Scandium as a pre-catalyst for the deoxygenative allylation of benzylic alcohols†
Ivan
Šolić
a,
Pattarakiat
Seankongsuk
b,
Joanna Kejun
Loh
a,
Tirayut
Vilaivan
b and
Roderick W.
Bates
 *a
*a
aDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371. E-mail: roderick@ntu.edu.sg
bOrganic Synthesis Research Unit, Department of Chemistry, Faculty of Science, Chulalongkorn University, Phayathai Road, Patumwan, Bangkok 10330, Thailand
First published on 6th December 2017
Abstract
Scandium triflate is an effective pre-catalyst for the deoxygenative allylation of benzylic alcohols with a narrow substrate window. The reaction is shown to proceed through a “hidden Brønsted acid” mechanism. The reaction is efficient provided that the aryl group is neither too electron rich nor too electron poor. It is shown that this allows useful selectivity. The reaction also works for benzyhydryl alcohols with broader scope. The reaction may also be catalysed by Nafion.
Introduction
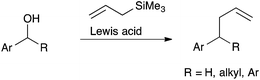
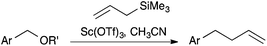
Numerous methods have been reported for the deoxygenative allylation of benzyl alcohols, benzhydryl alcohols and related compounds. All of these employ Lewis acids,1 including Brønsted acids,2 to mediate the reaction (Scheme 1). Not all of these reactions are catalytic as some Lewis acids employed are deactivated by the reaction conditions. Arising from our new-found interest in the use of lanthanide ions as Lewis acid catalysts,3 we were surprised to find no reports of their effective use in this reaction. To the best of our knowledge, there are just a few reports of their unsatisfactory or limited application.1c,g,r,t,2eResults and discussion
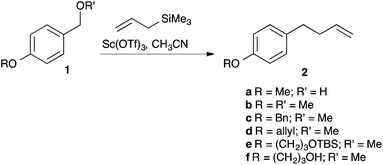
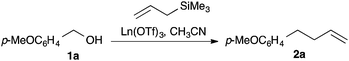
Anticipating a carbocation intermediate, we selected p-methoxybenzyl alcohol 1a for exploratory studies (Scheme 2). While treatment with allyltrimethylsilane and a sub-stoichiometric amount of scandium triflate4 in dichloromethane gave only modest results, a rapid reaction ensued when we employed acetonitrile5 as the solvent at 40 °C, giving alkene 2a in good yield (Table 1, entry 1).| Entry | Pre-catalyst | Solvent | Time | Yield (%) |
|---|---|---|---|---|
| a Solutions were 0.5 M in substrate, using 4 equivalents of the allyl silane; reactions were stirred at 40 °C for the indicated time. b Water content: 130 ppm. c Water content: 45 ppm. d Accompanied by some decomposition. | ||||
| 1 | Sc(OTf)3 | Bench CH3CNb | 1 h | 71 |
| 2 | Sc(OTf)3 | 2.5% H2O in CH3CN | 1 h | Traced |
| 3 | Sc(OTf)3 | 5% H2O in CH3CN | 2 h | — |
| 4 | Sc(OTf)3 | 10% H2O in CH3CN | 2 h | — |
| 5 | Sc(OTf)3 | Dried CH3CNc | 1 h | 24 |
| 6 | La(OTf)3 | Bench CH3CN | 1 h | 23 |
| 7 | Gd(OTf)3 | Bench CH3CN | 20 h | 15 |
| 8 | Lu(OTf)3 | Bench CH3CN | 20 h | 8d |
As we wished for a robust synthetic method, we employed “bench” acetonitrile, with no attempt at drying of the solvent or the glassware. Karl Fischer titration showed that this solvent contained approximately 130 ppm of water. Lanthanide triflates have been claimed to be water tolerant Lewis acids.6 We tested this by running the same reaction in acetonitrile to which a small amounts of water, from 2.5 to 10% by volume, had been added (entries 2–5). At most, only traces of the product could be observed. To our further surprise, the reaction using the 10% v/v mixture (entry 4) failed to proceed even at 90 °C. Interestingly, when dried acetonitrile (approx. 45 ppm water) was employed (entry 5), the reaction was much less efficient, giving only 24% yield in the same time as the original experiment. Three true lanthanide triflate salts were also tested, but gave much lower yields than scandium, even with extended reaction times (entries 6–8).
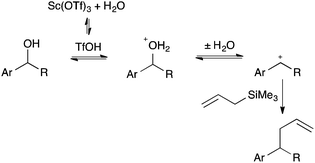
Spencer7 has demonstrated that the real catalyst when many metal salts are employed as Lewis acids is actually in situ generated H+i.e. a “hidden Brønsted acid”.8 Following the work of Spencer, we tested for this by addition of di-t-butyl-4-methylpyridine which, for steric reasons, is capable of sequestering a proton, but not a Lewis acid.9 In the presence of this additive, two equivalents relative to scandium, the reaction did not proceed at 40 °C. Even at 90 °C only a 60% conversion was observed after 22 hours. This experiment indicates that the reaction is actually catalysed by a Brønsted acid (Scheme 3), likely to have formed by hydrolysis of the scandium salt8b and that, in the absence of a proton source, substantially more forcing conditions are required. Scandium triflate is, therefore, a pre-catalyst unless those more forcing conditions are applied. Catalysis of this reaction by Brønsted acids is well documented.2 Indeed, the reaction was also catalysed by triflic acid giving 75% conversion in 2 hours. Given that triflic acid was a less effective catalyst than scandium triflate it seems that, although a hidden Brønsted acid mechanism operates, there is a modest synergistic effect due to the scandium. The addition of water, of course, inhibits the reaction by diluting the acid, while concentrations of water that are too low prevent the formation of a sufficient Brønsted acid concentration.
Consistent with the need to form a strong Brønsted acid by hydrolysis to catalyse the reaction, no reaction was observed when scandium acetate was employed.
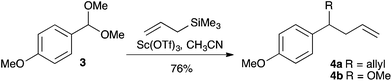
As would be expected from the mechanism, the corresponding methyl ether 1b performed equally well (Table 2, entry 2). In contrast, various other benzyl alcohols performed very poorly. No product could be obtained when benzyl alcohol 1g (entry 6), m-methoxybenzyl alcohol 1i (entry 8) and p-chlorobenzyl alcohol 1k (entry 10) were employed; low yields were obtained with o-methoxybenzyl alcohol 1h (entry 7) and piperonyl alcohol 1i (entry 9). These examples would be expected to be less electron rich or less effective at stabilising a benzylic cation. The lower reactivity is, therefore, to be expected. Such selectivity is, of course, valuable: when p-benzyloxybenzyl methyl ether 1c was employed as the substrate (entry 3), selective allylation at only the more electron rich benzylic position was observed. p-Allyloxybenzyl methyl ether 1d also underwent efficient deoxygenative allylation. A substrate 1e with a TBS group, however, did not survive the reaction conditions, predominantly yielding the corresponding allylated compound 1f with a free alcohol group.10 In addition, the dimethoxy acetal of p-methoxybenzaldehyde 3 underwent diallylation, giving diene 4a, contaminated by some of the mono-allylated product 4b in a 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Scheme 4).
1 ratio (Scheme 4).
| Entry | Substrate | Ar | R′ | Product, yield (%) |
|---|---|---|---|---|
| a Solutions were 0.5 M in substrate, using 4 equivalents of the allyl silane; reactions were stirred at 40 °C for the indicated time. b Desilylated product. c Oligomerisation was also observed: see Scheme 5. | ||||
| 1 | 1a | p-MeOC6H4 | H | 2a, 71 |
| 2 | 1b | p-MeOC6H4 | Me | 2a, 77 |
| 3 | 1c | p-BnOC6H4 | Me | 2c, 97 |
| 4 | 1d | p-Allyl O C6H4 | Me | 2d, 94 |
| 5 | 1e | p-TBSO(CH2)3OC6H4 | Me | 2e, 6 |
| 2f, 51b | ||||
| 6 | 1g | Ph | H | 0 |
| 7 | 1h | o-MeOC6H4 | H | 2h, 24 |
| 8 | 1i | m-MeOC6H4 | H | 0 |
| 9 | 1j | o,m-OCH2OC6H3 | H | 2j, 10 |
| 10 | 1k | p-ClC6H4 | H | 2k, 0 |
| 11 | 1l | 2-Thiophenyl | H | 2l, tracec |
| 12 | 1m | m,p-(MeO)2 | H | 2m, 15c |
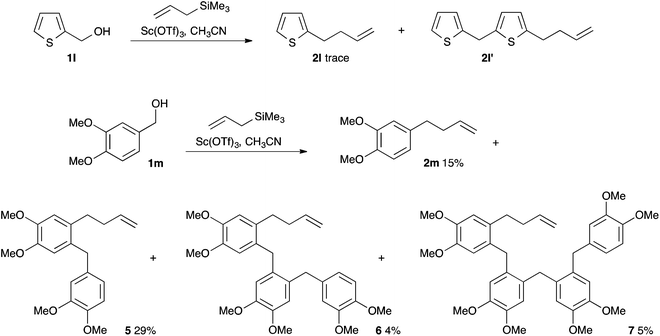
Thiophenylmethanol 1l (entry 11) gave a trace of the volatile allylation product 2l, accompanied by oligomerisation products, amongst which the pseudodimer 2l′ could be isolated (Scheme 5). We were interested to find what would happen with an aryl group bearing more electron donating groups. Veratryl alcohol 1m reacted rapidly under these conditions, but gave a mixture of products. The expected allylation product 2m was obtained in 15% yield (entry 12), but the pseudo-dimer 5, isolated in 29% yield, proved to be the major product. We were also able to isolate the pseudo-trimer 6 and pseudo-tetramer 7 in 4 and 5% yields respectively. Dimerisation, oligomerisation and polymerisation have been observed previously.1a,t In this case and that of the thiophenyl substrate, it appears that the aromatic ring of the allylation product is sufficiently nucleophilic to compete with the silane. We attempted to drive the selectivity by changing to allyl tri-n-butylstannane, a more reactive (but less green) nucleophile.11 Surprisingly, no product was obtained and starting material 1m was recovered. We suspect that this is due to destruction of Brønsted acids in the medium by reaction with the stannane. Indeed, when allyl tri-n-butylstannane was added to scandium triflate in d3-acetonitrile in an NMR tube, effervescence could be observed and the 1H NMR signals corresponding to propene could be identified.
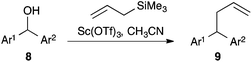
The dexoygenative allylation has been widely used with benzhydryl alcohols.12 Indeed, under our conditions, a range of benzhydryl alcohols 8 underwent deoxygenative allylation, giving the products 9 in good yields (Table 3). Notably, a ferrocenyl group could be one of the aromatic groups (entry 5). One exception was phenyl 2-furyl carbinol 8g (entry 7). We suspect this is due to the well-known acid sensitivity of furans. Thiophenes are regarded as more stable and phenyl 2-thiophenyl carbinol 8f did give a better yield (entry 6). Phenyl cyclopropyl carbinol 8h gave a modest yield of the allylated product 9h (entry 8). A small amount of 4-phenylbut-3-en-1-ol (4%) was also isolated. This appears to be a Julia product13 arising from cyclopropane ring opening during return of water to the carbocation intermediate.
| Entry | Substrate | Ar1 | Ar2 | Product, yield (%) |
|---|---|---|---|---|
| a Solutions were 0.5 M in substrate, using 4 equivalents of the allyl silane; reactions were stirred at 40 °C for the indicated time. b A small amount of ring opened product was also isolated. c The corresponding methyl ether was used as the substrate, rather than the alcohol. | ||||
| 1 | 8a | Ph | Ph | 9a, 96 |
| 2 | 8b | p-MeOC6H4 | Ph | 9b, 94 |
| 3 | 8c | p-MeC6H4 | Ph | 9c, 95 |
| 4 | 8d | p-MeOC6H4 | p-FC6H4 | 9d, 84 |
| 5 | 8e | Ph | Ferrocenyl | 9e, 93 |
| 6 | 8f | Ph | 2-Thiophenyl | 9f, 52 |
| 7 | 8g | Ph | 2-Furyl | 0 (dec.) |
| 8 | 8h | Ph | Cyclopropyl | 9h, 48b |
| 9 | 8i | p-MeOC6H4 | m-Br | 9i, 94 |
| 10 | 8j | p-MeOC6H4 | m-MeO2CC6H4 | 9j, 96 |
Returning to the concept of Brønsted acid catalysis, we wondered whether we could employ a solid supported catalyst. This modification would simplify the work-up procedure and be of future use in flow chemistry. Given that we were using the triflate of scandium, we employed Nafion, a perfluorinated sulfonic acid resin (Table 4).14 Pleasingly, under these conditions, benzhydryl alcohol 8a was converted to its allylated derivative 9a in 42% yield (entry 1). A number of other benzhydryl alcohols could also be allylated (entries 3–5). In contrast, no reaction was observed when Nafion-supported scandium(III)15 was used (entry 2), indicating that this polymer does not undergo significant hydrolysis under these conditions.
Conclusions
Scandium triflate is an effective catalyst for the deoxygenative allylation of benzhydryl alcohols, and displays significant and potentially useful selectivity in the same reaction with benzyl alcohols. The reaction proceeds through a hidden Brønsted acid mechanism. Scandium triflate is, therefore, better considered as a pre-catalyst! Claims in the literature that this allylation (and other) reactions are Lewis acid catalysed should be viewed with caution if no appropriate test has been carried out.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Agency for Science Technology and Research (A-Star) for financial support of this work (PSF grant number 1321202095) and the Graduate School, Chulalongkorn University (Overseas Research Experience Scholarship for Graduate Student, to PS). We thank Professor Richard Webster and Sher Li Gan for assistance with the Karl Fischer titration.Notes and references
- Lewis acids: BF3: (a) J. A. Cella, J. Org. Chem., 1982, 47, 2125 CrossRef CAS. B(C6F5)3: (b) M. Rubin and V. Gevorgyan, Org. Lett., 2001, 3, 2705 CrossRef CAS PubMed. Indium halides: (c) M. Yasuda, T. Saito, M. Ueba and A. Baba, Angew. Chem., Int. Ed., 2004, 43, 1414 CrossRef CAS PubMed; (d) S. H. Kim, C. Shin, A. N. Pae, H. Y. Koh, M. H. Chang, B. Y. Chung and Y. S. Cho, Synthesis, 2004, 1581 CAS; (e) T. Saito, M. Yasuda and A. Baba, Synlett, 2005, 1737 CAS; (f) T. Saito, Y. Nishimoto, M. Yasuda and A. Baba, J. Org. Chem., 2006, 71, 8516 CrossRef CAS PubMed. BiCl3: (g) S. K. De and R. A. Gibbs, Tetrahedron Lett., 2005, 46, 8345 CrossRef CAS n-BuLi + BCl3: (h) G. W. Kabalka, M.-L. Yao and S. Borella, J. Am. Chem. Soc., 2006, 128, 11320 CrossRef CAS PubMed; (i) G. W. Kabalka, S. Borella and M.-L. Yao, Synthesis, 2008, 325 CrossRef CAS. ZrCl4: (j) G. V. M. Sharma, K. L. Reddy, P. S. Laksmi, K. Ravi and C. Ajit, J. Org. Chem., 2006, 71, 3967 CrossRef CAS PubMed. I2: (k) J. S. Yadav, B. V. Subba Reddy, A. Srinivas Reddy and B. Eeshwaraiah, Chem. Lett., 2007, 36, 1500 CrossRef CAS. Me3SiBr-I2: (l) M. Saito, N. Tsuji, Y. Kobayashi and Y. Takemoto, Org. Lett., 2015, 17, 3000 CrossRef CAS PubMed. FeCl3: (m) J. Han, Z. Cui, J. Wang and Z. Liu, Synth. Commun., 2010, 40, 2042 CrossRef CAS; (n) X. Fan, X.-M. Cui, Y.-H. Guan, L.-A. Fu, H. Lv, K. Guo and H.-B. Zhu, Eur. J. Org. Chem., 2014, 498 CrossRef CAS; (o) Y. Sawama, R. Goto, S. Nagata, Y. Shishido, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2014, 20, 2631 CrossRef CAS PubMed. Fe(OTf)3: (p) L. Y. Chan, S. Kim, W. T. Chung, C. Long and S. Kim, Synlett, 2011, 415 CAS; Rh(I) complex: (q) G. Onodera, E. Yamamoto, S. Tonegawa, M. Iezumi and R. Takeuchi, Adv. Synth. Catal., 2011, 353, 2013 CrossRef CAS; Bi(OTf)3: (r) K. Nurayana and K. K. Laali, Org. Biomol. Chem., 2012, 10, 7347 RSC; TiCl4: (s) A. Hassner and C. R. Bandi, Synlett, 2013, 24, 1275 CrossRef CAS. The rhodium catalysed reaction of benzylic acetates has been reported: (t) A single example using La(OTf)3 in nitromethane at 100 °C, can be found in M. Noji, T. Ohno, K. Fuji, N. Futaba, H. Tajima and K. Ishii, J. Org. Chem., 2003, 68, 9340 CrossRef CAS PubMed.
- Brønsted acids: (a) G. Kaur, M. Kaushik and S. Trehan, Tetrahedron Lett., 1997, 38, 2521 CrossRef CAS; (b) H. A. I. Yoshihara, G. Chiellini, T. J. Michison and T. S. Scanlan, Bioorg. Med. Chem., 1998, 6, 1179 CrossRef CAS PubMed; (c) K. Motokura, N. Nakagiri, E. Tomoo, K. Ebitani and K. Kaneda, J. Org. Chem., 2007, 72, 6006 CrossRef CAS PubMed; (d) A. Devineau, G. Pousse, C. Tallier, J. Blanchet, J. Rouden and V. Dalla, Adv. Synth. Catal., 2010, 352, 2881 CrossRef CAS; (e) S. T. Kadam, H. Lee and S. S. Kim, Appl. Organomet. Chem., 2010, 24, 67 CAS; (f) J. Wang, Y. Masui and M. Onaka, Tetrahedron Lett., 2010, 51, 3300 CrossRef CAS; (g) M. Barbero, S. Bazzi, S. Cadamuro, S. Dughera and C. Piccinini, Synthesis, 2010, 315 CAS; (h) K. Murugan and C. Chen, Tetrahedron Lett., 2011, 52, 5827 CrossRef CAS; (i) I. Orizu and Y. Bolshan, Tetrahedron Lett., 2016, 57, 5798 CrossRef CAS.
- I. Šolić, D. Reich, J. Lim and R. W. Bates, Asian J. Org. Chem., 2017, 6, 658 CrossRef.
- S. Kobayashi, Eur. J. Org. Chem., 1999, 15 CrossRef CAS . Scandium may be considered as a pseudo-lanthanide.
- Despite the use of this solvent, we observed no products from the Ritter reaction.
- S. Kobayashi, M. Sugiura, H. Kitagawa and W. W.-L. Lam, Chem. Rev., 2002, 102, 2227 CrossRef CAS PubMed.
- T. C. Wabnitz, J.-Q. Yu and J. B. Spencer, Chem. – Eur. J., 2004, 10, 484 CrossRef CAS PubMed.
- For some recent studies of “hidden Brønsted acids”, see: (a) Z. Liu, R. Ganguly and D. Vidović, Dalton Trans., 2017, 46, 753 RSC; (b) J. Kaźmierczak, K. Kuciński and G. Hreczycho, Inorg. Chem., 2017, 56, 9337 CrossRef PubMed; (c) N. Kim, R. E. M. Brooner and R. A. Widenhoefer, Organometallics, 2017, 36, 673 CrossRef CAS; (d) H. J. Jin, J. H. Kim and E. J. Kang, Synthesis, 2017, 3137 CAS; (e) D. von der Heiden, S. Bozkus, M. Klussmann and M. J. Breugst, Org. Chem., 2017, 82, 4037 CrossRef CAS PubMed; (f) T. T. Dang, F. Boeck and L. Hintermann, J. Org. Chem., 2011, 76, 9353 CrossRef CAS PubMed; (g) J. G. Taylor, L. A. Adrio and K. K. Hii, Dalton Trans., 2010, 39, 1171 RSC; (h) J. L. McBee, A. T. Bell and T. D. J. Tilley, Am. Chem. Soc., 2008, 130, 16562 CrossRef CAS PubMed.
- B. Kanner and H. C. Brown, J. Am. Chem. Soc., 1953, 75, 3865 Search PubMed.
- T. Oriyama, Y. Kobayashi and K. Noda, Synlett, 1998, 1047 CrossRef CAS.
- More nucleophilic by four orders of magnitude: G. Hagen and H. Mayr, J. Am. Chem. Soc., 1991, 113, 4954 CrossRef CAS.
- For the most recent contribution see ref. 2i.
- M. Julia, S. Julia and R. Guegan, Bull. Soc. Chim. Fr., 1960, 1072 CAS.
- G. Gelbard, Ind. Eng. Chem. Res., 2005, 44, 8468 CrossRef CAS . Low yields have been reported for the use of Nafion (ref. 2e).
- L. Yu, D. Chen, J. Li and G. Wang, J. Org. Chem., 1997, 62, 3575 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures; experimental details and NMR spectra for compounds 1c, 1e, 1l, 8a–j, 2a, c, d, f, h, j, l′, m, 4–7, 9a, b, c, d, e, f, h, i, j. See DOI: 10.1039/c7ob02219k |
| This journal is © The Royal Society of Chemistry 2018 |