Base mediated green synthesis of enantiopure 2-C-spiro-glycosyl-3-nitrochromenes†
Sabita
Nayak
 *a,
Pravati
Panda
a,
Bishnu Prasad
Raiguru
a,
Seetaram
Mohapatra
a and
Chandra Shekhar
Purohit
*a,
Pravati
Panda
a,
Bishnu Prasad
Raiguru
a,
Seetaram
Mohapatra
a and
Chandra Shekhar
Purohit
 b
b
aDepartment of Chemistry, Ravenshaw University, Cuttack, Odisha, India. E-mail: sabitanayak18@gmail.com
bSchool of Chemical Sciences, National Institute of Science Education and Research, Bhubaneswar, Odisha 751 005, India
First published on 19th November 2018
Abstract
A novel green synthetic methodology has been developed to obtain enantiopure (2S)-2-C-spiro-glycosyl-3-nitrochromenes following the oxa-Michael–aldol condensation reaction of sugar derived 3-C-vinyl nitro olefins with substituted salicylaldehydes using Et3N as a base under neat conditions at rt–40 °C. The stereochemistry of the product is confirmed by a single crystal X-ray study. Several advantages are associated with this protocol such as cost effectiveness, easy accessibility, short reaction time, high yields, wide substrate scope and high enantiopurity.
Introduction
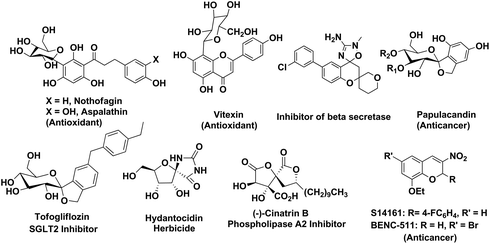
C-Glycosides represent an important class of bioactive compounds that are resistant to metabolic processing.1 They play a pivotal role in developing novel materials, surfactants and bioactive molecules.2 A broad class of natural products such as flavones,3 xanthones,4 chromenes5 and anthrones6 possess aryl-C-glycosides. The synthetic and natural products containing aryl-C-glycosides such as nothofagin, aspalathin, vitexin, papulacandin, tofogliflozin etc. are of great significance in medicinal chemistry owing to their stability toward enzymatic and chemical hydrolysis as compared to the O-glycosides (Fig. 1).7 These compounds possess a wide range of biological properties such as antioxidant, antidiabetic, potent enzyme inhibitor and anticancer properties.3–6,8Similarly, imparting stereochemistry in the quaternary centers of a carbohydrate building block for the generation of structurally intriguing and biologically potent natural products and building blocks is a daunting challenge in asymmetric synthesis and has attracted considerable attention in recent years due to their potential biological activities.9 Stereoselective formation of an oxa-quaternary carbon center at the anomeric position has been reported by various methods such as the semipinacol rearrangement reaction, intramolecular C–H insertion reaction, ring closing metathesis, palladium catalysed cross coupling reaction, 1,3-dipolar cycloaddition reaction, hetero-Michael addition reaction etc.10 The literature survey reveals a number of methods on the synthesis of analogues of natural spironucleoside (+)-hydantocidin, (−)-cinatrin B and papulacandin in which the heterocyclic ring was fused at the anomeric position.11 But the stereoselective synthesis of six- or seven-membered C-spiro-heterocycles at the 3-position of glucofuranose has not been investigated extensively although the six-membered heterocyclic ring exists in many drug molecules as the core substructure and their derivatives are of very important biological significance.12
Similarly, chromenes and their derivatives belong to an important family of heterocycles that are widespread in plants13 and possess a broad range of biological properties.14 The current literature report reveals that 2H-chromenes and their derivatives such as S14161 and BENC-511 show potent anticancer activities (Fig. 1).15,16 A key aspect is that the lipophilic nature of the benzopyran derivatives helps to cross the cell membrane more easily. Particularly, 3-nitro-2H-chromenes are of great importance because of their potential uses as pesticides, endothelin-A (ETA)-selective receptor antagonists, inhibitors of the proliferation of cancer cells, highly potent antihypertensive drugs, important intermediates (flavanol, chroman-3-amine) in the synthesis of medicinally active 2H-benzopyrans and potential candidates for nonlinear optical applications (Fig 1).17–20 Due to the advent of simple methods of synthesis and high reactivity of 3-nitro-2H-chromenes, they are used as precursors for the synthesis of various complex natural products.21
On account of the biological significance of both C-spiro glycosides and nitrochromenes and due to our enormous interest towards the synthesis of spiro-C-glycosides, we became interested to fuse both of them in a single molecular framework to synthesize 2-C-spiro-glycosyl-3-nitrochromenes.
Herein we first report the enantiopure synthesis of 2-C-spiro-glycosyl-3-nitrochromenes in a fewer number of steps with good to excellent yields at rt–40 °C in a very short period of time under neat conditions. Moreover, the present discovery will also provide access for further functional group interconversion to increase the molecular complexity.
Though the syntheses of enantiopure 2-C-glycosyl-3-nitrochromenes were reported by Raquel G. Soengas et al.22 in multiple steps with moderate yield, enantiopure synthesis of 2-C-spiro-glycosyl-3-nitrochromenes has not been reported yet.
To the best of our knowledge, this is the first report on the development of a general methodology for the enantiopure construction of sugar fused nitrochromene derivatives, which are new types of C-spiro-glycosides.
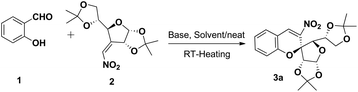
Our synthetic endeavour commenced from commercially available salicylaldehydes 1 and 3-C-vinyl sugar nitro olefins 2. Compound 2 was prepared from glucofuranose diacetonide in three steps following the reported literature procedure.23,24 The feasibility of the reaction between 1 and 2 was investigated by varying different reaction parameters such as in the presence of air, various bases, neat conditions and using organic solvents at rt–40 °C (Table 1).
| Entry | Base | Solvent | Time | Yieldb (%) |
|---|---|---|---|---|
| a 1 (1 mmol), 2 (1 mmol), base (1 mmol). b Isolated yield. c Stirring at room temperature–40 °C. d Oil bath heating, 80 °C. | ||||
| 1c | DABCO | Neat | 2 h | 55% |
| 2d | DABCO | Neat | 12 h | 30% |
| 3c | DABCO | CH3CN | 2 h | 50% |
| 4d | DABCO | CH3CN | 2 h | 40% |
| 5c | K2CO3 | Neat | 45 min | 80% |
| 6c | K2CO3 | Dioxane | 2 h | 55% |
| 7d | K2CO3 | Dioxane | 2 h | 50% |
| 8c | Et3N | Neat | 15 min | 85% |
| 9c | DBU | Neat | 2 h | 30% |
Initially o-hydroxy benzaldehyde 1 was subjected to the reaction with 3-C-vinyl nitro olefins of glucose diacetonide 2 in DABCO at rt–40 °C under neat conditions. Increasing the time and temperature was deleterious to the yield of the reaction (entries 1 and 2). The same reaction was again monitored with DABCO using CH3CN as a solvent from rt–40 °C (entries 3 and 4). But the yield of the product formed did not vary a lot. A mixture of 1, 2 and K2CO3 under neat conditions at room temperature was stirred for 45 min mechanically (entries 5) and the yield of the product was found to be 80%. The reaction was also tried in dioxane at room temperature and under heating conditions (entries 6 and 7). Extensive experiments revealed that the yield of product 3a was greatly affected by the use of solvents. The reaction was also performed in Et3N under neat conditions (entries 8). Rapid conversion was observed when triethyl amine was added to a mixture of 1 and 2, and the spirocyclised product 3a was formed with 85% yield, in 15 min encouraging us to move further. Interestingly, only one enantiomer 3a was observed under all different reaction conditions. The reaction proceeded well in air or under an oxygen atmosphere. The reaction took a longer time in DBU and the yield of the product formation decreased (entries 9). All the above screening results demonstrate that the reaction proceeded to completion within 15 min in a clean manner at rt–40 °C in triethylamine.
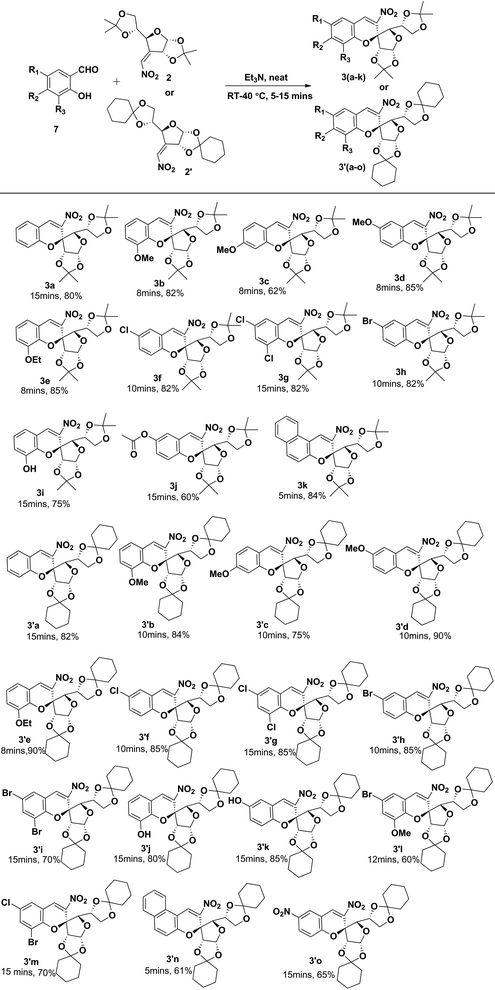
With the optimized reaction conditions established, we further explored the substrate scope of o-hydroxy benzaldehydes and the 3-C-nitro olefin of glucofuranose diisopropylidine/dicyclohexylidine. The results are shown in Scheme 1. To our delight, the reaction exhibited a broad generality and readily afforded a variety of different 2-C-spiro-glycosyl-3-nitrochromenes 3(a–k) and 3′(a–o) in moderate to good yields with specific enantioselectivities. Notably, the reaction displayed excellent functional group compatibility for the 3-C-nitro olefin of glucofuranose dicyclohexylidine containing electron-withdrawing groups (such as –NO2 groups) as well as electron donating groups (such as methoxy and ethoxy groups) and the corresponding products 3′(a–o) were produced in good yields in comparison to glucofuranose diacetonide 3(a–k). Generally the presence of electron rich substituents on the aromatic ring results in a higher yield of the product in both cases. The aromatic ring containing halogen substituents (dichloro, dibromo, monochloro, monobromo) were also tolerated without any difficulty except bromo-chloro substitution.
When bromo chloro salicylaldehyde and 5-nitro salicylaldehyde were reacted with the 3-C-nitro olefin of glucofuranose diacetonide 2 the expected product was not formed but excitingly good yield of the product was observed in the case of dicyclohexylidine 2′. Similarly when 4-hydroxy salicylaldehyde was reacted with the 3-C-nitro olefin of glucofuranose diacetonide 2/dicyclohexylidine 2′, the desired product was not formed. The feasibility of the reaction was monitored with different bases such as DABCO, K2CO3, DBU and solvents as well as under neat conditions. But unfortunately we were unable to obtain our target product. Furthermore, we have increased the time and temperature of the reaction, but we noticed that increasing the time as well as the temperature of the system leads to the decomposition of the 3-C-nitro olefin of glucofuranose diacetonide 2 and regretfully, the expected product was not formed. We also observed that when naphthyl salicylaldehyde was reacted with 3-C-vinyl nitro glucofuranose diisopropylidine good yield of the product 3k was obtained as compared to 3′n which might be due to the steric effect.
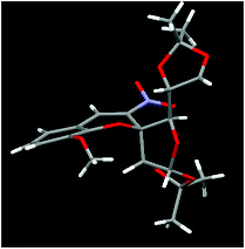
It is noteworthy that the current protocol represents a new and practical pathway for assembly of richly decorated 2-C-spiro-glycosyl-3-nitrochromenes with excellent enantioselectivities through the Et3N catalysed oxa-Michael–aldol condensation reaction of salicylaldehydes and the 3-C-nitro olefin of glucofuranose diisopropylidine/dicyclohexylidine. The structures of these spiro nitrochromenes were characterised by NMR spectroscopy and HRMS. The stereochemistry of the product was assigned by single crystal X-ray of compound 3b and the ‘S’-stereocentre was confirmed at the spirocarbon which is shown in Fig. 2. The exclusive formation of the ‘S’-stereoisomer at the spiro carbon might be controlled by the steric bulk of 1,2-diisopropylidine/cyclohexylidine of the sugar moiety.
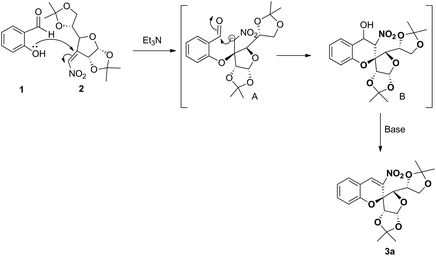
The reasonable mechanism for the formation of 3a is proposed as shown in Scheme 2. First, in the presence of a base the O-atom of 1 undergoes the Michael addition reaction with C-vinyl nitro 2 to form anion A, which then undergoes nucleophilic addition reaction with the carbonyl group of TS A to form the intermediate TS B. Subsequent dehydration of intermediate TS B in the base medium provided the desired product 3a.
In order to extend the substrate scope of this protocol the 3-C-vinyl nitro olefin was replaced by a 3-C-vinyl ester and 3-C-vinyl nitrile in order to obtain 2-C-spiro-glycosyl-3-chromene esters/nitriles. The Michael addition reaction was also monitored in different base media such as DBU, DABCO, K2CO3, Et3N etc. Unfortunately the desired product was not formed.
In summary, the first highly enantiopure synthesis of structurally diverse (2S)-2-C-spiro-glycosyl-3-nitrochromenes is described following oxa-Michael–Aldol condensation reaction. The products were obtained by the reaction of sugar derived 3C-vinyl nitro olefins with various substituted salicylaldehydes using Et3N as base at rt–40 °C in good to excellent yield with high purity. No other external reagent or solvent was required, and also no hazardous chemical wastes were generated during the progress of the reaction. To the best of our knowledge, this strategy involves the first approach for these types of 2-C-spiro-glycosyl-3-nitrochromene derivatives and also involves a series of novel processes that are of broad interest to synthetic organic chemists.
Experimental section
General procedure for the preparation of 2-C-spiro-glycosyl-3-nitrochromenes 3
Salicylaldehyde (0.08 mmol, 1.0 equiv.), 3-C-vinyl nitro olefin of glucofuranose diisopropylidine/glucofuranose dicyclohexylidine (0.08 mmol, 1.0 equiv.) and Et3N (0.01 mmol, 1.0 equiv.) were added in sequence and stirred mechanically at rt–40 °C under an air atmosphere for 5–15 min. After the completion of the reaction, the residue was adsorbed with silica gel and then transferred directly on to the flash column, without any additional work-up step, and was subjected to chromatographic purification using petroleum ether/ethyl acetate to give 3 in good yield (60–90%).Conflicts of interest
There are no conflicts to declare.Acknowledgements
SN and SRM are thankful to the CSIR New Delhi [02(0218)/14/EMR-II] and the DRDO New Delhi ERIP/ER/1203083/M/01/1643 for providing research grant and also thankful to DST-FIST New Delhi for providing NMR facility. PP is thankful to DST, Odisha, No. 27562800382017/202519 for Biju Patnaik research fellowship.References
- H. Gong, R. Sinisi and M. R. Gagne, J. Am. Chem. Soc., 2007, 129, 1908–1909 CrossRef CAS.
- K. Lalitha, K. Muthusamy, Y. Siva Prasad, P. K. Vemula and S. Nagarajan, Carbohydr. Res., 2015, 402, 158–171 CrossRef CAS.
- G. Franz and M. Grun, J. Med. Plants Res., 1983, 47, 131–140 CrossRef CAS.
- M. Kaaden, E. Breukink and R. J. Pieters, Beilstein J. Org. Chem., 2012, 8, 732–737 CrossRef PubMed.
- P. W. Snijman, E. Joubert, D. Ferreira, X.-C. Li, Y. Ding, I. R. Green and W. C. A. Gelderblom, J. Agric. Food Chem., 2009, 57, 6678–6684 CrossRef CAS.
- J. H. Kim, B. C. Lee, J. H. Kim, G. S. Sim, D. H. Lee, K. E. Lee, Y. P. Yun and H. B. Pyo, Arch. Pharmacal Res., 2005, 28, 195–202 CrossRef CAS.
- (a) N. Anand, K. Upadhyaya, A. Ajay, R. Mahar, S. K. Shukla, B. Kumar and R. P. Tripathi, J. Org. Chem., 2013, 78, 4685–4696 CrossRef CAS; (b) M. Liu, Y. Niu, Y. F. Wu and X. S. Ye, Org. Lett., 2016, 18, 1836–1839 CrossRef CAS; (c) K. R. Cavanaugh, S. Narayanasamy, J. R. Walker, M. Clagett-Dame and R. W. J. Curley, J. Carbohydr. Chem., 2016, 35, 249–260 CrossRef CAS; (d) A. Dutta, D. Dhara, P. K. Parida, A. Si, R. Yesuvadian, K. Jana and A. K. Misra, RSC Adv., 2017, 7, 28853–28864 RSC; (e) Y. Zou, S. Zhang, X. Wen, G. Wang, Y. Sun, S. Liu, T. Peng, Y. Gao and L. Wang, Tetrahedron Lett., 2017, 58, 2835–2837 CrossRef CAS; (f) G. H. Kuo, M. D. Gaul, Y. Liang, J. Z. Xu, F. Du, P. Hornby, G. Xu, J. Qi, N. Wallace, S. Lee, E. Grant, W. V. Murray and K. Demarest, Bioorg. Med. Chem. Lett., 2018, 28, 1182–1187 CrossRef CAS.
- S. K. Maurya and S. Hotha, Tetrahedron Lett., 2006, 47, 3307–3310 CrossRef CAS.
- X. Li, R. Wang, Y. Wang, H. Chen, Z. Li, C. Ba and J. Zhang, Tetrahedron, 2008, 64, 9911–9920 CrossRef CAS.
- (a) P. R. Sridhar, K. Seshadri and G. M. Reddy, Chem. Commun., 2012, 48, 756–758 RSC; (b) M. R. Lambu, A. Hussain, D. K. Sharma, S. K. Yousuf, B. Singh, A. K. Tripathib and D. Mukherjee, RSC Adv., 2014, 4, 11023–11028 RSC; (c) R. P. Robinson, V. Mascitti, C. M. Boustany-Kari, C. L. Carr, P. M. Foley, E. Kimoto, M. T. Leininger, A. Lowe, M. K. Klenotic, J. I. Macdonald, R. J. Maguire, V. M. Masterson, T. S. Maurer, Z. Miao, J. D. Patel, C. Préville, M. R. Reese, L. She, C. M. Steppan, B. A. Thuma and T. Zhu, Bioorg. Med. Chem. Lett., 2010, 20, 1569–1572 CrossRef CAS PubMed; (d) V. Mascitti, R. P. Robinson, C. Préville, B. A. Thuma, C. L. Carr, M. R. Reese, R. J. Maguire, M. T. Leininger, A. Lowe and M. Steppan, Tetrahedron Lett., 2010, 51, 1880–1883 CrossRef CAS; (e) M. R. Lambu, A. Hussain, D. K. Sharma, S. K. Yousuf, B. Singh, A. K. Tripathi and D. Mukherjee, RSC Adv., 2014, 4, 11023–11028 RSC; (f) K. Ohba and M. Nakata, Org. Lett., 2015, 17, 2890–2893 CrossRef CAS; (g) Y. Ohtake, T. Emura, M. Nishimoto, K. Takano, K. Yamamoto, S. Tsuchiya, S.-Y. Yeu, Y. Kito, N. Kimura, S. Takeda, M. Tsukazaki, M. Murakata and T. J. Sato, Org. Chem., 2016, 81, 2148–2153 CrossRef CAS.
- (a) C. Chatgilialoglu, C. Ferreri, T. Gimisis, M. Roberti, J. Balzarini and E. D. Clercq, Nucleosides, Nucleotides Nucleic Acids, 2004, 23, 1565–1581 CrossRef CAS; (b) J. Fuentes, B. A. B. Salameh, M. A. Pradera, F. J. Fernandez de Cordoba and C. Gasch, Tetrahedron, 2006, 62, 97–111 CrossRef CAS; (c) C. Gasch, B. A. B. Salameh, M. A. Pradera and J. Fuentes, Tetrahedron Lett., 2001, 42, 8615–8617 CrossRef CAS; (d) K. Singha, A. Roy, P. K. Dutta, S. Tripathi, S. Sahabuddin, B. Achari and S. B. Mandal, J. Org. Chem., 2004, 69, 6507–6510 CrossRef CAS; (e) R. Freire, A. Martın, I. Perez-Martın and E. Suarez, Tetrahedron Lett., 2002, 43, 5113–5116 CrossRef CAS; (f) T. Q. Pham, S. G. Pyne, B. W. Skelton and A. H. White, J. Org. Chem., 2005, 70, 6369–6377 CrossRef CAS; (g) A. Renard, J. Lhomme and M. Kotera, J. Org. Chem., 2002, 67, 1302–1307 CrossRef CAS; (h) G. Enderlin, C. Taillefumier, C. Didierjean and Y. Chapleur, Tetrahedron: Asymmetry, 2005, 16, 2459–2474 CrossRef CAS; (i) A. Roy, B. Achari and S. B. Mandal, Tetrahedron Lett., 2006, 47, 3875–3879 CrossRef CAS; (j) M. Shiozaki, Carbohydr. Res., 2002, 337, 2077–2088 CrossRef CAS; (k) L. Somsak, L. Kovacs, M. Toth, E. Osz, L. Szilagyi, Z. Gyoergydeak, Z. Dinya, T. Docsa, B. Toth and P. Gergely, J. Med. Chem., 2001, 44, 2843–2848 CrossRef CAS; (l) R. Fischer, E. Hyrosova, A. Druckova, L. Fisera, C. Hametner and M. K. Cyranski, Synlett, 2003, 2364–2368 CAS; (m) M. Benltifa, S. Vidal, D. Gueyard, P. G. Goekjian, M. Msaddek and J. P. Praly, Tetrahedron Lett., 2006, 47, 6143–6147 CrossRef CAS; (n) R. Elek, L. Kiss, J. P. Praly and L. Somasak, Carbohydr. Res., 2005, 340, 1397–1402 CrossRef CAS; (o) P. Z. Zhang, X. L. Li, H. Chen, Y. N. Li and R. Wang, Tetrahedron Lett., 2007, 48, 7813–7816 CrossRef CAS; (p) P. A. Colinas, V. Jager, A. Lieberknecht and R. D. Bravo, Tetrahedron Lett., 2003, 44, 1071–1074 CrossRef CAS.
- (a) C. Taillefumier, S. Thielges and Y. Chapleur, Tetrahedron, 2004, 60, 2213–2224 CrossRef CAS; (b) C. Gasch, M. A. Pradera, B. A. B. Salameh, J. L. Molina and J. Fuentes, Tetrahedron: Asymmetry, 2001, 12, 1267–1277 CrossRef CAS; (c) L. A. Paquette, S. Brand and C. Behrens, J. Org. Chem., 1999, 64, 2010–2025 CrossRef CAS; (d) A. Bartolozzi, G. Capozzi, C. Falciani, S. Menichetti, C. Nativi and A. P. Bacialli, J. Org. Chem., 1999, 64, 6490–6494 CrossRef CAS; (e) T. M. Krulle, K. A. Watson, M. Gregoriou, L. N. Johnson, S. Crook, D. J. Watkin, R. C. Griffiths, R. J. Nash, K. E. Tsitsanou, S. E. Zographos, N. G. Oikonomakos and G. W. J. Fleet, Tetrahedron Lett., 1995, 36, 8291–8294 CrossRef CAS; (f) H. Sano, S. Mio, J. Kitagawa and S. Sugai, Tetrahedron: Asymmetry, 1994, 5, 2233–2240 CrossRef CAS.
- G. P. Ellis, Chromenes, Chromanones and Chromones, Wiley, New York, 1977, p. 31 Search PubMed.
- G. X. Wang, N. X. Wang, T. Shi, J. L. Yu and X. L. Tang, Prog. Chem., 2008, 20, 518 CAS.
- P. Panda, S. Nayak, S. K. Sahoo, S. Mohapatra, D. Nayak, R. Pradhan and C. N. Kundu, RSC Adv., 2018, 8, 16802–16814 RSC.
- P. Panda, S. Nayak, S. Bhakta, S. Mohapatra and T. R. Murthy, J. Chem. Sci., 2018, 130, 127–142 CrossRef.
- (a) W. S. Bowers, in Comprehensive Insect Physiology, Biochemistry, and Pharmacology, ed. G. A. Kerkut and L. I. Gilbert, Pergamon Press, Oxford, 1985, vol. 8, p. 551 Search PubMed; (b) W. S. Bowers, T. Ohta, J. S. Cleere and P. A. Marsella, Science, 1976, 193, 542–547 CrossRef CAS; (c) N. Ishizuka, K. Matsumura, K. Sakai, M. Fujimoto, S. Mihara and T. J. Yamamori, J. Med. Chem., 2002, 45, 2041–2055 CrossRef CAS; (d) R. Bergmann and R. Gericke, J. Med. Chem., 1990, 33, 492–504 CrossRef CAS.
- (a) G. Burrell, F. Cassidy, J. M. Evans, D. Lightowler and G. Stemp, J. Med. Chem., 1990, 33, 3023–3027 CrossRef CAS; (b) R. Gericke, J. Harting, I. Lues and C. Schittenhelm, J. Med. Chem., 1991, 34, 3074–3085 CrossRef CAS; (c) T. S. Rao, A. K. Singth and G. K. Trievdi, Heterocycles, 1984, 22, 1377–1382 CrossRef CAS.
- (a) S. R. Deshpande, H. H. Mathur and G. K. Trivedi, Synthesis, 1983, 835 CrossRef CAS; (b) G.-Q. Xiao, B.-X. Liang, S.-H. Chen, T.-M. Ou, X.-Z. Bu and M. Yan, Arch. Pharm. Chem. Life Sci., 2012, 345, 767–770 CrossRef CAS; (c) M.-C. Yan, Y.-J. Jang and C.-F. Yao, Tetrahedron Lett., 2001, 42, 2717–2721 CrossRef CAS.
- (a) S. Rahmani-Nezhad, M. Safavi, M. Pordeli, S. K. Ardestani, L. Khosravani, Y. Pourshojaei, M. Mahdavi, S. Emami, A. Foroumadi and A. Shafiee, Eur. J. Med. Chem., 2014, 86, 562–569 CrossRef CAS; (b) P.-A. Wang, D.-X. Zhang and X.-Y. Liua, ARKIVOC, 2014, 408 Search PubMed; (c) L. W. Dillard, J. Yuan, L. Jia and Y. Zheng, PATENT, PCT/US2009/004686, 2009 Search PubMed.
- (a) V. Y. Korotaev, V. Y. Sosnovskikh and A. Y. Barkov, Russ. Chem. Rev., 2013, 82, 1081–1116 CrossRef CAS; (b) S. Mohapatra, S. Bhakta, S. Chakroborty, M. Tripathy and S. Nayak, Res. Chem. Intermed., 2015, 41, 7799–7813 CrossRef CAS.
- R. G. Soengas, H. Rodríguez-Solla, A. M. S. Silva, R. Llavona and F. A. A. Paz, J. Org. Chem., 2013, 78, 12831–12836 CrossRef CAS.
- P. Merino-Montiel, O. Lopez and J. G. Fernandez-Bolanos, RSC Adv., 2012, 2, 11326–11335 RSC.
- J. Luginina, V. Rjabovs, S. Belyakov and M. Turks, Carbohydr. Res., 2012, 350, 86–89 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1869383. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ob02278j |
| This journal is © The Royal Society of Chemistry 2019 |