, 1H, NH), 7.74 (s, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.21 (d, J = 7.5 Hz, 2H, ArH), 6.95 (t, J = 15.0 Hz, 1H, ArH), 6.89 (d, J = 7.5 Hz, 1H, ArH), 6.84 (t, J = 14.5 Hz, 1H, ArH), 6.44 (d, J = 7.5 Hz, 1H, ArH), 6.23 (t, J = 20.0 Hz, 1H, CH), 4.87 (d, J = 10.5 Hz, 1H, CH), 4.66–4.61 (m, 1H, CH), 3.42–3.39 (m, 1H, CH2), 2.65–2.63 (m, 1H, CH2), 2.27 (s, 3H, CH3), 2.02–1.98 (m, 1H, CH2), 1.94–1.93 (m, 1H, CH2), 1.69–1.66 (m, 1H, CH2), 1.44–1.40 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 178.6, 141.2, 136.1, 130.7, 130.2, 127.9, 127.6, 126.1, 123.8, 121.5, 118.9, 118.8, 111.8, 109.6, 107.6, 94.2, 74.7, 63.7, 51.0, 44.4, 27.9, 25.7, 21.2.
2′-(1H-Indol-3-yl)-7-methyl-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8c). Yellow solid; 93% yield; mp 213–216 °C; IR (KBr) 698, 756, 1190, 1341, 1458, 1541, 1719, 3360 cm−1; HRMS (EI) calcd for C23H22NaN4O3 [M + Na]+ 425.1584, found 425.1580. 1H NMR (500 MHz, DMSO-D6): δ 11.00 (s, 1H, NH), 10.31 (s, 1H, NH), 7.72 (d, J = 7.0 Hz, 1H, ArH), 7.53 (d, J = 8.0 Hz, 1H, ArH), 6.22 (d, J = 9.5 Hz, 2H, ArH), 6.96 (t, J = 14.0 Hz, 1H, ArH), 6.92 (d, J = 7.5 Hz, 1H, ArH), 6.87 (d, J = 7.0 Hz, 2H, ArH), 6.26 (t, J = 20.0 Hz, 1H, CH), 4.91 (d, J = 10.5 Hz, 1H, CH), 4.66–4.62 (m, 1H, CH), 3.35 (s, 1H, CH2), 2.59–2.57 (m, 1H, CH2), 2.02–2.00 (m, 1H, CH2), 1.99 (s, 3H, CH3), 1.94–1.92 (m, 1H, CH2), 1.66–1.64 (m, 1H, CH2), 1.44–1.42 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 179.1, 142.3, 136.0, 131.4, 127.7, 125.7, 124.4, 123.8, 121.7, 121.6, 119.2, 119.0, 118.8, 111.8, 107.8, 94.6, 74.8, 63.5, 51.0, 43.8, 27.9, 25.6, 16.7.
2′-(1H-Indol-3-yl)-5-methoxy-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8d). Yellow solid; 92% yield; mp 217–220 °C; IR (KBr) 689, 752, 1028, 1209, 1342, 1493, 1539, 1607, 1718, 2953, 3389 cm−1; HRMS (EI) calcd for C23H23N4O4 [M + H]+ 419.1714, found 419.1718. 1H NMR (500 MHz, DMSO-D6): δ 11.00 (s, 1H, NH), 10.08 (s, 1H, NH), 7.62 (d, J = 8.0 Hz, 2H, ArH), 7.22 (d, J = 6.5 Hz, 2H, ArH), 6.96 (t, J = 14.5 Hz, 1H, ArH), 6.86 (t, J = 15.0 Hz, 1H, ArH), 6.66 (d, J = 8.0 Hz, 1H, ArH), 6.47 (d, J = 8.5 Hz, 1H, ArH), 6.26 (t, J = 20.0 Hz, 1H, CH), 4.93 (d, J = 11.0 Hz, 1H, CH), 4.64–4.59 (m, 1H, CH), 3.74 (s, 3H, OCH3), 3.48–3.43 (m, 1H, CH2), 2.64–2.61 (m, 1H, CH2), 2.02–1.93 (m, 2H, CH2), 1.67–1.64 (m, 1H, CH2), 1.48–1.42 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 178.6, 155.0, 136.9, 136.0, 127.7, 127.3, 123.8, 121.5, 119.0, 118.9, 115.3, 114.3, 111.8, 110.2, 107.7, 94.1, 75.1, 63.6, 56.3, 50.8, 43.8, 27.9, 25.7.
5-Fluoro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8e). Yellow solid; 93% yield; mp 223–226 °C; IR (KBr) 754, 1188, 1341, 1485, 1541, 1721, 3375 cm−1; HRMS (EI) calcd for C22H19NaFN4O3 [M + Na]+ 429.1333, found 429.1330. 1H NMR (500 MHz, DMSO-D6): δ 11.02 (s, 1H, NH), 10.28 (s, 1H, NH), 7.97 (d, J = 9.0 Hz, 1H, ArH), 7.59 (d, J = 7.5 Hz, 1H, ArH), 7.22 (d, J = 10.0 Hz, 2H, ArH), 6.98–6.92 (m, 2H, ArH), 6.87 (t, J = 15.0 Hz, 1H, ArH), 6.55–6.53 (m, 1H, ArH), 6.27 (t, J = 20.0 Hz, 1H, CH), 4.95 (d, J = 10.5 Hz, 1H, CH), 4.65–4.60 (m, 1H, CH), 3.47–3.42 (m, 1H, CH2), 2.66–2.63 (m, 1H, CH2), 2.02–1.94 (m, 2H, CH2), 1.69–1.65 (m, 1H, CH2), 1.48–1.40 (m, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 178.7, 159.1, 157.2, 139.9, 136.0, 127.6, 123.8, 121.7, 119.0, 118.8, 116.5, 116.3, 115.5, 111.8, 110.5, 107.4, 93.7, 75.0, 63.7, 50.9, 44.0, 27.9, 25.7.
2′-(1H-Indol-3-yl)-7-methyl-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8f). Yellow solid; 92% yield; mp 112–114 °C; IR (KBr) 744, 1140, 1341, 1506, 1549, 1638, 1724, 2972, 3418 cm−1; HRMS (EI) calcd for C22H19F2N4O3 [M + H]+ 425.1420, found 425.1425. 1H NMR (400 MHz, DMSO-D6): δ 11.04 (d, J = 1.2 Hz, 1H, NH), 10.39 (s, 1H, NH), 8.31–8.27 (m, 1H, ArH), 7.61 (d, J = 8.0 Hz, 1H, ArH), 7.24 (d, J = 8.4 Hz, 2H, ArH), 6.98 (t, J = 14.8 Hz, 1H, ArH), 6.89 (t, J = 14.8 Hz, 1H, ArH), 6.61–6.56 (m, 1H, ArH), 6.27 (t, J = 20.0 Hz, 1H, CH), 4.95 (d, J = 10.8 Hz, 1H, CH), 4.63–4.57 (m, 1H, CH), 3.47–3.41 (m, 1H, CH2), 2.69–2.63 (m, 1H, CH2), 2.03–1.92 (m, 2H, CH2), 1.70–1.63 (m, 1H, CH2), 1.46–1.43 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 179.0, 140.5, 140.4, 136.0, 127.5, 123.8, 122.1, 121.6, 119.0, 118.8, 117.6, 117.4, 111.9, 107.2, 99.8, 99.6, 93.3, 74.7, 63.6, 50.8, 43.8, 28.0, 25.6.
6-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8g). Yellow solid; 95% yield; mp 217–219 °C; IR (KBr) 754, 926, 1074, 1194, 1339, 1454, 1541, 1618, 1726, 2976, 3347 cm−1; HRMS (EI) calcd for C22H19NaClN4O3 [M + Na]+ 445.1038, found 445.1042. 1H NMR (400 MHz, DMSO-D6): δ 11.06 (s, 1H, NH), 10.46 (s, 1H, NH), 8.01 (d, J = 8.0 Hz, 1H, ArH), 7.53 (d, J = 8.0 Hz, 1H, ArH), 7.24 (t, J = 7.2 Hz, 2H, ArH), 7.02–6.96 (m, 2H, ArH), 6.87 (t, J = 14.8 Hz, 1H, ArH), 6.59 (d, J = 2.0 Hz, 1H, ArH), 6.27 (t, J = 20.4 Hz, 1H, CH), 4.94 (d, J = 10.8 Hz, 1H, CH), 4.65–4.59 (m, 1H, CH), 2.63 (t, J = 14.0 Hz, 1H, CH2), 2.06–1.93 (m, 3H, CH2), 1.72–1.63 (m, 1H, CH2), 1.48–1.39 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 178.6, 145.2, 136.0, 134.5, 128.9, 127.5, 125.0, 123.9, 121.6, 121.4, 119.0, 118.7, 111.9, 110.1, 107.3, 93.7, 74.4, 63.6, 51.0, 43.8, 27.9, 25.6.
7-Chloro-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8h). Yellow solid; 93% yield; mp 180–182 °C; IR (KBr) 741, 1142, 1177, 1337, 1458, 1547, 1620, 1726, 2976, 3372, 3424 cm−1; HRMS (EI) calcd for C22H20ClN4O3 [M + H]+ 423.1218, found 423.1213. 1H NMR (400 MHz, DMSO-D6): δ 11.07 (s, 1H, NH), 10.75 (s, 1H, NH), 7.98 (d, J = 7.6 Hz, 1H, ArH), 7.53 (d, J = 8.0 Hz, 1H, ArH), 7.27–7.19 (m, 3H, ArH), 7.02–6.96 (m, 2H, ArH), 6.86 (t, J = 14.8 Hz, 1H, ArH), 6.28 (m, 1H, CH), 4.76 (d, J = 10.4 Hz 1H, CH), 4.67–4.61 (m, 1H, CH), 2.61 (d, J = 14.0 Hz, 1H, CH2), 2.07–2.02 (m, 1H, CH2), 2.00–1.93 (m, 2H, CH2), 1.71–1.48 (m, 1H, CH2), 1.48–1.42 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 178.5, 141.4, 136.0, 130.2, 127.9, 127.5, 126.1, 123.9, 123.0, 121.6, 119.1, 118.7, 114.1, 111.9, 107.3, 93.9, 75.3, 63.6, 50.9, 44.0, 27.9, 25.6.
6-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8i). Yellow solid; 91% yield; mp 190–192 °C; IR (KBr) 745, 914, 1126, 1337, 1543, 1612, 1724, 2974, 3418 cm−1; HRMS (EI) calcd for C22H19NaBrN4O3 [M + Na]+ 489.0533, found 489.0537. 1H NMR (400 MHz, DMSO-D6): δ 11.05 (s, 1H, NH), 10.43 (s, 1H, NH), 7.95 (d, J = 8.0 Hz, 1H, ArH), 7.53 (d, J = 8.0 Hz, 1H, ArH), 7.25–7.23 (m, 2H, ArH), 7.15 (t, J = 9.2 Hz, 1H, ArH), 6.98 (t, J = 15.2 Hz, 1H, ArH), 6.87 (m, 1H, ArH), 6.72 (d, J = 1.6 Hz, 1H, ArH), 6.26 (t, J = 20.0 Hz, 1H, CH), 4.93 (d, J = 10.8 Hz, 1H, CH), 4.65–4.58 (m, 1H, CH), 2.63 (t, J = 14.0 Hz, 1H, CH2), 2.05–1.91 (m, 3H, CH2), 1.70–1.63 (m, 1H, CH2), 1.48–1.41 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 178.5, 145.3, 136.0, 129.2, 127.5, 125.4, 124.3, 123.9, 123.0, 121.6, 119.0, 118.7, 112.8, 111.9, 107.3, 93.7, 74.5, 63.6, 51.0, 43.8, 27.9, 25.6.
7-Bromo-2′-(1H-indol-3-yl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (8j). Yellow solid; 95% yield; mp 155–157 °C; IR (KBr) 741, 1138, 1179, 1337, 1456, 1545, 1618, 1724, 2972, 3414 cm−1; HRMS (EI) calcd for C22H20BrN4O3 [M + H]+ 467.0713, found 467.0709. 1H NMR (400 MHz, DMSO-D6): δ 11.07 (s, 1H, NH), 10.62 (s, 1H, NH), 8.01 (d, J = 7.2 Hz, 1H, ArH), 7.54 (d, J = 8.0 Hz, 1H, ArH), 7.32 (d, J = 8.4 Hz, 1H, ArH), 7.25 (m, 2H, ArH), 6.99 (d, J = 7.2 Hz, 1H, ArH), 6.96–6.91 (m, 1H, ArH), 6.86 (t, J = 14.8 Hz, 1H, ArH), 6.27 (t, J = 20.0 Hz, 1H, ArH), 4.95 (d, J = 10.8 Hz, 1H, CH), 4.66–4.60 (m, 1H, CH), 2.60 (t, J = 14.0 Hz, 1H, CH), 2.06–2.01 (m, 1H, CH2), 1.98–1.92 (m, 2H, CH2), 1.73–1.64 (m, 1H, CH2), 1.47–1.43 (m, 1H, CH2); 13C NMR (100 MHz, DMSO): δ 178.4, 143.1, 136.0, 133.1, 127.8, 127.6, 126.5, 123.9, 123.4, 121.6, 119.0, 118.7, 111.9, 107.3, 102.2, 93.9, 75.4, 63.6, 50.9, 43.9, 28.0, 25.6.
6′-(1H-Indol-3-yl)-7′-nitro-1′,6′,7′,7a′-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8k). Yellow solid; 89% yield; mp 252–254 °C; IR (KBr) 679, 737, 750, 1196, 1339, 1369, 1472, 1549, 1618, 1715, 3248, 3401 cm−1; HRMS (EI) calcd for C21H18NaN4O3S [M + Na]+ 429.0992, found 429.0997. 1H NMR (500 MHz, DMSO-D6): δ 11.04 (s, 1H, NH), 9.98 (s, 1H, NH), 7.86 (d, J = 7.0 Hz, 1H, ArH), 7.32 (d, J = 2.5 Hz, 1H, ArH), 7.26–7.22 (m, 2H, ArH), 7.15 (t, J = 15 Hz, 1H, ArH), 6.94–6.90 (m, 1H, ArH), 6.67–6.63 (m, 2H, CH), 6.54 (d, J = 7.5 Hz, 1H, ArH), 6.47–6.43 (m, 1H, CH), 4.65 (d, J = 11.5 Hz, 1H, CH), 4.52–4.48 (m, 1H, CH), 4.05 (t, J = 16.5 Hz, 1H, CH2), 3.82 (d, J = 10.0 Hz, 1H, CH2), 3.12–3.08 (m, 1H, CH2), 2.97 (t, J = 19.0 Hz, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 177.6, 143.6, 136.1, 130.7, 127.3, 126.2, 125.8, 124.4, 122.3, 121.4, 118.9, 118.2, 111.8, 110.3, 106.7, 86.9, 74.5, 68.0, 55.0, 47.1, 33.4.
6-Chloro-6′-(1H-indol-3-yl)-7′-nitro-1′,6′,7′,7a′-tetrahydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazol]-2-one (8l). Yellow solid; 88% yield; mp 190–192 °C; IR (KBr) 752, 1074, 1128, 1323, 1549, 1612, 1726, 3352 cm−1; HRMS (EI) calcd for C21H17NaClN4O3S [M + Na]+ 463.0602, found 463.0606. 1H NMR (500 MHz, DMSO-D6): δ 11.07 (s, 1H, NH), 10.13 (s, 1H, NH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.33 (d, J = 2.5 Hz, 1H, ArH), 7.26–7.21 (m, 1H, ArH), 7.19 (d, J = 2.0 Hz, 1H, ArH), 6.96–6.93 (m, 1H, ArH), 6.72 (d, J = 6.5 Hz, 2H, ArH), 6.55 (d, J = 1.5 Hz, 1H, ArH), 6.47–6.43 (m, 1H, CH), 4.67 (d, J = 11.5 Hz, 1H, CH), 4.52–4.47 (m, 1H, CH), 4.06 (d, J = 10.0 Hz, 1H, CH2), 3.82 (d, J = 10.0 Hz, 1H, CH2), 3.12–3.08 (m, 1H, CH2), 3.00 (t, J = 19.0 Hz, 1H, CH2); 13C NMR (125 MHz, DMSO): δ 177.6, 145.2, 136.2, 135.0, 127.9, 127.2, 124.7, 124.6, 122.0, 121.5, 119.0, 118.1, 111.9, 110.3, 106.4, 86.6, 74.2, 68.1, 55.1, 46.9, 33.4.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31700179), Applied Basic Research Project of Yunnan (Nos. 2017FD073, 2017FD156), Scientific Research Foundation of Yunnan Provincial Education Department (No. 2017ZZX075) and PhD research startup foundation of Yunnan Normal University (Nos. 150025, 160056). We are grateful to Dr Yu-Xin Yan and Xiao-Di Hong (School of Vocational and Technical Education, Yunnan Normal University) for their assistance in the characterized experiment of IR spectra and melting points.
Notes and references
-
(a) R. S. Varma in Green chemistry: challenging perspectives, ed. P. Tundo and P. T. Anastas, Oxford University Press, Oxford, 2000, pp. 221–244 Search PubMed;
(b) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
-
(a) S. L. Y. Tang, R. L. Smith and M. Poliakoff, Green Chem., 2005, 7, 761–762 RSC;
(b) C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC;
(c) P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
-
(a) R. S. Varma, Green Chem., 2008, 10, 1129–1130 RSC;
(b) M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522–5551 RSC.
-
(a) M. Khoobi, T. M. Delshad, M. Vosooghi, M. Alipour, H. Hamadi, E. Alipour, M. P. Hamedani, Z. Safaei, A. Foroumadi and A. Shafiee, J. Magn. Magn. Mater., 2015, 375, 217–226 CrossRef CAS;
(b) A. Khazaei, M. A. Zolfigol, F. Karimitabar, I. Nikokar and A. R. Moosavi-Zare, RSC Adv., 2015, 5, 71402–71412 RSC;
(c) Y.-L. Ma, K.-M. Wang, R. Huang, J. Lin and S.-J. Yan, Green Chem., 2017, 19, 3574–3584 RSC;
(d) H. Wu, X.-M. Chen, Y. Wan, L. Ye, H.-Q. Xin, H.-H. Xu, C.-H. Yue, L.-L. Pang, R. Ma and D.-Q. Shi, Tetrahedron Lett., 2009, 50, 1062–1065 CrossRef CAS;
(e) L.-J. Yan, J.-L. Wang, D. Xu, K. S. Burgess, A.-F. Zhu, Y.-Y. Rao, X.-B. Chen and Y.-C. Wang, ChemistrySelect, 2018, 3, 662–665 CrossRef CAS.
-
(a) C. M. R. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390–2431 CrossRef CAS PubMed;
(b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed;
(c) B. M. Trost and A. J. Frontier, J. Am. Chem. Soc., 2000, 122, 11727–11728 CrossRef CAS;
(d) B. M. Trost, A. C. Gutierrez and R. C. Livingston, Org. Lett., 2009, 11, 2539–2542 CrossRef CAS PubMed;
(e) B. Jiang, S.-J. Tu, P. Kaur, W. Wever and G.-G. Li, J. Am. Chem. Soc., 2009, 131, 11660–11661 CrossRef CAS PubMed;
(f) B. Jiang, M.-S. Yi, F. Shi, S.-J. Tu, S. Pindi, P. McDowell and G. Li, Chem. Commun., 2012, 48, 808–810 RSC;
(g) J. Sun, Y. Sun, H. Gong, Y.-J. Xie and C.-G. Yan, Org. Lett., 2012, 14, 5172–5175 CrossRef CAS PubMed;
(h) X. Feng, Q. Wang, W. Lin, G.-L. Dou, Z.-B. Huang and D.-Q. Shi, Org. Lett., 2013, 15, 2542–2545 CrossRef CAS PubMed.
-
(a) C. de Graaff, E. Ruijte and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969–4009 RSC;
(b) P. Slobbe, E. Ruijte and R. V. A. Orru, MedChemComm, 2012, 3, 1189–1218 RSC.
-
(a) C.-B. Cui, H. Kakeya and H. Osada, J. Antibiot., 1996, 49, 832–835 CrossRef CAS PubMed;
(b) H. Conroy and J. K. Chakrabarti, Tetrahedron Lett., 1959, 1, 6–13 CrossRef;
(c) F. M. Lovell, R. Pepinsky and A. J. C. Wilson, Tetrahedron Lett., 1959, 1, 1–5 CrossRef.
-
(a) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC;
(b) C. B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1996, 52, 12651–12666 CrossRef CAS;
(c) J. Leclercq, M. C. de Pauw-Gillet, R. Bassleer and L. Angenot, J. Ethnopharmacol., 1986, 15, 305–316 CrossRef CAS PubMed.
-
(a) K. Ding, Y.-P. Lu, Z. Nikolovska-Coleska, S. Qiu, Y.-S. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S.-M. Wang, J. Am. Chem. Soc., 2005, 127, 10130–10131 CrossRef CAS PubMed;
(b) S. Rana, E. C. Blowers, C. Tebbe, J. I. Contreras, P. Radhakrishnan, S. Kizhake, T. Zhou, R. N. Rajule, J. L. Arnst, A. R. Munkarah, R. Rattan and A. Natarajan, J. Med. Chem., 2016, 59, 5121–5127 CrossRef CAS PubMed;
(c) R. F. George, N. S. M. Ismail, J. Stawinski and A. S. Girgis, Eur. J. Med. Chem., 2013, 68, 339–351 CrossRef CAS PubMed;
(d) Y. Arun, K. Saranraj, C. Balachandran and P. T. Perumal, Eur. J. Med. Chem., 2014, 74, 50–64 CrossRef CAS PubMed.
-
(a) A. Thangamani, Eur. J. Med. Chem., 2010, 45, 6120–6126 CrossRef CAS PubMed;
(b) D. Kathirvelan, J. Haribabu, B. S. R. Reddy, C. Balachandran and V. Duraipandiyan, Bioorg. Med. Chem. Lett., 2015, 25, 389–399 CrossRef CAS PubMed;
(c) S. U. Maheswari, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 7278–7282 CrossRef CAS PubMed;
(d) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswar and D. Sriram, J. Med. Chem., 2008, 51, 5731–5735 CrossRef CAS PubMed;
(e) A. Nandakumar, P. Thirumurugan, P. T. Perumal, P. Vembu, M. N. Ponnuswamy and P. Ramesh, Bioorg. Med. Chem. Lett., 2010, 20, 4252–4258 CrossRef CAS PubMed.
- B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, R. Brun, V. Dartois, T. T. Diagana and T. H. Keller, J. Med. Chem., 2010, 53, 5155–5164 CrossRef CAS PubMed.
- R. Murugan, S. Anbazhagan and S. S. Narayanan, Eur. J. Med. Chem., 2009, 44, 3272–3279 CrossRef CAS PubMed.
-
(a) P. Prasanna, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 5653–5661 CrossRef CAS PubMed;
(b) R. S. Kumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411–422 CrossRef CAS PubMed.
- G. Kumari, M. Modi, S. K. Gupta and R. K. Singh, Eur. J. Med. Chem., 2011, 46, 1181–1188 CrossRef CAS PubMed.
- N. Karalı, Ö. Güzel, N. Özsoy, S. Özbey and A. Salman, Eur. J. Med. Chem., 2010, 45, 1068–1077 CrossRef PubMed.
-
(a) S.-Y. Li, J. M. Finefield, J. D. Sunderhaus, T. J. Mcafoos, R. M. Williams and D. H. Sherman, J. Med. Chem., 2012, 134, 788–791 CAS;
(b) S. Crosignani, C. Jorand-Lebrun, P. Page, G. Campbell, V. Colovray, M. Missotten, Y. Humbert, C. Cleva, J.-F. Arrighi, M. Gaudet, Z. Johnson, P. Ferro and A. Chollet, J. Med. Chem., 2011, 2, 644–649 CAS;
(c) K. Karthikeyan, P. M. Sivakumar, M. Doble and P. T. Perumal, Eur. J. Med. Chem., 2010, 45, 3446–3452 CrossRef CAS PubMed.
-
(a) P. B. Alper, C. Meyers, A. Lerchner, D. R. Siegel and E. M. Carreira, Angew. Chem., Int. Ed., 1999, 38, 3186–3189 CrossRef CAS;
(b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed.
-
(a) J.-Y. Li, A. Corma and J.-H. Yu, Chem. Soc. Rev., 2015, 44, 7112–7127 RSC;
(b) J.-J. Feng, T.-Y. Lin, C.-Z. Zhu, H.-M. Wang, H.-H. Wu and J.-L. Zhang, J. Am. Chem. Soc., 2016, 138, 2178–2181 CrossRef CAS PubMed;
(c) X.-X. Guo, D.-W. Gu, Z.-X. Wu and W.-B. Zhang, Chem. Rev., 2014, 115, 1622–1651 CrossRef PubMed;
(d) J.-J. Feng and J.-L. Zhang, ACS Catal., 2016, 6, 6651–6661 CrossRef CAS;
(e) M. Bakthadoss and N. Sivakumar, Synlett, 2009, 6, 1014–1018 CrossRef;
(f) M. Bakthadoss, N. Sivakumar, A. Devaraj and D. S. Sharada, Synthesis, 2011, 13, 2136–2146 CrossRef.
-
(a) K. Ding, Y.-P. Lu, Z. Nikolovska-Coleska, G.-P. Wang, S. Qiu, S. Shangary, W. Gao, D.-G. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS PubMed;
(b) V. V. Vintonyak, K. Warburg, H. Kruse, S. Grimme, K. Hübel, D. Rauth and H. Waldmann, Angew. Chem., Int. Ed., 2010, 49, 5902–5905 CrossRef CAS PubMed;
(c) A. Fensome, W. R. Adams, A. L. Adams, T. J. Berrodin, J. Cohen, C. Huselton, A. Illenberger, J. C. Karen, M. A. Hudak, A. G. Marella, E. G. Melenski, C. C. McComas, C. A. Mugford, O. D. Slayeden, M. Yudt, J. Zhang, P. Zhang, Y. Zhu, R. C. Winneker and J. E. Wrobel, J. Med. Chem., 2008, 51, 1861–1873 CrossRef CAS PubMed.
-
(a) K. Debnath, K. Singha and A. Pramanik, RSC Adv., 2015, 5, 31866–31877 RSC;
(b) C. A. Maier and B. Wüensch, J. Med. Chem., 2002, 45, 438–448 CrossRef CAS PubMed;
(c) G. Lang, A. Pinkert, J. W. Blunt and M. H. G. Munro, J. Nat. Prod., 2005, 68, 1796–1798 CrossRef CAS PubMed;
(d) C. Macleod, B. I. Martinez-Teipel, W. M. Barker and R. E. Dolle, J. Comb. Chem., 2006, 8, 132–140 CrossRef CAS PubMed.
- H.-Y. Wang and D.-Q. Shi, ACS Comb. Sci., 2013, 15, 261–266 CrossRef CAS PubMed.
-
(a) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef PubMed;
(b) S. M. Paul, D. S. Mytelka, C. T. Dunwiddie, C. C. Persinger, B. H. Munos, S. R. Lindborg and A. L. Schacht, Nat. Rev. Drug Discovery, 2010, 9, 203–214 CrossRef CAS PubMed;
(c) Y. Arun, G. Bhaskar, C. Balachandran, S. Ignacimuthu and P. T. Perumal, Bioorg. Med. Chem. Lett., 2013, 23, 1839–1845 CrossRef CAS PubMed.
-
(a) G. Chen, Y.-Q. Miao, R. Zhou, L. Zhang, J. Zhang and X.-J. Hao, Res. Chem. Intermed., 2013, 39, 2445–2450 CrossRef CAS;
(b) R. T. Pardasani, P. Pardasani, V. Chaturvedi, S. K. Yadav, A. Saxena and I. Sharma, Heteroat. Chem., 2003, 14, 36–41 CrossRef CAS;
(c) S. N. Singh, S. Regati, A. K. Paul, M. Layek, S. Jayaprakash, K. V. Reddy, G. S. Deora, S. Mukherjee and M. Pal, Tetrahedron Lett., 2013, 54, 5448–5452 CrossRef CAS;
(d) G. P. Rizzi, J. Org. Chem., 1970, 35, 2069–2070 CrossRef CAS.
-
(a) P. R. Mali, L. C. Rao, V. M. Bangade, P. K. Shirsat, S. A. George, N. Jaqadeesh babu and H. M. Meshram, New J. Chem., 2016, 40, 2225–2232 RSC;
(b) S. Haddad, S. Boudriga, T. N. Akhaja, J. P. Raval, F. Porzio, A. Soldera, M. Askri, M. Knorr, Y. Rousselin, M. M. Kubicki and D. Rajani, New J. Chem., 2015, 39, 520–528 RSC.
-
(a) S. M. Rajesh, S. Perumal, J. C. Menéndez, P. Yogeeswari and D. Sriram, MedChemComm, 2011, 2, 626–630 RSC;
(b) A. Y. Barkov, N. S. Zimnitskiy, V. Y. Korotaev, I. B. Kutyashev, V. S. Moshkin and V. Y. Sosnovskikh, Tetrahedron, 2016, 72, 6825–6836 CrossRef CAS;
(c) S. Kanchithalaivan, M. A. Rani and R. R. Kumar, Synth. Commun., 2014, 44, 3122–3129 CrossRef CAS.
-
(a) D. Xu, J.-L. Wang, L.-J. Yan, M.-Q. Yuan, X.-T. Xie and Y.-C. Wang, Tetrahedron: Asymmetry, 2016, 27, 1121–1132 CrossRef CAS;
(b) Y.-C. Wang, D. Li, J. Lin and K. Wei, RSC Adv., 2015, 5, 5863–5874 RSC;
(c) Y.-C. Wang, S. Ji, K. Wei and J. Lin, RSC Adv., 2014, 4, 30850–30856 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for compounds 5a–5e, 4a–4w, 8a–8l. CCDC 1817780. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra13207g |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Jun-Liang Wangc,
Kevin S. Burgessd,
Jiang-Wei Zhange,
Qiu-Mei Zhenga,
Ya-Dan Pua,
Li-Jun Yan*a and
Xue-Bing Chen
*a,
Jun-Liang Wangc,
Kevin S. Burgessd,
Jiang-Wei Zhange,
Qiu-Mei Zhenga,
Ya-Dan Pua,
Li-Jun Yan*a and
Xue-Bing Chen *b
*b

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be the most suitable solvent for the model reaction (94% yield, Table 1, entry 16). To maximize the product yield, we then tested the best reaction temperature (Table 1, entries 16, 18–19). Yields were not significantly different when the reaction temperature was increased from room temperature to 50 °C or to reflux (94% vs. 95% vs. 95% yields, respectively, Table 1, entries 16, 18–19): room temperature was the ideal reaction temperature for the synthesis of polycyclic pyrrolidine-fused spirooxindole derivative 4a (94% yield). There was no significant increase in yield when the reaction time was increased from 6 h to 12 h (95% vs. 94% yields, respectively, entries 16, 20). It is worth mentioning that the highly purified target compound could be obtained by filtering the reaction precipitates and then washing with EtOH/H2O (1
1) was found to be the most suitable solvent for the model reaction (94% yield, Table 1, entry 16). To maximize the product yield, we then tested the best reaction temperature (Table 1, entries 16, 18–19). Yields were not significantly different when the reaction temperature was increased from room temperature to 50 °C or to reflux (94% vs. 95% vs. 95% yields, respectively, Table 1, entries 16, 18–19): room temperature was the ideal reaction temperature for the synthesis of polycyclic pyrrolidine-fused spirooxindole derivative 4a (94% yield). There was no significant increase in yield when the reaction time was increased from 6 h to 12 h (95% vs. 94% yields, respectively, entries 16, 20). It is worth mentioning that the highly purified target compound could be obtained by filtering the reaction precipitates and then washing with EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 2–3 times in the absence of traditional purification techniques such as column chromatography or recrystallization. Overall, the best reaction conditions for synthesizing polycyclic pyrrolidine-fused spirooxindole compound 4a are achieved by employing (E)-1-methyl-3-(2-nitrovinyl)-1H-indole (5a, 1.0 mmol), isatin (6a, 1.1 mmol) and (2S,3aS,7aS)-octahydroindole-2-carboxylic acid (7a, 1.2 mmol) in EtOH/H2O (1
1) for 2–3 times in the absence of traditional purification techniques such as column chromatography or recrystallization. Overall, the best reaction conditions for synthesizing polycyclic pyrrolidine-fused spirooxindole compound 4a are achieved by employing (E)-1-methyl-3-(2-nitrovinyl)-1H-indole (5a, 1.0 mmol), isatin (6a, 1.1 mmol) and (2S,3aS,7aS)-octahydroindole-2-carboxylic acid (7a, 1.2 mmol) in EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent at room temperature for 6 h.
1) solvent at room temperature for 6 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5.0 mL) at room temperatures for 6 hours.b The resulting precipitates were filtered and washed with 3–5 mL EtOH/H2O (v/v = 1
1) (5.0 mL) at room temperatures for 6 hours.b The resulting precipitates were filtered and washed with 3–5 mL EtOH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the yields based on β-nitrostyrene (5).
1), the yields based on β-nitrostyrene (5).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5.0 mL) at room temperatures for 6 hours.b The resulting precipitates were filtered and washed with 3–5 mL EtOH/H2O (v/v = 1
1) (5.0 mL) at room temperatures for 6 hours.b The resulting precipitates were filtered and washed with 3–5 mL EtOH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the yields based on β-nitrostyrene (5d).
1), the yields based on β-nitrostyrene (5d).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mL) was stirred at reflux for 6 h at room temperature. The resulting precipitate was collected by filtration and washed with cold EtOH/H2O (v/v = 1
1 v/v, 10 mL) was stirred at reflux for 6 h at room temperature. The resulting precipitate was collected by filtration and washed with cold EtOH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3–5 mL) for 2–3 times to yield pure products (4 and 8).
1, 3–5 mL) for 2–3 times to yield pure products (4 and 8).