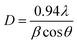Are organic templates responsible for the optical and magnetic response of MgO nanoparticles?†
Jitendra Pal
Singh
 a,
So Hee
Kim
a,
So Hee
Kim
 a,
Hee Kyoung
Kang
a,
Hee Kyoung
Kang
 a,
Sung Ok
Won
a,
Sung Ok
Won
 a,
Ik-Jae
Lee
a,
Ik-Jae
Lee
 b and
Keun Hwa
Chae
b and
Keun Hwa
Chae
 *a
*a
aAdvanced Analysis Centre, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea. E-mail: khchae@kist.re.kr
bBeamline Division, Pohang Accelerator Lab, Pohang 37673, Republic of Korea
First published on 2nd July 2018
Abstract
Herein, we report the optical and magnetic response of magnesium oxide nanoparticles synthesized using magnesium nitrate in a citric acid host. The precursor obtained during the synthesis process was annealed at 350 °C, 400 °C, 500 °C, 700 °C, 900 °C and 1100 °C to obtain magnesium oxide nanoparticles. The rock salt phase of the synthesized nanoparticles was investigated by Rietveld analysis of their X-ray diffraction (XRD) patterns. The temperature-dependent XRD investigation revealed a stable rock salt phase at the measured temperature of 300 °C for these nanoparticles. The nanoparticles exhibited varying crystallite sizes in the range from 6.9 ± 0.2 nm to 29.7 ± 3.9 nm as the annealing temperature increased from 350 °C to 1100 °C. Furthermore, scanning electron microscopy and transmission electron microscopy revealed the formation of regular particles above 500 °C; however, below this temperature, the particles appeared to be embedded in a carbonaceous-like matrix. A variation in color with annealing temperature was visually observed for the nanoparticles, which was associated with the presence of the carbonaceous-like matrix. Near-edge X-ray absorption spectroscopy (NEXAFS) measurements carried out at the O K-edge also revealed a carbonaceous-like matrix, and these results were supported by C and N K-edge measurements. These measurements indicated the presence of C–O–, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–, C–N–, and C–H-like bonds attached to MgO crystallites having dominant contribution at low annealing temperatures. This behavior was also reflected by the X-ray photoelectron spectroscopic measurements at O 1s, C 1s and N 1s. The Mg K-edge NEXAFS measurements exhibited slight distortion in the Mg2+ ion coordination at lower annealing temperatures, which may be due to the presence of Mg2+ ions in MgO- and MgCO3-like co-ordinations, as investigated from Mg 1s spectra of these nanoparticles. Diffuse reflectance measurements showed almost 100% reflectance for the nanoparticles at an annealing temperature of 900 °C and wavelength of 600 nm; the reflectance of these nanoparticles reduced when the annealing temperature was lowered, and it reduced to almost 8% at an annealing temperature of 350 °C. Additionally, O K-edge X-ray magnetic circular dichroism (XMCD) measurements obtained for these materials showed the onset of magnetism in these C-rich materials.
O–, C–N–, and C–H-like bonds attached to MgO crystallites having dominant contribution at low annealing temperatures. This behavior was also reflected by the X-ray photoelectron spectroscopic measurements at O 1s, C 1s and N 1s. The Mg K-edge NEXAFS measurements exhibited slight distortion in the Mg2+ ion coordination at lower annealing temperatures, which may be due to the presence of Mg2+ ions in MgO- and MgCO3-like co-ordinations, as investigated from Mg 1s spectra of these nanoparticles. Diffuse reflectance measurements showed almost 100% reflectance for the nanoparticles at an annealing temperature of 900 °C and wavelength of 600 nm; the reflectance of these nanoparticles reduced when the annealing temperature was lowered, and it reduced to almost 8% at an annealing temperature of 350 °C. Additionally, O K-edge X-ray magnetic circular dichroism (XMCD) measurements obtained for these materials showed the onset of magnetism in these C-rich materials.
Introduction
In recent years, MgO has attracted increasing attention due to its interesting properties such as ferromagnetism combined with multilevel switching characteristics,1 broadband laser emission in the near-ultraviolet to blue-green spectral range2 and room temperature ferromagnetism (RTFM).3,4 RTFM in band gap tunable MgxZn1−xO (x ≤ 0.22) is ascribed to cation (zinc) vacancies.5 Al-doped MgO exhibits weak RTFM with its saturation magnetization (Ms) reaching a maximum value of 0.023 emu g−1 at 2 at% of Al, which is ascribed to oxygen vacancies.6 In MgO doped with O, C and N, magnetic moment occurs from the localized wave function of unpaired electrons, and the exchange interaction forms a long-ranged magnetic order.7 MgO films with different thicknesses show a thickness-dependent transition from the ferromagnetic to paramagnetic state, which is due to cation vacancies.8,9 Similar observations are reported for homogeneous (200)-oriented MgO thin films deposited on Si substrates,10 MgO thin films deposited by pulsed laser deposition,11 and MgO nanoparticles and hollow spheres.12–15 Kumar et al. reported that oxygen vacancies, namely, singly ionized anionic vacancies (Fþ) and dimers (F22þ) induce RTFM spin order in MgO nanocrystallites,16 which has also been reported for highly defective MgO nanosheets.17 Ferromagnetism in MgO films may be closely related to the hydrogen-driven instability of V-type centers in this material.18 Thus, numerous reports highlight the magnetic behavior of MgO; however, the optical behaviors of MgO thin films have not been studied. Recently, Bindhu et al. reported that MgO nanoparticles with a crystallite size of 16 nm exhibit an optical band gap of 5.25 eV,19 which is less than the value of bulk MgO (7.65 eV).20 Monodispersed nanoparticles 2–3 nm in size exhibit broad absorption centered at 325 nm in the visible region,21 and MgO nanoparticles 9 nm in size exhibit a band gap of ∼4.8 eV.22 Wobbe et al. showed theoretically that the optical excitation of MgO nanoparticles is affected by the cluster size.23 Thus, MgO exhibits interesting magnetic and optical behaviors, which depend on the size of the nanoparticles; however, there is no consensus for the explanation of their origin. Hence, there is a need for deep investigation of this system with appropriate techniques considering the magnetic and optical behaviors.In the present study, we focus on MgO nanoparticles synthesized using a chemical method. Chemical methods generally utilize an organic matrix to promote chemical reactions; these reactions start at a temperature of around 100 °C. Continuous heating at this temperature up to several hours leads to the formation of a precursor. This precursor is then annealed to remove water and other phases. However, it is reported that materials synthesized using these methods contain organic impurities.24 Considering the magnetism in nonmagnetic oxides, this behavior in organic systems has recently attracted increasing attention from researchers.25,26 Orbital hybridization between carbon and oxygen is considered to be crucial for this type of magnetism. Thus, the nature of the organic matrix associated with synthesized nanoparticles requires much deeper investigation with appropriate techniques to shed light on the exact source of magnetism.
Thus, investigations related to the chemical state, hybridization and interaction between neighboring atoms are prerequisites for this study. It is well-established that X-ray absorption spectroscopy (XAS) is widely used for the investigation of valence state, crystalline field, disturbance of bonds between two atoms and effect of any adsorbed species on the particle surface.24,27 On the other hand, X-ray photoelectron spectroscopy (XPS) is more effective for obtaining chemical information of atoms.28,29 Thus, the combination of these techniques can help identify the possible mechanisms of the various fundamental aspects of materials.30–32 X-ray magnetic circular dichroism (XMCD) can help identify the atomic species responsible for magnetism.33,34 This technique is also effective for establishing the role of vacancies and defects in magnetism in organic systems35 and non-magnetic oxides.36,37 Thus, in the present study, we report the synthesis of MgO nanoparticles and their investigations using UV-Vis spectroscopy, XAS, XPS and XMCD.
Experimental
Synthesis of nanoparticles
A solution of magnesium nitrate hexahydrate (AR Grade) in a stoichiometric proportion was mixed with citric acid solution by keeping the ratio of cations to citric acid as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.24 The mixture was magnetically stirred at 90 °C for 6 h to obtain a viscous solution. This viscous solution was then dried at 100 °C for almost 16 h. The precursor obtained was subsequently annealed at different temperatures ranging from 350 °C to 1100 °C for 1 h. These materials were coded as M350, M400, M500, M700, M900 and M1100.
3.24 The mixture was magnetically stirred at 90 °C for 6 h to obtain a viscous solution. This viscous solution was then dried at 100 °C for almost 16 h. The precursor obtained was subsequently annealed at different temperatures ranging from 350 °C to 1100 °C for 1 h. These materials were coded as M350, M400, M500, M700, M900 and M1100.
Characterization of nanoparticles
X-ray diffraction (XRD) patterns of the synthesized materials were recorded using a D/MAX2500 (RIGAKU, Japan) X-ray diffractometer with Cu-Kα (λ = 1.5418 Å) radiation; these patterns were refined using the FullProf program.38 Temperature-dependent XRD measurements were obtained using a Dmax2500/PC diffractometer. Scanning electron microscopy (SEM) measurements were obtained using a Hitachi (S-4200) field emission scanning electron microscope (FE-SEM) equipped with a Horiba 6853H element dispersive spectrometer (EDS) for the determination of the morphology and elemental composition of the synthesized materials. A Techni F20 G2 (FEI, The Netherlands) transmission electron microscope (TEM) with an operating voltage at 150 kV was utilized for further estimation of particle size.Near-edge X-ray absorption fine structure (NEXAFS) measurements of the synthesized materials were conducted in ultrahigh vacuum at the 10D XAS KIST (Korea Institute of Science and Technology) beamline in Pohang Light Source, Korea. This beamline was operated at 3.0 GeV energy with a maximum storage ring current of 400 mA.39 Grating monochromators with 400 lines per mm, 1000 lines per mm and 1800 lines per mm with entrance and exist slit openings of 100 μm × 100 μm were used for recoding C K-, N K-, O K- and Mg-K-edge NEXAFS spectra of the synthesized materials. The obtained spectra were normalized with respect to incident photon flux.24,39 XPS measurements were obtained using a PHI 5000 VersaProbe (Ulvac-PHI) X-ray photoelectron spectrometer under a high vacuum of 2.0 × 10−7 Torr; this spectrometer uses Al-Kα (1486.6 eV) as the monochromator source. Before measurements, the spectrometer was calibrated using the C 1s peak of energy 284.6 eV. The spot size was maintained at 100 μm × 100 μm during measurements. The detection limit of the spectrometer was 0.5 at%. XPS survey scan spectra is shown in Fig. S1 (ESI†).
UV-Vis spectroscopic measurements were carried out using a Carry 5000 (Agilent, US) UV/VIS/NIR spectrometer in the diffuse reflectance (DRS) mode. To compare our results with that of reference MgO, MgO procured from Alfa Aesar was also used for the optical investigations. XMCD experiments at O K-edges were performed at the 2A MS beamline in Pohang Light Source, Korea using circularly polarized light with a degree of circular polarization of around 80%. Absorption data were collected in the total electron yield (TEY) mode by applying a voltage of 200 V. The photon beam and the magnetic field were perpendicular to the sample plane. The base pressure of the system was 10−9 Torr. The absorption was normalized to the incoming photon beam intensity by simultaneously measuring the photocurrent at a gold grid. The magnetic field strength, μ0H, (between −0.75 and 0.75 T) was generated by an electromagnet.40 All measurements were obtained at room temperature. The absorption cross-section of the circularly polarized X-rays was labeled as μαβ, where α denotes the helicity of the photon (α = ↑(↓) when the photon is right-handed (left-handed) polarized), and β denotes the direction of the magnetic field (β = ↑(↓) when the magnetic field is parallel (antiparallel) to the propagation vector). In the electric dipole approximation, reversing the magnetic field is equivalent to changing the beam helicity; thus, μ↑↑ = μ↓↓ and μ↑↓ = μ↓↑. To compensate for systematic uncertainties inherent to the measurement process of the absorption cross section, the XMCD signal is obtained as μXMCD = (μ↑↑ + μ↓↓ − μ↑↓ − μ↓↑)/2.40
Results and discussion
The synthesized nanoparticles exhibit various colors (Fig. 1). Nanoparticles M1100 and M900 exhibit a white color, which is similar to that of bulk MgO.41 The color of M700 is light brown, whereas that of M500 is brown. M400 and M350 are brown and dark brown, respectively. Thus, the color variation of these nanoparticles with annealing temperatures motivated our group to investigate the underlying mechanism.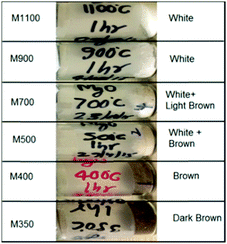 | ||
| Fig. 1 Color variation of the synthesized MgO nanoparticle systems, namely, M350, M400, M500, M700, M900 and M1100. | ||
Phase analysis, size, distribution and morphology
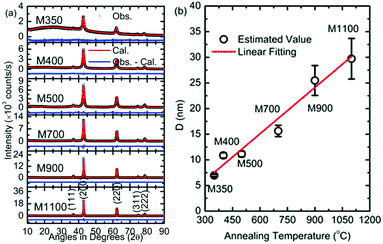
Fig. 2a shows the XRD patterns refined using the Rietveld program along with JCPDS No. #78-0430. The refined patterns show the presence of the Fm3m space group. Thus, the XRD studies reveal the formation of a pure phase of the synthesized nanoparticles, which indicates that the color at a lower annealing temperature is not associated with any impure phase.To further investigate the responsible factor, the parameters obtained from Rietveld refinement are discussed. The values of the lattice parameters obtained from the simulation are shown in Table 1. The lattice parameter is 4.233 ± 0.008 Å for M350; this value decreases with an increase in the annealing temperature and attains a value of 4.213 ± 0.001 Å for M1100, which is close to the value of bulk MgO. The variation in lattice parameters with annealing temperature is ascribed to the distortion of the unit cell at a lower temperature.22
| Sample | R b (%) | R f (%) | χ 2 | GOF | a (Å) |
|---|---|---|---|---|---|
| M350 | 14.3 | 10.3 | 1.49 | 1.2 | 4.233 ± 0.008 |
| M400 | 3.35 | 3.75 | 2.03 | 1.4 | 4.225 ± 0.002 |
| M500 | 2.07 | 1.40 | 4.87 | 2.2 | 4.221 ± 0.003 |
| M700 | 6.96 | 5.01 | 5.52 | 2.3 | 4.219 ± 0.002 |
| M900 | 1.83 | 2.55 | 1.8 | 3.44 | 4.215 ± 0.002 |
| M1100 | 2.82 | 2.00 | 2.9 | 8.29 | 4.213 ± 0.001 |
The crystallite size of the synthesized nanoparticles was estimated using Scherrer's formula from the most intense peak.
The values for the crystallite size are 6.9 ± 0.2, 10.8 ± 0.5, 11.1 ± 0.6, 16.0 ± 1.0, 25.5 ± 2.9, and 29.7 ± 3.9 nm for M350, M400, M500, M700, M900, and M1100 nanoparticles, respectively (Fig. 2b). The variation in crystallite size with annealing temperature is fitted by a linear function. An increase in crystallite size with annealing temperature is generally observed for nanoparticle growth, which has been discussed by numerous authors for oxide materials synthesized using the sol–gel auto combustion synthesis process.24,42
To shed light on the phase stability of the synthesized nanoparticles, XRD measurements were performed for M350, M500 and M1100, as shown in Fig. 3. The presence of peaks associated with the rock salt phase was observed in the XRD patterns of these nanoparticles at different temperatures. Additionally, these measurements further demonstrated that the rock salt phase in these nanoparticles was stable up to 300 °C (573 K).
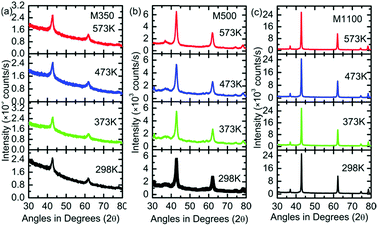 | ||
| Fig. 3 (a) XRD patterns of (a) M350, (b) M500 and (c) M1100 at the temperatures of 298, 373, 473 and 573 K. | ||
Fig. 4 shows the SEM micrographs of M350, M400, M900, and M1100 at two different magnifications: (a) 5k and (b) 30k. All the nanoparticles exhibit distinct differences in their morphologies. M350 exhibits micro-sized particles having an irregular shape, where some spherical-shaped particles are present on these particles. It seems that large irregular-shaped particles are present due to the formation of organic impurities because of the synthesis process; however, the cluster of small particles is due to MgO nanoparticles. In the present case, annealing of the precursor at 350 °C is sufficient to initiate crystallization, but this temperature is too low to remove organic impurities. The annealing of the precursor at 400 °C leads to a significant decrease in the concentration of large particles. At this temperature, the cluster of small particles is significant in number. Upon further increasing the annealing temperature, the number of micron-sized particles decreases significantly, and MgO nanoparticles appear clearly in the micrographs (Fig. 4). Thus, a threshold temperature is required for the removal of organic impurities when materials are synthesized using the nitrate method.24
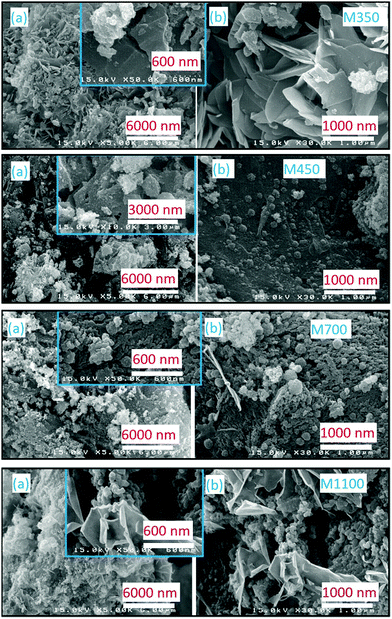 | ||
| Fig. 4 Scanning electron micrographs of M350, M400, M700 and M1100. Panels (a) and (b) show the morphological behaviors at different magnifications. | ||
The EDS spectra for M350, M400, M700, and M1100 are shown in Fig. 5a; these spectra clearly indicate the presence of Mg, O and C atoms in the synthesized nanoparticles (Fig. 5a). To further elucidate the effect of annealing temperature on the stoichiometry (although this is not an accurate method to determine stoichiometry, it gives a rough idea) of the synthesized nanoparticles, elemental compositions of M350, M400, M700 and M1100 were estimated, as shown in Fig. 5b. The elemental composition estimated from SEM-EDS shows the presence of carbon in the nanoparticles in addition to Mg and O. The content of carbon was significantly high in M350 and M400 nanoparticles. Besides, oxygen was also present in excess, which led to the Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O stoichiometric ratio of 1
O stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x (x > 1). Thus, excess amounts of carbon and oxygen clearly indicated the presence of organic templates in these nanoparticle systems.
x (x > 1). Thus, excess amounts of carbon and oxygen clearly indicated the presence of organic templates in these nanoparticle systems.
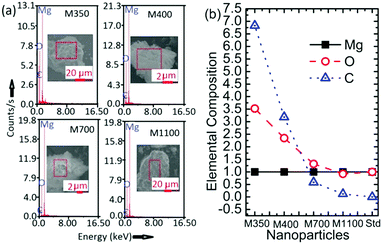 | ||
| Fig. 5 (a) EDS spectra and (b) elemental compositions of M350, M400, M700 and M1100 along with that of the MgO standard (Std). | ||
Further investigations on the size and shape of the nanoparticles were conducted using transmission electron microscopy. Fig. 6 shows the TEM images of M350, M400 and M500 at two different magnifications; these micrographs clearly indicated the presence of small particles in some matrix (Fig. 6). Thus, these results supported the results obtained from the SEM images.
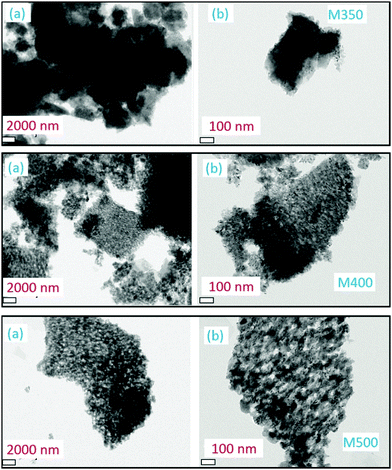 | ||
| Fig. 6 Transmission electron micrographs of M350, M400 and M500 at (a) lower and (b) higher magnifications. | ||
Element specific information
Fig. 7a shows O K-edge spectra of M350, M400, M500, M700, M900 and M1100 nanoparticles; these spectra show spectral features Ao, A1, A2 and A3 centered at 534, 537, 541, and 547 eV, respectively. According to the band structure calculation and previous reports, we can infer that the spectral features Ao, A1, A2 and A3 arise from the combination of charges on both the Mg and O sites corresponding to Mg–O covalent bonding orbitals or states, O 2p nonbonding states, O 2p–Mg 3s antibonding states, and Mg 3p states.43,44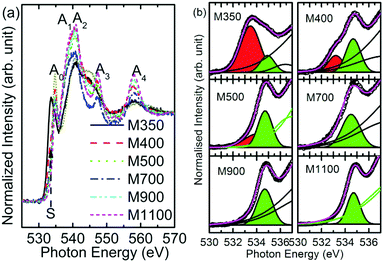 | ||
| Fig. 7 (a) O K-edge spectra and (b) pre-edge region of O K-edge spectra for the M350, M400, M500, M700, M900 and M1100 nanoparticle systems. | ||
The pre-edge region of the O K-edge spectrum of MgO arises due to excitation to the localized bound state and the presence of surface states or intermixing.45 In the present case, the pre-edge region consists of the Ao spectral feature followed by a shoulder S in the spectra of the nanoparticles. To further elucidate the behavior of the shoulder S, the pre-edge regions of O K-edge spectra of these nanoparticles are de-convoluted, as shown in Fig. 7b. The shoulder S shown in red is distinguishable in the spectra of M350, M400 and M500, but it is dominant in the spectrum of the M350 nanoparticles. As the annealing temperature increases, the shoulder S disappears systematically. The origin of this shoulder is due to the interaction of O2− ions with carbon-like impurities.46 These impurities in the nanoparticles occur as a result of the chemical reaction for the formation of MgO in the citric acid matrix. The normalized intensity obtained from the area under the curve for this shoulder S is shown in Fig. 8a. The normalized intensity decreases exponentially with the annealing temperature and seems to be related to the variation obtained from SEM-EDX (Fig. 4). Apart from the intensity of this pre-edge spectral feature, Ao increases exponentially with the annealing temperature except for M1000 (Fig. 8b).
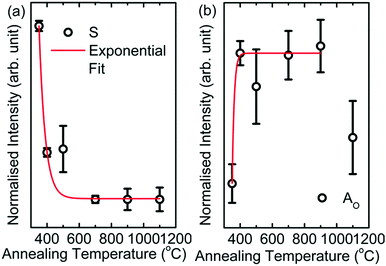 | ||
| Fig. 8 (a) Intensity variation of the shoulder (S) with annealing temperature and (b) intensity variation of Ao with annealing temperature for M350, M400, M500, M700, M900 and M1100. | ||
To obtain exact information on the interaction of oxygen ions with the constituent ions, O 1s spectra of different nanoparticles were analyzed, as shown in Fig. 9. The deconvoluted spectra exhibited two features, A1′ and A2′, centered at 531.5 and 532.6 eV, respectively, for all the synthesized nanoparticles (Fig. 8a). These spectral features were associated with the presence of oxygen in MgO and MgCO3 (Table S1, ESI†).47–49 The area of the A1′ spectral feature increased exponentially with annealing temperature (Fig. 8b: upper panel); this may be due to the enhancement in oxygen associated with MgO with annealing temperature. Additionally, the area of the A2′ spectral feature was also modified with annealing temperature (Table S1, ESI†).
We further calculated the ratio of the spectral features (A2′/A1′) corresponding to oxygen in carbonate and oxygen in MgO, as shown in Fig. 9b (lower panel). The ratio of area (A2′/A1′) decreased with annealing temperature except at 1100 °C; this indicated that oxygen ions in the form of MgCO3 relative to those in the form of MgO decreased with annealing temperature, which revealed a decrease in carbonaceous-like impurities with an increase in annealing temperature.
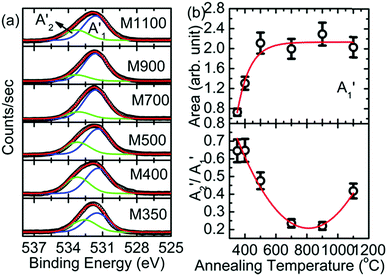
Since Mg ion is located at the center of a regular oxygen octahedron in MgO, the lowest empty Mg 3p orbitals should be degenerate to cause a sharp transition of electrons from the Mg 1s orbital. As a result, the spectral features of B1, B2, B3, and B5 appear in Mg K-edge NEXAFS spectra of MgO50,51 at 1305, 1311, 1318 and 1350 eV, respectively (Fig. 10a). Besides, a shoulder, B4 (∼1339 eV), also appears in the Mg K-edge spectra of all the nanoparticles. A visual inspection shows that these spectral features dominate with an increase in annealing temperature; this indicates that the Mg2+ ion co-ordination strengthens with an increase in the annealing temperature. To gain further insights into the Mg2+ ion co-ordination, the difference in the B1 and B2 spectral features (B2–B1) is estimated, as shown in Fig. 10b. The inset in Fig. 10b shows the de-convoluted spectrum for one representative nanoparticle system. The B1 and B2 spectral features are highlighted in red and green, respectively. The nanoparticle systems M400, M500, M700, M900 and M1100 exhibit difference (B2–B1) values of 6.05 ± 0.02, 6.04 ± 0.02, 6.15 ± 0.02, 6.10 ± 0.02 and 6.21 ± 0.02 eV, respectively, which are close to that for bulk MgO. Hence, these nanoparticles exhibit Mg2+ ion co-ordination similar to that of bulk MgO.52
The B1B2 difference (δB1B2) value is 5.31 ± 0.05 eV for M350, which indicates reduced co-ordination compared to those for the other nanoparticle systems. To further corroborate the chemical nature of Mg2+ ions in these nanoparticles, the Mg 1s spectra for various nanoparticles are de-convoluted, as shown in Fig. 11a. Mg 1s spectra are consistent with O 1s spectra, and they also reveal two different types of Mg2+ ion co-ordinations: one is associated with Mg2+ in MgO and the other is associated with Mg2+ in MgCO3 (Table S2, ESI†).47–49 The spectral features associated with two different types of Mg2+ ions are B1′ and B2′ centered around 1304.2 and 1305.2 eV, respectively (Table S2, ESI†). The spectral feature B3′ (∼1306.6 eV) is also observed in the Mg 1s spectra of these nanoparticles. Thus, lowering of difference value is associated with the presence of Mg2+ ions in the carbonate form. Moreover, the area ratio B2′/B1′ decreases up to 900 °C and then increases at 1100 °C; this behavior is analogous to that obtained from the O 1s spectra for these nanoparticles, which indicates an excess content of Mg2+ ions in the form of MgCO3.
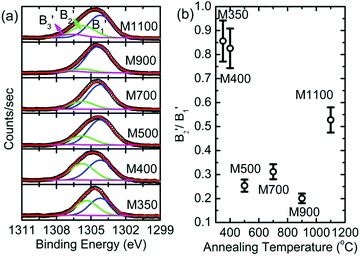 | ||
| Fig. 11 (a) Deconvoluted Mg 1s spectra and (b) variation of B2′/B1′ with annealing temperature for the M350, M400, M500, M700, M900 and M1100 nanoparticles. | ||
Thus, these measurements provide information that is specific to the chemical environment in the MgO lattice for these nanoparticles. Since the onset of organic impurities is observed from the O K-edge and O 1s spectra, which is supported by the Mg K-edge and Mg 1s spectra, the exact nature of these impurities is determined by measuring C K-edge NEXAFS spectra and C 1s XPS spectra for these nanoparticle systems (Fig. 12). These spectra exhibit spectral features C1, C2, C3, C4 and C5 centered at 287, 291, 295, 298 and 301 eV, respectively (Fig. 12a), which are ascribed to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O bonds. This reveals the presence of organic impurities in the synthesized nanoparticles.53 To further, corroborate the results obtained from the C K-edge spectra, the deconvoluted C 1s spectra for the nanoparticles are shown in Fig. 12b. The C 1s spectra of M350 and M400 nanoparticles exhibit deconvoluted spectral features C1′, C2′, C3′, C4′, C5′, C6′ and C7′ centered at 283.0, 284.2, 285.6, 286.1, 288.6, 289.9 and 291.7 eV, respectively (Fig. 12b). M500, M700, M900 and M1100 nanoparticles exhibit deconvoluted spectral features C2′, C3′, C5′, C6′ and C7′ centered at 283.5, 284.7, 286.1, 289.7 and 291.7 eV, respectively (Fig. 12b). All these spectral features are associated with the C–O and C
O and C–O bonds. This reveals the presence of organic impurities in the synthesized nanoparticles.53 To further, corroborate the results obtained from the C K-edge spectra, the deconvoluted C 1s spectra for the nanoparticles are shown in Fig. 12b. The C 1s spectra of M350 and M400 nanoparticles exhibit deconvoluted spectral features C1′, C2′, C3′, C4′, C5′, C6′ and C7′ centered at 283.0, 284.2, 285.6, 286.1, 288.6, 289.9 and 291.7 eV, respectively (Fig. 12b). M500, M700, M900 and M1100 nanoparticles exhibit deconvoluted spectral features C2′, C3′, C5′, C6′ and C7′ centered at 283.5, 284.7, 286.1, 289.7 and 291.7 eV, respectively (Fig. 12b). All these spectral features are associated with the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds present in the nanoparticles (Table S3, ESI†).47–50,54,55 Thus, the XPS measurements also reveal the presence of carbon-like impurities in these nanoparticles.
O bonds present in the nanoparticles (Table S3, ESI†).47–50,54,55 Thus, the XPS measurements also reveal the presence of carbon-like impurities in these nanoparticles.
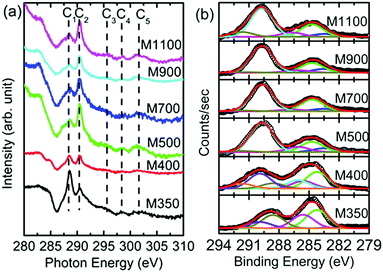 | ||
| Fig. 12 (a) C K-edge spectra and (b) deconvoluted C 1s spectra for the M350, M400, M500, M700, M900 and M1100 nanoparticles. | ||
The N K-edge and N 1s spectra for these materials are shown in Fig. 13. The N K-edge spectra consist of spectral features D1 and D2 (Fig. 13a). The D1 spectral feature is associated with amino-type species in these nanoparticles,56,57 and the D2 spectral feature appears due to the presence of N–O and nitride carbon.56–58 Thus, the presence of these spectral features is associated with N in the form of amino-type, N–O and nitride carbon in these nanoparticles. However, N 1s spectra of these nanoparticles do not exhibit any features associated with these types of bonds (Fig. 13b).
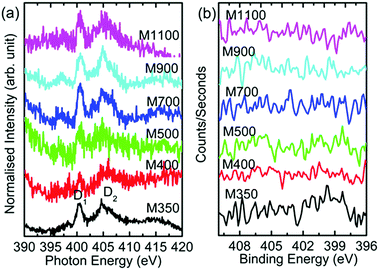 | ||
| Fig. 13 (a) N K-edge spectra and (b) N 1s spectra for the M350, M400, M500, M700, M900 and M1100 nanoparticles. | ||
Thus, measurements obtained from XRD, SEM, TEM, NEXAFS and XPS provide information about the crystal phase, size, and local electronic structure of synthesized nanoparticles. On the basis of these measurements, a schematic representing the growth and Mg2+ co-ordination with annealing temperature is shown in Fig. 14.
Optical response
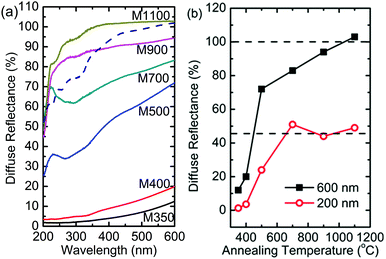
The optical response of the nanoparticles was studied by measuring their UV-Vis spectra in the diffuse reflectance mode. Fig. 15a shows the diffuse reflectance UV-Vis spectra of the nanoparticles and MgO-ref (MgO procured from Alfa Aesar). A distinct difference was observed between the UV-Vis spectra of these nanoparticles. In the measured range, the maximum reflectance of MgO-ref was almost 100% at the wavelength of 600 nm (Fig. 15a).59 A similar value was obtained for M1100; however, the spectra of both samples were not very similar. The maximum reflectance values for M900, M700 and M500 were 90%, 78% and 62%, respectively (Fig. 15b). A similar change in optical properties with the reduction in crystallite size was also reported by numerous researchers.21,23,60,61Besides, a peak is observed for M700 and M500 at a wavelength of 240 nm, which is not observed for M900. Also, the diffuse reflectance values drop significantly for M900, M700 and M500 in the measured range (Fig. 15a). The diffuse reflectance spectra for M400 and M350 are very different from those observed for other nanoparticle systems; the reflectance for these nanoparticles reduces from 15% to 7% at a wavelength of 600 nm. Thus, this suggests that the reflectance for these nanoparticle systems is lower when the carbon concentration is high. In another report for similar samples, we have shown that the optical band gap of MgO nanoparticles is lower than that of MgO-ref, which is an adverse effect of optical confinement.62 We have associated this effect to the presence of organic impurities. Thus, reduced reflectance is associated with the presence of carbon-like templates in these nanoparticles.
Magnetic response
Since the reduced dimensions of MgO make it a strong candidate for do ferromagnetism, this effect was studied for the synthesized nanoparticles using XMCD measurements. Fig. 15 shows the O K-edge spectra of M400, M500 and M1100 under right- and left-handed circular helicities. The spectral features Ao, A1, A2, A3, and A4 appeared in the spectra of these materials (Fig. 16a), which corresponded to those shown in Fig. 8a, even though the spectra were measured at different beamlines. The green line in the spectrum of each material shows the difference in absorption under different helicities, which is the XMCD signal for these materials.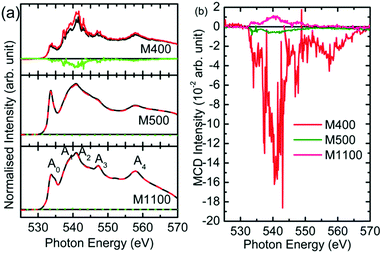 | ||
| Fig. 16 (a) O K-edge absorption under right-handed (black line) and left-handed (red line) circular helicity and (b) XMCD signals for the M400, M500 and M1100 nanoparticles. | ||
These signals are shown in Fig. 16b for clarity. It is clear that M400 nanoparticles show a dominant XMCD signal. Thus, the dominant XMCD signal was observed for carbon-rich nanoparticles.63 Similarly, the magnetism of carbon-rich ZnO has also been reported.64 Hence, this suggests that the optical and magnetic response is ascribed to the presence of organic impurities rather than crystallite size.
Conclusions
In the present study, we have synthesized MgO nanoparticles from magnesium nitrate. These nanoparticles exhibit the pure rock salt phase of MgO. Both the crystallite size and particle size increase with annealing temperature. EDX spectra show that the presence of organic impurities is dominant at lower annealing temperatures. The near-edge X-ray absorption fine structure measurements indicate the strengthening of Mg2+ and O2− ion co-ordination with an increase in the annealing temperature. These measurements also reflect the onset of organic impurities in the nanoparticles at low annealing temperatures. The diffuse reflectance spectra of the nanoparticles reveal a huge reduction of reflectance compared with that of reference MgO. Also, the X-ray magnetic circular dichroism signal is dominant for the organic impurity-rich nanoparticles. Thus, the magnetic and optical response is associated with organic templates in MgO nanoparticles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Korea Institute of Science and Technology (KIST Project No.: 2V06030).Notes and references
- O. Jambois, P. Carreras, A. Antony, J. Bertomeu and C. Martínez-Boubeta, Solid State Commun., 2011, 151, 1856 CrossRef.
- T. Uchino and D. Okutsu, Phys. Rev. Lett., 2008, 101, 117401 CrossRef PubMed.
- J. P. Singh and K. H. Chae, Condens. Matter, 2017, 2, 36 CrossRef.
- J. P. Singh, C. L. Chen, C. L. Dong, J. Prakash, D. Kabiraj, D. Kanjilal, W. F. Pong and K. Asokan, Superlattices Microstruct., 2015, 77, 313 CrossRef.
- Y. Li, R. Deng, B. Yao, G. Xing, D. Wang and T. Wu, Appl. Phys. Lett., 2010, 97, 102506 CrossRef.
- D. Mishra, B. P. Mandal, R. Mukherjee, R. Naik, G. Lawes and B. Nadgorny, Appl. Phys. Lett., 2013, 102, 182404 CrossRef.
- Q. Li, B. Ye, Y. Hao, J. Liu, J. Zhang, L. Zhang, W. Kong, H. Weng and B. Ye, Chem. Phys. Lett., 2013, 556, 237–241 CrossRef.
- C. M. Araujo, M. Kapilashrami, X. Jun, O. D. Jayakumar, S. Nagar, Y. Wu, C. Århammar, B. Johansson, L. Belova, R. Ahuja, G. A. Gehring and K. V. Rao, Appl. Phys. Lett., 2010, 96, 232505 CrossRef.
- M. Kapilashrami, J. Xu, K. V. Rao, L. Belova, E. Carlegrim and M. Fahlman, J. Phys.: Condens. Matter, 2010, 22, 345004 CrossRef PubMed.
- Y. Wu, Y. Zhan, M. Fahlman, M. Fang, K. V. Rao and L. Belova, MRS Online Proc. Libr., 2011, 1292, K12–K26 Search PubMed.
- J. Li, Y. Jiang, Y. Li, D. Yang, Y. Xu and M. Yan, Appl. Phys. Lett., 2013, 102, 072406 CrossRef.
- B. Choudhury and A. Choudhury, Mater. Res. Express, 2014, 1, 025026 CrossRef.
- J. Hu, Z. Zhang, M. Zhao, H. Qin and M. Jiang, Appl. Phys. Lett., 2008, 93, 192503 CrossRef.
- N. Kumar, D. Jagadeesan, P. B. Pillai, M. Chacko, M. Eswaramoorthy and A. Sundaresan, Chem. Phys. Lett., 2011, 504, 189–192 CrossRef.
- S. K. Mahadeva, J. Fan, A. Biswas, K. S. Sreelath, L. Belova and K. V. Rao, Nanomaterials, 2013, 3, 486–497 CrossRef PubMed.
- A. Kmar, J. Kumar and S. Priya, Appl. Phys. Lett., 2012, 100, 192404 CrossRef.
- B. M. Maoz, E. Tirosh, M. B. Sadan and G. Markovich, Phys. Rev. B, 2011, 83, 161201(R) CrossRef.
- L. Balcells, J. I. Beltrán, C. Martínez-Boubeta, Z. Konstantinović, J. Arbiol and B. Martínez, Appl. Phys. Lett., 2011, 97, 252503 CrossRef.
- M. R. Bindhu, M. Umadevi, M. Kavin Micheal, M. V. Arasu and N. A. Al-Dhabi, Mater. Lett., 2016, 166, 19–22 CrossRef.
- M. W. Williams and E. T. Arakawa, J. Appl. Phys., 1967, 38, 5272–5276 CrossRef.
- H. R. Moon, J. J. Urban and D. J. Milliron, Angew. Chem., Int. Ed., 2009, 48, 6278–6281 CrossRef PubMed.
- K. R. Nemade and S. A. Waghuley, Int. J. Met., 2014, 389416 Search PubMed.
- M. C. C. Wobbe, A. Kerridge and M. A. Zwijnenburg, Phys. Chem. Chem. Phys., 2014, 16, 22052–22061 RSC.
- J. P. Singh, S. H. Lee, S. O. Won, W. C. Li, I.-J. Lee and K. H. Chae, CrystEngComm, 2016, 18, 2701–2711 RSC.
- F. Kagawa, S. Horiuchi, M. Tokunaga, J. Fujioka and Y. Tokura, Nat. Phys., 2010, 6, 169–172 Search PubMed.
- E. Dormann, H. Winter, B. Gotschy and H. Naarmann, Phys. Scr., T, 1993, 49, 731–734 Search PubMed.
- J. P. Singh, M.-J. Ji, M. Kumar, I.-J. Lee and K. H. Chae, J. Alloys Compd., 2018, 748, 355–362 CrossRef.
- J.-C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
- L. Armelao, D. Barreca, G. Bottaro and S. Gross, Surf. Sci. Spectra, 2003, 10, 137–142 CrossRef.
- P. Jiang, D. Prendergast, F. Borondics, S. Porsgaard, L. Giovanetti, E. Pach, J. Newberg, H. Bluhm, F. Besenbacher and M. Salmeron, J. Chem. Phys., 2013, 138, 024704 CrossRef PubMed.
- D. Schmeisser, M. Tallarida, K. Henkel, K. Muller, D. Mandal, D. Chumakov and E. Zschch, Mater. Sci.-Pol., 2009, 27, 141–157 Search PubMed.
- A. G. Thomas and K. L. Syres, Chem. Soc. Rev., 2012, 41, 4207–4217 RSC.
- G. van der Laan and A. I. Figueroa, Coord. Chem. Rev., 2014, 277–278, 95–129 CrossRef.
- C. E. R. Torres, F. Golmar, M. Ziese, P. Esquinazi and S. P. Heluani, Phys. Rev. B, 2011, 84, 064404 CrossRef.
- Y. F. Wang, S. B. Singh, M. V. Limaye, Y. C. Shao, S. H. Hsieh, L. Y. Chen, H. C. Hsueh, H. T. Wang, J. W. Chiou, Y. C. Yeh, C. W. Chen, C. H. Chen, S. C. Ray, J. Wang, W. F. Pong, Y. Takagi, T. Ohigashi, T. Yokoyama and N. Kosugi, Sci. Rep., 2015, 5, 15439 CrossRef PubMed.
- C. Guglieri, M. A. Laguna-Marco, M. A. García, N. Carmona, E. Céspedes, M. García-Hernández, A. Espinosa and J. Chaboy, J. Phys. Chem. C, 2012, 116, 6608–6614 CrossRef.
- S. B. Singh, Y.-F. Wang, Y.-C. Shao, H.-Y. Lai, S.-H. Hsieh, M. V. Limaye, C.-H. Chuang, H.-C. Hsueh, H. Wang, J.-W. Chiou, H.-M. Tsai, C.-W. Pao, C.-H. Chen, H.-J. Lin, J.-F. Lee, C.-T. Wu, J.-J. Wu, W.-F. Pong, T. Ohigashi, N. Kosugi, J. Wang, J. Zhou, T. Regier and T.-K. Sham, Nanoscale, 2014, 6, 9166–9176 RSC.
- X. Cui, Z. Feng, Y. Jin, A. Y. Cao, D. Deng, H. Chu, S. Cao, C. Dong and J. c. Zhang, J. Appl. Crystallogr., 2015, 48, 1581–1586 CrossRef.
- H.-N. Hwang, H.-S. Kim, B. Kim, C. C. Hwang, S. W. Moon, S. M. Chung, C. Jeon, C.-Y. Park, K. H. Chae and W. K. Choi, Nucl. Instrum. Methods Phys. Res., Sect. A, 2007, 581, 850–855 CrossRef.
- C. H. Sohn, D.-Y. Cho, C.-T. Kuo, L. J. Sandilands, T. F. Qi, G. Cao and T. W. Noh, Sci. Rep., 2016, 6, 23856 CrossRef PubMed.
- M. A. Shand, Physical and chemical properties of magnesium oxide, The Chemistry and Technology of Magnesia, Wiley, 2006 Search PubMed.
- J. P. Singh, S. O. Won, W. C. Lim, I. J. Lee and K. H. Chae, J. Mol. Struct., 2016, 1108, 444–450 CrossRef.
- J. A. McLeod, R. G. Wilks, N. A. Skorikov and L. D. Finkelstein, Phys. Rev. B, 2010, 81, 245123 CrossRef.
- J. P. Singh, I. Sulania, J. Prakash, S. Gautam, K. H. Chae, D. Kanjilal and K. Asokan, Adv. Mater. Lett., 2012, 3, 112–117 CrossRef.
- T. Linder, H. Sauer, W. Engel and K. Kmabe, Phys. Rev. B, 2016, 33, 22–27 CrossRef.
- D. Gador, C. Buchberger, A. Soukopp, M. Sokolowski, R. Fink and E. Umbach, J. Electron Spectrosc. Relat. Phenom., 1999, 101-103, 532–537 Search PubMed.
- A. R. Head and J. Schnadt, JOM, 2016, 68, 3070–3077 CrossRef.
- F. Khairallah and A. Glisenti, Surf. Sci. Spectra, 2006, 13, 58–71 CrossRef.
- J. H. Lee, J. H. Eun, S. G. Kim, S. J. Park, M. J. Lee and H. J. Kim, J. Mater. Res., 2003, 18, 2895–2903 CrossRef.
- T. Yoshida, T. Tanaka, H. Yoshida, T. Funabiki and S. Yoshid, J. Phys. Chem., 1995, 99, 10890–10896 CrossRef.
- J. P. Singh, S. Gautam, B. B. Singh, S. Chaudhary, D. Kabiraj, D. Kanjilal, K. H. Chae, R. Kotnala, J.-M. Lee, J.-M. Chen and K. Asokan, Adv. Mater. Lett., 2014, 5, 372–377 CrossRef.
- J. P. Singh, W. C. Lim, J. Lee, J. Song and K. H. Chae, Appl. Surf. Sci., 2018, 432, 131–139 CrossRef.
- J. P. Singh, M.-J. Ji, C.-H. Shim, S. O. Kim and K. H. Chae, Particuology, 2017, 33, 47–49 CrossRef.
- A. Canneva, I. S. Giordana, G. Erra and A. Calvo, Energy Fuels, 2017, 31, 10414–10419 CrossRef.
- J. Larrieu, F. Clément, B. Held, N. Soulem, F. Luthon, C. Guimon and H. Martinez, Surf. Interface Anal., 2005, 37, 544–554 CrossRef.
- P. D. Johnson, Phys. Rev., 1954, 94, 845–849 CrossRef.
- I.-C. Ho, Y. Xu and J. D. Mackenzie, J. Sol-Gel Sci. Technol., 1997, 9, 295–301 Search PubMed.
- S. Stankic, M. Müller, O. Diwald, M. Sterrer, E. Knözinger and J. Bernardi, Angew. Chem., Int. Ed., 2005, 44, 4917–4920 CrossRef PubMed.
- J. P. Singh, S. O. Won, W. C. Lim and K. H. Chae, Mater. Lett., 2017, 198, 34–37 CrossRef.
- P. Zhu, J. Song, D. Lv, D. Wang, C. Jaye, D. A. Fischer, T. Wu and Y. Chen, J. Phys. Chem. C, 2014, 118, 7765–7771 CrossRef.
- S. Yang, X. Feng, X. Wang and K. Mllen, Angew. Chem., Int. Ed., 2011, 50, 5339–5343 CrossRef PubMed.
- S. Yang, X. Feng, X. Wang and K. Mülle, Angew. Chem., 2011, 123, 5451–5455 CrossRef.
- J. Lee, N. G. Subramaniam, I. A. Kowalik, J. Nisar, J. Lee, Y. Kwon, J. Lee, T. Kang, X. Peng, D. Arvanitis and R. Ahuja, Sci. Rep., 2015, 5, 17053 CrossRef PubMed.
- D.-T. Ngo, L. T. Cuong, N. H. Cuong, C. T. Son, P. T. Huy and N. D. Dung, Adv. Funct. Mater., 2018, 17, 1704567 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00145f |
| This journal is © the Partner Organisations 2018 |