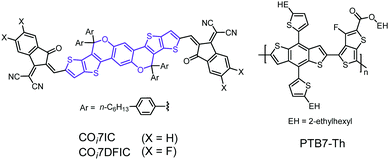
A heptacyclic carbon–oxygen-bridged ladder-type building block for A–D–A acceptors†
Ke
Jin‡
ab,
Chenyu
Deng‡
b,
Lei
Zhang
b,
Dan
Li
b,
Ting
Li
b,
Feng
Wang
*a,
Yongbo
Yuan
*c,
Zuo
Xiao
 *b and
Liming
Ding
*b and
Liming
Ding
 *b
*b
aKey Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan 430073, China. E-mail: psfwang@wit.edu.cn
bCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: xiaoz@nanoctr.cn; ding@nanoctr.cn
cSchool of Physics & Electronics, Central South University, Changsha 410083, China. E-mail: yuanyb@csu.edu.cn
First published on 13th July 2018
Abstract
A heptacyclic carbon–oxygen-bridged ladder-type unit, COi7, was designed. Two corresponding nonfullerene acceptors, COi7IC and COi7DFIC, were developed. The single crystal structure of COi7IC indicates an S-shaped backbone and a packing via π–π interaction of the end groups. PTB7-Th:COi7DFIC solar cells gave a power conversion efficiency of 8.32%, with a decent fill factor of 71.9%.
Owing to their strong light-harvesting capability, tunable energy levels and good morphological stability, nonfullerene acceptors (NFAs) show greater potential than fullerene acceptors in organic solar cells.1 The power conversion efficiency (PCE) for NFA-based solar cells has exceeded 14%.2 Acceptor–donor–acceptor (A–D–A) small molecule acceptors could be the most successful class of NFAs. They generally consist of a carbon-bridged (C-bridged) ladder-type electron-donating core unit, such as indacenodithiophene (IDT), and two strong electron-withdrawing end units, such as 1,1-dicyanomethylene-3-indanone (IC).3 The C-bridged core units are crucial for making high-performance A–D–A NFAs: (1) the extended conjugation systems facilitate π electron delocalization, reducing the bandgaps and enhancing the light absorption of the acceptors; (2) the rigid molecular structures favour improvement in the electron mobility;4 and (3) the out-of-plane side chains avoid overaggregation of the molecules, helping to realize an optimal morphology.5 However, the medium electron-donating capability of C-bridged units is unfavourable for making NFAs with stronger light-harvesting capability.6 In this regard, our group started using carbon–oxygen-bridged (CO-bridged) ladder-type core units for constructing NFAs with strong light absorption. The rationale is that electron-rich oxygen atoms can greatly improve the electron-donating capability of the unit via the conjugation effect. We first reported the synthesis of pentacyclic, hexacyclic and octacyclic CO-bridged units, and the corresponding A–D–A NFAs.7 CO-Bridged NFAs exhibited strong light-harvesting capability and high performance in solar cells. A PCE of 14.62% was achieved.8 Here, we report a heptacyclic CO-bridged ladder-type unit, COi7 (“i” indicates that C–O bonds point to the core of the molecule (inward), and “7” stands for seven fused rings), and its use in preparing two A–D–A acceptors, COi7IC and COi7DFIC (Fig. 1). The single crystal structure of COi7IC indicates an S-shaped backbone of the molecule and a packing via π–π interaction of the end groups. Solar cells based on COi7DFIC and a copolymer donor, PTB7-Th, gave a PCE of 8.32%, with a decent fill factor of 71.9%.
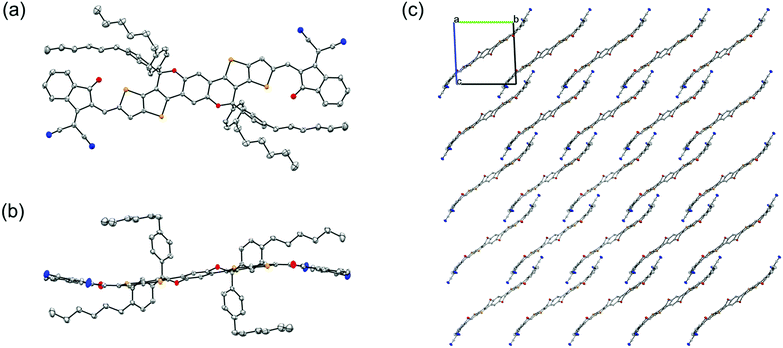
The synthesis of COi7IC and COi7DFIC is similar to that of previously reported CO-bridged NFAs (Scheme S1, ESI†).7 The details are given in the ESI.† COi7IC and COi7DFIC were characterized by nuclear magnetic resonance (NMR) and mass spectroscopy (see the ESI†). The structure of COi7IC was also confirmed by the single crystal XRD analysis (Fig. 2). The top view of COi7IC indicates the Z configuration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds connecting COi7 and IC units, and the locked conformation between COi7 and IC, in which the carbonyl group is close to the sulfur atom of thiophene. Such a molecular geometry is consistent with previous theoretical calculations,9 and S⋯O interaction plays an important role. The side view of the molecule indicates an S-shaped backbone of COi7IC, which is quite different from those of C-bridged A–D–A NFAs.9–11 The S-shaped geometry could result from the two non-coplanar CO-bridges, which might induce a twist of the backbone plane. Being viewed along the a-axis, the material presents a one-dimensional stacking via terminal π–π interaction.11 The π–π interaction distance is around 3.5 Å.
C double bonds connecting COi7 and IC units, and the locked conformation between COi7 and IC, in which the carbonyl group is close to the sulfur atom of thiophene. Such a molecular geometry is consistent with previous theoretical calculations,9 and S⋯O interaction plays an important role. The side view of the molecule indicates an S-shaped backbone of COi7IC, which is quite different from those of C-bridged A–D–A NFAs.9–11 The S-shaped geometry could result from the two non-coplanar CO-bridges, which might induce a twist of the backbone plane. Being viewed along the a-axis, the material presents a one-dimensional stacking via terminal π–π interaction.11 The π–π interaction distance is around 3.5 Å.
The absorption spectra for COi7IC and COi7DFIC in chloroform and as films are shown in Fig. S11 (ESI†). In solution, COi7IC and COi7DFIC show a strong intramolecular charge transfer (ICT) band at 500–750 nm, presenting an absorption maximum at 676 nm and 689 nm, respectively. For films, the absorption maximum shifts to 698 nm and 717 nm, respectively, suggesting enhanced intermolecular interaction in the solid state. The absorption onsets for COi7IC and COi7DFIC films are 775 nm and 802 nm, respectively, corresponding to optical bandgaps (Eoptg) of 1.60 eV and 1.55 eV, respectively. The fluorine atoms enhance the electron-withdrawing capability of the end units and strengthen the ICT, thus leading to a smaller Eoptg and stronger light-harvesting capability for COi7DFIC.12 The energy levels estimated from cyclic voltammetry measurements are shown in Fig. S13 (ESI†).13 The highest occupied molecular orbital (HOMO) levels for COi7IC and COi7DFIC are −5.66 eV and −5.78 eV, respectively, and the lowest unoccupied molecular orbital (LUMO) levels are −3.92 eV and −4.06 eV, respectively. The donor PTB7-Th exhibits a HOMO at −5.38 eV and a LUMO at −3.11 eV.
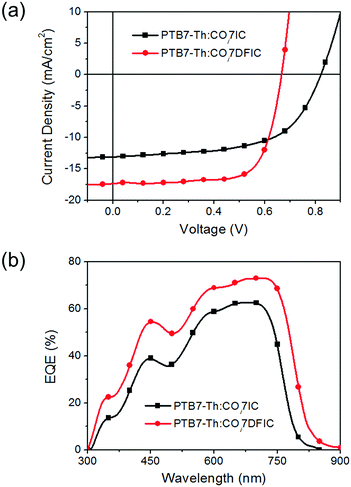
Bulk heterojunction solar cells with an ITO/ZnO/PTB7-Th:NFA/MoO3/Ag structure were made to evaluate the photovoltaic performance of COi7IC and COi7DFIC.14J–V curves and external quantum efficiency (EQE) spectra are shown in Fig. 3, and the performance data are listed in Table 1. The best PTB7-Th:COi7IC cells gave a PCE of 6.36%, with an open-circuit voltage (Voc) of 0.82 V, a short-circuit current density (Jsc) of 13.12 mA cm−2 and a fill factor (FF) of 58.9%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 (w/w), an active layer thickness of 105 nm and 0.2 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S3, ESI†). The best PTB7-Th:COi7DFIC cells gave a PCE of 8.32%, with a Voc of 0.67 V, a Jsc of 17.36 mA cm−2 and a FF of 71.9%. These cells have a D/A ratio of 1
1.4 (w/w), an active layer thickness of 105 nm and 0.2 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S3, ESI†). The best PTB7-Th:COi7DFIC cells gave a PCE of 8.32%, with a Voc of 0.67 V, a Jsc of 17.36 mA cm−2 and a FF of 71.9%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 (w/w), an active layer thickness of 83 nm and 0.4 vol% DIO as the additive (Tables S4–S6, ESI†). COi7DFIC cells gave a lower Voc than COi7IC cells because of the deeper LUMO of COi7DFIC, since Voc is proportional to (LUMOacceptor – HOMOdonor).15 In contrast, COi7DFIC cells gave much higher Jsc and FF than COi7IC cells. From COi7IC cells to COi7DFIC cells, the EQE spectra broaden and intensify (Fig. 3b). The broadening of the EQE spectra is consistent with the enhanced light-harvesting capability of COi7DFIC, while the enhanced EQE might be due to improved charge generation and transport in COi7DFIC cells. We investigated exciton dissociation probabilities (Pdiss) (Fig. S14, ESI†). Pdiss for COi7IC and COi7DFIC cells are 90.7% and 92.4%, respectively, suggesting more efficient charge generation in COi7DFIC cells.2 The 71.9% FF for COi7DFIC cells is among the highest values reported for PTB7-Th:NFA solar cells to date.9,16 A high FF suggests balanced charge transport and suppressed charge recombination in the active layer. We measured hole and electron mobilities (μh and μe) using the space charge limited current (SCLC) method (Fig. S15 and S16 and Table S7, ESI†).17 Compared with the PTB7-Th:COi7IC blend film, the PTB7-Th:COi7DFIC blend film shows higher μh and μe of 4.73 × 10−4 cm2 V−1 s−1 and 6.95 × 10−5 cm2 V−1 s−1, respectively, and more balanced charge carrier transport (μh/μe is closer to 1). We studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. S17, ESI†).18 The data were fitted to a power law: Jsc ∝ Pαlight. The α values for COi7IC and COi7DFIC cells are 0.933 and 0.969, respectively, suggesting less charge recombination in the latter. Enhanced mobilities, balanced charge transport and reduced charge recombination lead to a high FF for COi7DFIC cells. The film morphology was studied using an atomic force microscope (AFM) (Fig. S18, ESI†). Compared with the PTB7-Th:COi7IC blend film, the PTB7-Th:COi7DFIC blend film presents very clear nanofiber structures with ∼16 nm diameter. Such an optimal nanoscale phase separation favors charge generation and transport, also accounting for the higher Jsc and FF for PTB7-Th:COi7DFIC cells. DIO additive can promote phase separation during the film-forming process due to its low dissolving capability to NFAs.18a The XRD profiles for the pure and blend films are shown in Fig. S19 (ESI†). The COi7IC and COi7DFIC films gave a broad diffraction peak centered at 2θ of ∼24°, corresponding to a π–π stacking d-spacing of ∼3.7 Å. Sharp diffraction peaks at 2θ of 9.04°, 10.80°, 22.96° and 26.34° were also observed for the COi7DFIC film, suggesting a higher crystallinity of COi7DFIC. The PTB7-Th:COi7IC and PTB7-Th:COi7DFIC blend films show similar XRD profiles, with a broad diffraction peak centered at 2θ of ∼24°.
1.4 (w/w), an active layer thickness of 83 nm and 0.4 vol% DIO as the additive (Tables S4–S6, ESI†). COi7DFIC cells gave a lower Voc than COi7IC cells because of the deeper LUMO of COi7DFIC, since Voc is proportional to (LUMOacceptor – HOMOdonor).15 In contrast, COi7DFIC cells gave much higher Jsc and FF than COi7IC cells. From COi7IC cells to COi7DFIC cells, the EQE spectra broaden and intensify (Fig. 3b). The broadening of the EQE spectra is consistent with the enhanced light-harvesting capability of COi7DFIC, while the enhanced EQE might be due to improved charge generation and transport in COi7DFIC cells. We investigated exciton dissociation probabilities (Pdiss) (Fig. S14, ESI†). Pdiss for COi7IC and COi7DFIC cells are 90.7% and 92.4%, respectively, suggesting more efficient charge generation in COi7DFIC cells.2 The 71.9% FF for COi7DFIC cells is among the highest values reported for PTB7-Th:NFA solar cells to date.9,16 A high FF suggests balanced charge transport and suppressed charge recombination in the active layer. We measured hole and electron mobilities (μh and μe) using the space charge limited current (SCLC) method (Fig. S15 and S16 and Table S7, ESI†).17 Compared with the PTB7-Th:COi7IC blend film, the PTB7-Th:COi7DFIC blend film shows higher μh and μe of 4.73 × 10−4 cm2 V−1 s−1 and 6.95 × 10−5 cm2 V−1 s−1, respectively, and more balanced charge carrier transport (μh/μe is closer to 1). We studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. S17, ESI†).18 The data were fitted to a power law: Jsc ∝ Pαlight. The α values for COi7IC and COi7DFIC cells are 0.933 and 0.969, respectively, suggesting less charge recombination in the latter. Enhanced mobilities, balanced charge transport and reduced charge recombination lead to a high FF for COi7DFIC cells. The film morphology was studied using an atomic force microscope (AFM) (Fig. S18, ESI†). Compared with the PTB7-Th:COi7IC blend film, the PTB7-Th:COi7DFIC blend film presents very clear nanofiber structures with ∼16 nm diameter. Such an optimal nanoscale phase separation favors charge generation and transport, also accounting for the higher Jsc and FF for PTB7-Th:COi7DFIC cells. DIO additive can promote phase separation during the film-forming process due to its low dissolving capability to NFAs.18a The XRD profiles for the pure and blend films are shown in Fig. S19 (ESI†). The COi7IC and COi7DFIC films gave a broad diffraction peak centered at 2θ of ∼24°, corresponding to a π–π stacking d-spacing of ∼3.7 Å. Sharp diffraction peaks at 2θ of 9.04°, 10.80°, 22.96° and 26.34° were also observed for the COi7DFIC film, suggesting a higher crystallinity of COi7DFIC. The PTB7-Th:COi7IC and PTB7-Th:COi7DFIC blend films show similar XRD profiles, with a broad diffraction peak centered at 2θ of ∼24°.
Conclusions
In summary, a heptacyclic carbon–oxygen-bridged ladder-type unit, COi7, was designed. Two corresponding A–D–A acceptors, COi7IC and COi7DFIC, were prepared. The single crystal structure of COi7IC indicates an S-shaped backbone and a one-dimensional stacking via terminal π–π interaction. A PCE of 8.32% and a decent FF of 71.9% obtained from PTB7-Th:COi7DFIC solar cells demonstrate the potential of the COi7 building block for developing efficient nonfullerene acceptors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We greatly acknowledge the National Natural Science Foundation of China (U1401244, 21572041, 51503050, 51773045, 21772030 and 21704021), the National Key Research and Development Program of China (2017YFA0206600) and the Youth Association for Promoting Innovation (CAS) for financial support.References
- (a) P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131 CrossRef; (b) D. Baran, T. Kirchartz, S. Wheeler, S. Dimitrov, M. Abdelsamie, J. Gorman, R. S. Ashraf, S. Holliday, A. Wadsworth, N. Gasparini, P. Kaienburg, H. Yan, A. Amassian, C. J. Brabec, J. R. Durrant and I. McCulloch, Energy Environ. Sci., 2016, 9, 3783 RSC.
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef PubMed.
- (a) G. Han, Y. Yi and Z. Shuai, Adv. Energy Mater., 2018, 8, 1702743 CrossRef; (b) L. Wang, G. Nan, X. Yang, Q. Peng, Q. Lia and Z. Shuai, Chem. Soc. Rev., 2010, 39, 423 RSC.
- Y. Lin, F. Zhao, Y. Wu, K. Chen, Y. Xia, G. Li, S. K. K. Prasad, J. Zhu, L. Huo, H. Bin, Z. Zhang, X. Guo, M. Zhang, Y. Sun, F. Gao, Z. Wei, W. Ma, C. Wang, J. Hodgkiss, Z. Bo, O. Inganäs, Y. Li and X. Zhan, Adv. Mater., 2017, 29, 1604155 CrossRef PubMed.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef.
- (a) Z. Xiao, F. Liu, X. Geng, J. Zhang, S. Wang, Y. Xie, Z. Li, H. Yang, Y. Yuan and L. Ding, Sci. Bull., 2017, 62, 1331 CrossRef; (b) T. Li, H. Zhang, Z. Xiao, J. J. Rech, H. Niu, W. You and L. Ding, Mater. Chem. Front., 2018, 2, 700 RSC; (c) Z. Xiao, X. Jia, D. Li, S. Wang, X. Geng, F. Liu, J. Chen, S. Yang, T. P. Russell and L. Ding, Sci. Bull., 2017, 62, 1494 CrossRef.
- H. Li, Z. Xiao, L. Ding and J. Wang, Sci. Bull., 2018, 63, 340 CrossRef.
- X. Shi, L. Zuo, S. B. Jo, K. Gao, F. Lin, F. Liu and A. K.-Y. Jen, Chem. Mater., 2017, 29, 8369 CrossRef.
- Z. Liang, M. Li, X. Zhang, Q. Wang, Y. Jiang, H. Tian and Y. Geng, J. Mater. Chem. A, 2018, 6, 8059 RSC.
- G. Han, Y. Guo, X. Song, Y. Wang and Y. Yi, J. Mater. Chem. C, 2017, 5, 4852 RSC.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef PubMed.
- J. Cao, Q. Liao, X. Du, J. Chen, Z. Xiao, Q. Zuo and L. Ding, Energy Environ. Sci., 2013, 6, 3224 RSC.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 RSC.
- B. P. Rand, D. P. Burk and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 115327 CrossRef.
- (a) H.-H. Gao, Y. Sun, X. Wan, X. Ke, H. Feng, B. Kan, Y. Wang, Y. Zhang, C. Li and Y. Chen, Adv. Sci., 2018, 5, 1800307 CrossRef PubMed; (b) Y. Zhang, B. Kan, Y. Sun, Y. Wang, R. Xia, X. Ke, Y. Yi, C. Li, H.-L. Yip, X. Wan, Y. Cao and Y. Chen, Adv. Mater., 2018, 30, 1707508 CrossRef PubMed.
- (a) X. Wang, J. Tong, P. Guo, Y. Li, H. Li, Y. Xia and F. Wang, Polymer, 2017, 122, 96 CrossRef; (b) X. Wang, C. Cheng, Y. Li and F. Wang, Polymers, 2018, 10, 331 CrossRef.
- (a) M. An, F. Xie, X. Geng, J. Zhang, J. Jiang, Z. Lei, D. He, Z. Xiao and L. Ding, Adv. Energy Mater., 2017, 7, 1602509 CrossRef; (b) D. Li, Z. Xiao, S. Wang, X. Geng, S. Yang, J. Fang, H. Yang and L. Ding, Adv. Energy Mater., 2018, 8, 1800397 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Material preparation and characterization, solar cell fabrication and measurements. CCDC 1837067. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8qm00285a |
| ‡ K. Jin and C. Deng contributed equally to this work. |
| This journal is © the Partner Organisations 2018 |