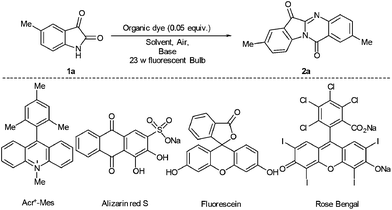
Synthesis of visible-light mediated tryptanthrin derivatives from isatin and isatoic anhydride under transition metal-free conditions†
Hong
Hou
 *,
Hengxue
Li
,
Ying
Han
and
Chaoguo
Yan
*,
Hengxue
Li
,
Ying
Han
and
Chaoguo
Yan
 *
*
College of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Road, Yangzhou, China. E-mail: hhou@yzu.edu.cn; cgyan@yzu.edu.cn
First published on 18th September 2017
Abstract
Visible-light mediated synthesis of diverse tryptanthrin derivatives catalyzed by an organic dye has been achieved. An energy transfer reaction process leads to the self-condensation of isatin, as well as the cross-condensation of isatin and isatoic anhydride. The irradiation of the reaction mixture with a 23 W fluorescent bulb in the presence of Rose Bengal provides a transition metal-free, environmentally friendly and inexpensive alternative approach to synthesize this important heterocycle.
Tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione) presents a kind of natural alkaloid.1 It possesses a series of biological and medicinal activities including antibacterial2 and potential anticancer properties3 and so on. Meanwhile, the substructures of tryptanthrin, for example the indole core4 and the quinazolinone nucleus5 are very important building blocks for many naturally occurring alkaloids and medicine, so it is of great value and meaning to develop more effective methods to synthesize structurally diverse tryptanthrin derivatives.
Traditionally, tryptanthrin can be achieved by self-condensation using isatin as a single starting material. Different approaches including cathodic reduction,6 promoted by POCl3,7 KMnO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 or other oxidants9 have been developed. Meanwhile, tryptanthrin derivatives could also be obtained by the cross-condensation reaction between isatins/indoles and isatoic anhydrides10 in the presence of a base at high temperature or with a cobalt complex as a catalyst. The coupling of ortho-aminobenzoic acid with isatin/indole or 2-aminoacetophenones with isatoic anhydrides has been developed to synthesize tryptanthrin.11,12 Substituted indoles have also been reported towards the tryptanthrin synthesis, and different catalyst systems including copper catalysis,13 oxone induced dimerization14 and visible light photocatalysis15 have been reported. However, most of these methods suffered from the use of strong and toxic oxidants and high temperature, or relied on transition metals as the catalyst. Therefore, the development of a convenient method leading to the synthesis of tryptanthrin derivatives under mild and transition metal-free reaction conditions would be desirable.
8 or other oxidants9 have been developed. Meanwhile, tryptanthrin derivatives could also be obtained by the cross-condensation reaction between isatins/indoles and isatoic anhydrides10 in the presence of a base at high temperature or with a cobalt complex as a catalyst. The coupling of ortho-aminobenzoic acid with isatin/indole or 2-aminoacetophenones with isatoic anhydrides has been developed to synthesize tryptanthrin.11,12 Substituted indoles have also been reported towards the tryptanthrin synthesis, and different catalyst systems including copper catalysis,13 oxone induced dimerization14 and visible light photocatalysis15 have been reported. However, most of these methods suffered from the use of strong and toxic oxidants and high temperature, or relied on transition metals as the catalyst. Therefore, the development of a convenient method leading to the synthesis of tryptanthrin derivatives under mild and transition metal-free reaction conditions would be desirable.
With the development of photoredox catalysis, organic dyes16 serving as a kind of economical, green and non-transition metal catalyst have attracted significant attention for the new reaction development.17 So, an organic dye was selected as the catalyst to explore an effective method for tryptanthrin synthesis.
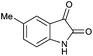
Initially, different organic dyes, including Acr+-Mes, Alizarin Red S, Fluorescein and Rose Bengal as catalysts were examined, with 5-methylisatin 1a as the model substrate, DMF as the solvent and K2CO3 (0.2 equiv.) as the base under the irradiation of a 23 W fluorescent bulb (Table 1, entries 1–4). The reaction proceeded smoothly to afford the desired product 2,8-dimethylindolo[2,1-b]quinazoline-6,12-dione 2a in modest to good yield. Rose Bengal showed the best performance for this transformation and 2a was isolated in 77% yield (Table 1, entry 3). When Acr+-Mes, Fluorescein and Alizarin Red S were used, the yield of 2a was much lower (10–20% yield).
| Entry | Organic dye | Solvent | K2CO3 (equiv.) | Yieldb [%] |
|---|---|---|---|---|
| a Reactions were conducted with 1a (0.20 mmol), organic dye (0.01 mmol, 0.05 equiv.) and base in solvent (2.0 mL). b Yield of isolated product. c The reaction was performed in the dark. d The reaction was performed under an argon atmosphere. | ||||
| 1 | Acr+-Mes | DMF | 0.2 | 20 |
| 2 | Alizarin Red S | DMF | 0.2 | 13 |
| 3 | Fluorescein | DMF | 0.2 | 10 |
| 4 | Rose Bengal | DMF | 0.2 | 77 |
| 5 | Rose Bengal | DCM | 0.2 | Trace |
| 6 | Rose Bengal | Ethyl acetate | 0.2 | 8 |
| 7 | Rose Bengal | THF | 0.2 | 40 |
| 8 | Rose Bengal | Toluene | 0.2 | Trace |
| 9 | Rose Bengal | DMSO | 0.2 | 65 |
| 10 | Rose Bengal | MeCN | 0.2 | 9 |
| 11 | Rose Bengal | DMF | 0.5 | 44 |
| 12 | Rose Bengal | DMF | 1.0 | 88 |
| 13 | — | DMF | 1.0 | Trace |
| 14 | Rose Bengal | DMF | — | NR |
| 15c | Rose Bengal | DMF | 1.0 | Trace |
| 16d | Rose Bengal | DMF | 1.0 | 40 |
Different solvents including DCM, ethyl acetate, THF, toluene, DMSO and MeCN were evaluated next (Table 1, entries 5–10) and no improvement was observed. Changing the amount of K2CO3 had great effects on the reaction, 0.5 equivalents of K2CO3 reduced the yield of 2a (44% yield, Table 1, entry 11). To our great delight, when 1.0 equivalent of K2CO3 was used, the yield was obviously increased and 2a was isolated in 88% yield (Table 1, entry 12).
The background reactions showed that the Rose Bengal catalyst, a base and light were essential in this transformation (Table 1, entries 13–15). The reaction could proceed under an argon atmosphere, and the desired product 2a was formed in a lower isolated yield (Table 1, entry 16).
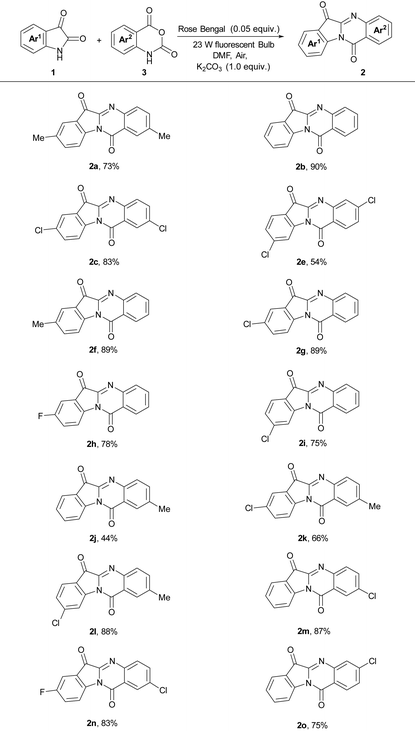
With the best reaction conditions for this transition metal-free tryptanthrin approach in hand, the scope of isatins was explored. A range of isatins bearing various substituents at different positions were subjected to the optimal reaction conditions and successfully yielded the corresponding tryptanthrins 2a–2e (Table 2).
Both electron-donating and electron-withdrawing groups were proved to be compatible for this self-condensation process. When 5-methylindoline-2,3-dione was used, the reaction provided the corresponding product 2a in 88% yield. When indoline-2,3-dione 1b was employed, it successfully gave the desired product 2b in 76% yield. Different electron withdrawing groups on the phenyl ring of isatin (5-Cl, 5-F) were introduced under the reaction conditions, affording the corresponding products 2c–2e in good to excellent yields (52–98%). Significantly, the steric properties of substituents on the benzene ring influence the reaction efficiency dramatically. Compared with the reaction of 5-chloroindoline-2,3-dione (Table 2, entry 3), 6-chloroindoline-2,3-dione gave the corresponding product 2e in lower yield (Table 2, entry 5).
As the single starting material isatins were used for this self-condensation approach, the structural diversity of tryptanthrin products was much confined. In order to synthesize tryptanthrin derivatives with different substituents, we next turned our attention to employ isatoic anhydride and isatin as substrates under the above standard reaction conditions. To our great delight, the reaction could undergo a cross-condensation reaction and yield the desired product 2a in good yield (73%, Table 3) and the scope of isatins and isatoic anhydride was studied and summarized in Table 3.
At first, the reaction of 2H-benzo[d][1,3]oxazine-2,4(1H)-dione 3a with different isatins was probed and the results indicated that both electron-withdrawing groups (5-Cl, 5-F, 6-Cl) and the electron-donating group (5-Me) could be applicable and gave the corresponding products 2f–2i in good yields (75–89%). The reaction of 2H-benzo[d][1,3]oxazine-2,4(1H)-dione with indoline-2,3-dione successfully gave the desired product 2b in 90% yield. Different isatoic anhydrides with 5-substituent (Me, Cl) and 6-substituent (Cl) were investigated and successfully yielded the desired products 2e, 2j–2o in good yields. Remarkably, it looks like either isatoic anhydrides or isatins with an electron-withdrawing group could provide the corresponding products in good yields.
It should be noted that in the reactions of isatins with isatoic anhydrides, the products from the self-condensation of the isatins were not observed. It is probably because the activation of the isatoic anhydride was more feasible than the activation of isatin.
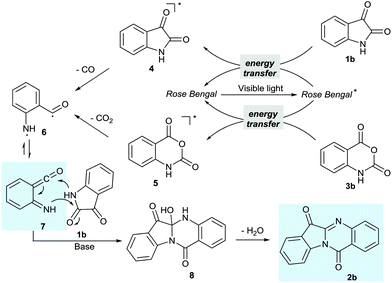
Based on the previous reports on the tryptanthrin synthesis,6–14 and the photoredox chemistry literature,16,18 herein we propose a visible light photochemical energy transfer mechanism for this transformation. Although a single-electron-transfer mechanism for the self-condensation reaction of isatin using Ru(bpy)3Cl2 as the catalyst was proposed by B. A. Shah and co-workers recently,19 comparing the reaction conditions with B. A. Shah's report, it was found that an electron donor such as an organic amine was not necessary under our reaction conditions and the reaction could occur under an argon atmosphere. We consider that the above reaction under transition metal-free conditions operated through an energy transfer mechanism pathway.20 The triplet energy transfer from the photoexcited organic photocatalyst to isatin and isatoic anhydride gave the triple states 4 and 5 respectively which then underwent fragmentative formation resulting in di-radical 6, which would then rearrange to the highly reactive species iminoketene intermediate 7,21 followed by the nucleophilic addition of the nitrogen atom to the ketene and the carbonyl group giving intermediate 8, which then underwent aromatization reaction giving the tryptanthrin derivatives 2 (Scheme 1).
Conclusions
In conclusion, we have developed a visible-light mediated transition metal-free protocol for the synthesis of tryptanthrin derivatives. Using commercially available isatin and isatoic anhydride as starting materials afforded the highly valuable heteroatom cyclic compounds under mild reaction conditions. The use of this methodology for other important valuable heteroatom cyclic compounds is underway in our laboratory.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Project supported by the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (Grant No. 17KJB150042). We also thank the Analysis and Test Centre of Yangzhou University instruments for analysis.Notes and references
- (a) L. A. Mitscher, W. C. Wong, T. De Meulenere, J. Sulko and S. Drake, Heterocycles, 1981, 15, 1017 CrossRef CAS; (b) A. Witt and J. Bergman, Curr. Org. Chem., 2003, 7, 1–19 CrossRef.
- S.-T. Yu, T.-M. Chen, S.-Y. Teng and Y.-H. Chen, Biochem. Biophys. Res. Commun., 2007, 358, 79–84 CrossRef CAS PubMed.
- (a) T. Yasutaka, S. Takao, W. Nobuhisa, A. Hideyuki, S. Shigeru and S. Isao, J. Med. Chem., 1994, 37, 2106–2111 CrossRef; (b) L. A. Mitscher and W. Baker, Med. Res. Rev., 1998, 18, 363–374 CrossRef CAS PubMed; (c) A. K. Bhattacharjee, D. J. Skanchy, B. Jennings, T. H. Hudson, J. J. Brendle and K. A. Werbovetz, Bioorg. Med. Chem., 2002, 10, 1979–1989 CrossRef CAS PubMed.
- (a) R. W. Brown, in Indoles, ed. W. J. Houlihan, Wiley-Interscience, New York, 1972 Search PubMed; (b) The Chemistry of Heterocyclic Compounds, ed. E. C. Taylor and J. E. Saxto, Wiley-Interscience, New York, 1983, vol. 25, 1994 Search PubMed; (c) R. J. Sundberg, in The Chemistry of Indoles, Academic Press, New York, 1996 Search PubMed; (d) A. R. Katritzky, C. A. Ramsden, J. A. Joule and V. V. Zhdankin, Handbook of Heterocyclic Chemistry, Elsevier, Oxford, 3rd edn, 2010 Search PubMed.
- (a) S. Yoshida, T. Aoyagi, S. Harada, N. Matsuda, T. Ikeda, H. Naganawa, M. Hamada and T. Takeuchi, J. Antibiot., 1991, 44, 111–112 CrossRef CAS PubMed; (b) T. Nomura, Z. Z. Ma, Y. Hano and Y. J. Chen, Heterocycles, 1997, 46, 541–546 CrossRef; (c) J. P. Michael, Nat. Prod. Rep., 2007, 24, 223–246 RSC.
- B. Batanero and F. Barba, Tetrahedron Lett., 2006, 47, 8201–8203 CrossRef CAS.
- C.-W. Jao, W.-C. Lin, Y.-T. Wu and P.-L. Wu, J. Nat. Prod., 2008, 71, 1275–1279 CrossRef CAS PubMed.
- G. Honda and M. Tabata, Planta Med., 1979, 36, 85–86 CrossRef CAS PubMed.
- (a) M. A. E. A. A. A. El-Remaily and O. M. Elhady, Tetrahedron Lett., 2016, 57, 435–437 CrossRef; (b) F.-C. Jia, Z.-W. Zhou, C. Xu, Y.-D. Wu and A.-X. Wu, Org. Lett., 2016, 18, 2942–2945 CrossRef CAS PubMed.
- (a) A. Kumar, V. D. Tripathi and P. Kumar, Green Chem., 2011, 13, 51–54 RSC; (b) J.-M. Hwang, T. Oh, T. Kaneko, A. M. Upton, S. G. Franzblau, Z. Ma, S.-N. Cho and P. Kim, J. Nat. Prod., 2013, 76, 354–367 CrossRef CAS PubMed; (c) K. Seya, A. Yamaya, S. Kamachi, M. Murakami, H. Kitahara, J. Kawakami, K. Okumura, M. Murakami, S. Motomura and K.-I. Furukawa, J. Nat. Prod., 2014, 77, 1413–1419 CrossRef CAS PubMed.
- (a) K. C. Jahng, S. I. Kim, D. H. Kim, C. S. Seo, J.-K. Son, S. H. Lee, E. S. Lee and Y. Jahng, Chem. Pharm. Bull., 2008, 56, 607–609 CrossRef CAS PubMed; (b) J. L. Liang, S.-E. Park, Y. Kwon and Y. Jahng, Bioorg. Med. Chem., 2012, 20, 4962–4967 CrossRef CAS PubMed; (c) X. Li, H. Huang, C. Yu, Y. Zhang, H. Li and W. Wang, Org. Lett., 2016, 18, 5744–5747 CrossRef CAS PubMed.
- B. V. S. Reddy, D. M. Reddy, G. N. Reddy, M. R. Reddy and V. K. Reddy, Eur. J. Org. Chem., 2015, 8018–8022 CrossRef CAS.
- C. Wang, L. Zhang, A. Ren, P. Lu and Y. Wang, Org. Lett., 2013, 15, 2982–2985 CrossRef CAS PubMed.
- A. C. Nelson, E. S. Kalinowski, T. L. Jacobson and P. Grundt, Tetrahedron Lett., 2013, 54, 6804–6806 CrossRef CAS.
- (a) C. Zhang, S. Li, F. Bureš, R. Lee, X. Ye and Z. Jiang, ACS Catal., 2016, 6, 6853–6860 CrossRef CAS; (b) X. Li, H. Huang, C. Yu, Y. Zhang, H. Li and W. Wang, Org. Lett., 2016, 18, 5744–5747 CrossRef CAS PubMed.
- (a) P. Esser, B. Pohlmann and H.-D. Scharf, Angew. Chem., Int. Ed. Engl., 1994, 33, 2009–2023 CrossRef; (b) A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS PubMed; (c) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903–2934 CrossRef CAS; (d) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed.
- For selected examples, see: (a) M. Neumann, S. Füldner, B. König and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951–954 CrossRef CAS PubMed; (b) K. Ohkubo, K. Mizushima, R. Iwata and S. Fukuzumi, Chem. Sci., 2011, 2, 715–722 RSC; (c) H. Liu, W. Feng, C. W. Kee, Y. Zhao, D. Leow, Y. Pan and C.-H. Tan, Green Chem., 2010, 12, 953–956 RSC; (d) D. Dondi, S. Protti, A. Albini, S. M. Carpio and M. Fagnoni, Green Chem., 2009, 11, 1653–1659 RSC; (e) A. G. Griesbeck, M. Reckenthäler and J. Uhlig, Photochem. Photobiol. Sci., 2010, 9, 775–778 RSC; (f) Y. Pan, C. W. Kee, L. Chen and C.-H. Tan, Green Chem., 2011, 13, 2682–2685 RSC; (g) J. Xuan, X.-D. Xia, T.-T. Zeng, Z.-J. Feng, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 5653–5656 CrossRef CAS PubMed; (h) H.-T. Song, W. Ding, Q.-Q. Zhou, J. Liu, L.-Q. Lu and W.-J. Xiao, J. Org. Chem., 2016, 81, 7250–7255 CrossRef CAS PubMed; (i) Y.-Q. Zou, S.-W. Duan, X.-G. Meng, X.-Q. Hu, S. Gao, J.-R. Chen and W.-J. Xiao, Tetrahedron, 2012, 68, 6914–6919 CrossRef CAS.
- For selected reviews of visible light photoredox catalysis in organic synthesis, see: (a) M. Fagnoni, D. Dondi, D. Ravelli and A. Albini, Chem. Rev., 2007, 107, 2725–2756 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed; (c) J. M. Narayanam and C. R. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (d) J. W. Tucker and C. R. Stephenson, J. Org. Chem., 2012, 77, 1617–1622 CrossRef CAS PubMed; (e) C. K. Prier, D. A. Rankic and D. W. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (f) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed; (g) R. A. Angnes, Z. Li, C. R. D. Correia and G. B. Hammond, Org. Biomol. Chem., 2015, 13, 9152–9167 RSC; (h) X. Lang, J. Zhao and X. Chen, Chem. Soc. Rev., 2016, 45, 3026–3038 RSC; (i) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed; (j) J.-R. Chen, D.-M. Yan, Q. Wei and W.-J. Xiao, ChemPhotoChem, 2017, 1, 148–158 CrossRef CAS.
- S. Sultan, V. Gupta and B. A. Shah, ChemPhotoChem, 2017, 1, 120–124 CrossRef CAS.
- M. Majek and A. J. v. Wangelin, Acc. Chem. Res., 2016, 49, 2316–2327 CrossRef CAS PubMed.
- (a) T. Kametani, C. V. Loc, T. Higa, M. Koizumi, M. Ihara and K. Fukumoto, J. Am. Chem. Soc., 1977, 99, 2306–2309 CrossRef CAS; (b) K. C. Jahng, S. Kim, D. H. Kim, C. S. Seo, J.-K. Son, S. H. Lee, E. S. Lee and Y. Jahng, Chem. Pharm. Bull., 2008, 56, 607–609 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction conditions and NMR spectra of the new compounds. See DOI: 10.1039/c7qo00740j |
| This journal is © the Partner Organisations 2018 |