Enhanced n-dodecane hydroisomerization performance by tailoring acid sites on bifunctional Pt/ZSM-22 via alkaline treatment†
Xian
Wu
ab,
Minghuang
Qiu
b,
Xinqing
Chen
 *b,
Gan
Yu
b,
Xing
Yu
ab,
Chengguang
Yang
b,
Jian
Sun
a,
Ziyu
Liu
*b and
Yuhan
Sun
b
*b,
Gan
Yu
b,
Xing
Yu
ab,
Chengguang
Yang
b,
Jian
Sun
a,
Ziyu
Liu
*b and
Yuhan
Sun
b
aSchool of Environmental and Chemical Engineering, Shanghai University, Shanghai 201900, China
bCAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China. E-mail: chenxq@sari.ac.cn; liuziyu@sari.ac.cn
First published on 27th November 2017
Abstract
Acidity controlled Pt/ZSM-22 catalysts for n-dodecane hydroisomerization were explored through a simple and efficient post-treatment with different NaOH concentrations, and then the resulting samples were characterized using acid measurement, XRD, SEM, TEM, XRF and XPS. It appeared that the high crystallinity, uniform morphology and textural structure almost remained even after the alkaline-treatment of the parent zeolite with a low Si/Al ratio (∼20). However, the distribution of acid sites, and in particular the Brønsted acidity, changed which then affected the catalytic performance. This originates from the Brønsted acid sites of the parent catalyst being partially blocked. With the acid sites optimized using the alkali treatment, the selectivity for isomers increased from 59.5% (parent sample) to 87.3% (treated sample) with the yield increasing from 43.3% to 69.8%. Thus, such an ordinary alkaline treatment proved to be promising in the n-paraffin hydroisomerization for the cleaner production of lubricating oil based stocks.
1. Introduction
Lubricating oil is regarded as the “blood” in maintaining mechanical operation, playing an important role in the development of modern social economies.1,2 Hydroisomerization of n-paraffin is a productive way to offer lubricating oil based stocks with an ideal cold flow point and high viscosity index.3–5 However, a poor yield of lubricating oil based stocks is usually obtained from the isomerization process as the cracking of raw materials also occurs during hydroisomerization.6 To maximize the yield of desired products from long chain n-paraffin, promising hydroisomerization catalysts with a high degree of activity and isomer selectivity are desirable.Typically, the hydroisomerization reaction employs bifunctional catalysts with an optimum balance of metal and acidic property.7 Normally, precious metals are supported on acidic zeolites to perform isomerization reactions, with noble metallic sites for hydrogenation/dehydrogenation, and acid sites on the zeolite supports for the skeletal isomerization of n-paraffin through alkylcarbenium ions.8 In view of this, the excellent contributors for metallic activity are Pt and Pd. Besides, other transition metals such as Ni9 and Mo10 have also been reported as potential substitutes due to their low costs. ZSM-22,11 ZSM-23,12 and SAPO-11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 were used as ideal acidic supports due to their 1-dimensional 10-membered ring channels which provide a specific molecular shape selectivity for isomers via the pore mouth-key lock catalysis mechanism. Thus, the selection of an appropriate zeolite support with suitable acid sites to avoid cracking reactions is of great importance for the hydroisomerization of long chain n-paraffin. To achieve the minimum cracking selectivity, fewer Brønsted acidic sites in the micropores could reduce the cracking of the products, and then increase the isomer selectivity.11,14–16 Therefore, it is useful to block the Brønsted acid sites in the micropores of zeolites using non-acidic and/or weak acidic species. Many efforts have been devoted to this using in situ synthesis and post-treatment processing. Liu et al. reported that a ZSM-22 framework, which was partly substituted with iron and exhibited a suitable acidity compared to the parent catalyst, showed an enhanced performance in the hydroisomerization of n-dodecane.15 One reason for this is that the Brønsted acidity decreases due to the poor acidity of Si–OH–Fe bridge hydroxyls compared to that of Si–OH–Al. Newalkar et al. adjusted the ratio of total acidity/Brønsted acidity of ZSM-22 zeolite by varying the bulk molar Si/Al ratio and obtained an increased hydroisomerization selectivity for n-hexadecane.17 In another aspect, the post-treatment process using an alkali solution can adjust the acid distribution and decrease the number of Brønsted acid sites on zeolites. However, the zeolites after NaOH etching produce an Al-rich structure with poor acidity due to the desilication and blocked micropores of the zeolite.18 To overcome this problem, a two-step post-synthetic approach to prepare a ZSM-22 (Si/Al ratio of 40) catalyst with NaOH etching followed by the recovery of micropores by mild HCl washing has been reported, and showed enhanced hydroisomerization of long chain n-paraffin.11,19 Meanwhile, a mesoporous structure in the zeolite framework was generated after the desilication process in alkali solution. However, the weight loss of the zeolite during the alkali treatment was as high as 30%, which increased the cost of the final catalyst.20 A catalyst with high hydroisomerization performance but low zeolite weight loss is strongly desired. It was reported that a zeolite framework with a low Si/Al ratio (<25) would prevent the Si component from being extracted.21,22 As a result, a high zeolite yield may be obtained after the post-treatment. In this article, ZSM-22 with a low Si/Al ratio (∼20) was chosen as the parent zeolite to increase the zeolite yield and retain the pore system after the alkaline-treatment, meanwhile the type and strength of the acid sites were optimized to improve its performance for long chain n-paraffin hydroisomerization.
13 were used as ideal acidic supports due to their 1-dimensional 10-membered ring channels which provide a specific molecular shape selectivity for isomers via the pore mouth-key lock catalysis mechanism. Thus, the selection of an appropriate zeolite support with suitable acid sites to avoid cracking reactions is of great importance for the hydroisomerization of long chain n-paraffin. To achieve the minimum cracking selectivity, fewer Brønsted acidic sites in the micropores could reduce the cracking of the products, and then increase the isomer selectivity.11,14–16 Therefore, it is useful to block the Brønsted acid sites in the micropores of zeolites using non-acidic and/or weak acidic species. Many efforts have been devoted to this using in situ synthesis and post-treatment processing. Liu et al. reported that a ZSM-22 framework, which was partly substituted with iron and exhibited a suitable acidity compared to the parent catalyst, showed an enhanced performance in the hydroisomerization of n-dodecane.15 One reason for this is that the Brønsted acidity decreases due to the poor acidity of Si–OH–Fe bridge hydroxyls compared to that of Si–OH–Al. Newalkar et al. adjusted the ratio of total acidity/Brønsted acidity of ZSM-22 zeolite by varying the bulk molar Si/Al ratio and obtained an increased hydroisomerization selectivity for n-hexadecane.17 In another aspect, the post-treatment process using an alkali solution can adjust the acid distribution and decrease the number of Brønsted acid sites on zeolites. However, the zeolites after NaOH etching produce an Al-rich structure with poor acidity due to the desilication and blocked micropores of the zeolite.18 To overcome this problem, a two-step post-synthetic approach to prepare a ZSM-22 (Si/Al ratio of 40) catalyst with NaOH etching followed by the recovery of micropores by mild HCl washing has been reported, and showed enhanced hydroisomerization of long chain n-paraffin.11,19 Meanwhile, a mesoporous structure in the zeolite framework was generated after the desilication process in alkali solution. However, the weight loss of the zeolite during the alkali treatment was as high as 30%, which increased the cost of the final catalyst.20 A catalyst with high hydroisomerization performance but low zeolite weight loss is strongly desired. It was reported that a zeolite framework with a low Si/Al ratio (<25) would prevent the Si component from being extracted.21,22 As a result, a high zeolite yield may be obtained after the post-treatment. In this article, ZSM-22 with a low Si/Al ratio (∼20) was chosen as the parent zeolite to increase the zeolite yield and retain the pore system after the alkaline-treatment, meanwhile the type and strength of the acid sites were optimized to improve its performance for long chain n-paraffin hydroisomerization.
Herein, ZSM-22 with a low Si/Al ratio (∼20) was selected as the zeolite support in the present work. The parent ZSM-22 was treated in alkaline media with 0.05, 0.07, 0.1 and 0.2 M of NaOH. The prepared ZSM-22 samples were characterized using XRD, SEM, TEM, NH3-TPD, FT-IR, Py-IR, XPS, XRF, etc. Subsequently, the obtained zeolites were employed as supports to synthesize hydroisomerization catalysts with a Pt loading of 0.5 wt% and the catalytic activity was tested using n-dodecane as a model feed. The effect of the acid sites in the ZSM-22 framework after alkaline treatment on the isomer selectivity has been investigated.
2. Experimental
2.1 Catalyst preparation
HZSM-22 (Si/Al = 20) was provided by the Shanghai Novel Chemical Technology Co., Ltd, China. In a typical post-treatment process, 20 g of HZSM-22 was treated in NaOH solutions with different concentrations (0.05, 0.07, 0.1, and 0.2 M) under agitation at 90 °C for 2 h. Then the products were ion-exchanged with NH4Cl under vigorous stirring at 80 °C for 5 h three times. The exchanged samples were dried at 100 °C for 12 h and subsequently calcined at 550 °C for 5 h in air. The obtained zeolites were identified as HZAT-0.05, HZAT-0.07, HZAT-0.1 and HZAT-0.2 respectively. Finally, the prepared samples were loaded with 0.5 wt% Pt by impregnation using H2PtCl6·6H2O aqueous solution. The resultant suspensions were evaporated under stirring at 70 °C, followed by drying at 100 °C for 12 h and calcination at 450 °C for 4 h in air to get Pt/ZSM-22, Pt/ZAT-0.05, Pt/ZAT-0.07, Pt/ZAT-0.1 and Pt/ZAT-0.2.2.2 Characterization
X-ray diffraction patterns of the samples were measured using an X-ray diffractometer (XRD, Rigaku, Ultima IV) with Cu Kα (λ = 0.154 nm) radiation at a tube current of 40 mA and a voltage of 40 kV. The crystal morphologies of the ZSM-22 samples were observed using a scanning electron microscope (SEM, Zeiss SUPRA 55 SAPPHIRE) with acceleration voltages of 2 kV and 20 kV. TEM images were obtained on a high resolution transmission electron microscope with an acceleration voltage of 120 kV (JEOL, JEM-2010). The samples were degassed under vacuum at 300 °C for at least 10 h for each run, then the textural data of the samples derived from N2 adsorption–desorption measurements were measured at −196 °C using an automatic physisorption analyser (Tristar 3020, Micromeritics). The functional groups of the samples were detected using Fourier-transform infrared spectroscopy (FT-IR, Thermo Scientific, Nicolet 6700). The elemental compositions of the samples were analysed using X-ray fluorescence (XRF, Bruker S4PIONEER). Solid-state MAS NMR experiments were performed at 5 kHz on a Bruker AVANCE 400 spectrometer operating at frequencies of 79.5 and 104.22 MHz for 29Si and 27Al, respectively. The near-surface chemical information of the samples was analysed using X-ray photoelectron spectroscopy (XPS, K-Alpha, Al Kα radiation, 1486.6 eV, 12 kV, 3 mA) with an organic elemental analyser (FLASH 2000). The acidic strengths of the samples were measured using the temperature programmed desorption of ammonia (NH3-TPD, Tianjin Xianquan TP-5080) with a flame ionization detector (TCD) under nitrogen flow. CO-chemisorption was performed with a chemisorption analyser (Tristar 2920, Micromeritics) using the same method as previous reports.15 The types of acid site were estimated from Pyridine-adsorbed IR (Py-IR) spectra obtained on an EQUINOX 70 (Bruker, Germany).11,13 Wafers of compressed zeolite samples were mounted in FT-IR cells followed by being degassed under a vacuum of 10−2 Pa at 400 °C for 2 h. The samples were saturated with pyridine vapour at room temperature. The samples were degassed for 30 min at 200 °C and 350 °C to remove the excess probe molecules before the IR spectra were recorded. The molar extinction coefficients of pyridine for Brønsted acid (ε = 1.67 cm μmol−1) and Lewis acid (ε = 2.22 cm μmol−1) sites were used; the acid sites were calculated according to the following equations (eqn (1) and (2)).23| C (pyridine on Brønsted acid sites) = 1.88 IA(Brønsted)R2/W | (1) |
| C (pyridine on Lewis acid sites) = 1.42 IA(Lewis)R2/W | (2) |
2.3 Catalytic reaction
The hydroisomerization of n-dodecane reaction was carried out in a tubular fixed-bed stainless steel reactor with an I.D. of 12 mm. Typically, 2.0 g of catalyst (20–40 mesh) was loaded in the isothermal zone of the reactor. The catalytic performance was tested under a pressure of 2.0 MPa and a H2/n-C12 volume ratio of 600. The catalyst was reduced by hydrogen at 400 °C for 4 h under ambient pressure. After the catalyst reduction, the reactor was adjusted to the target reaction conditions, and then n-dodecane was fed into the reactor by a pump at the desired flow rate. The liquid products were collected using a cooling trap and then analysed using a GC with a flame ionization detector (FID) and a HP-1 capillary column (30 m × 0.32 mm). The gaseous products were analysed using GC-2014C gas chromatographs equipped with a FID. The conversion and selectivity of n-dodecane were calculated according to eqn (3) and (4). | (3) |
 | (4) |
3. Results and discussion
3.1 Characterization of ZSM-22 with alkaline treatment
The powder XRD patterns of the ZSM-22 samples before and after alkaline treatment are shown in Fig. 1. All of the samples present the typical peaks of ZSM-22 which are in good agreement with the simulated XRD pattern of TON zeolite.24 The diffractions at 2θ = 13.86, 17.41 and 5.04 indicate a framework consisting of 5-, 6-, and 10-rings within ZSM-22. Furthermore, no impurity crystals or amorphous phases are found, indicating that the pure ZSM-22 is well preserved after NaOH etching. However, the intensity of the diffraction peaks reduces slightly. Compared with the parent ZSM-22 (defined as 100% relative crystallinity), the relative crystallinities of the treated samples are 97.3%, 95.7%, 92.6% and 90.6% for HZAT-0.05, HZAT-0.07, HZAT-0.1 and HZAT-0.2, respectively. In addition, the recovered weights of ZSM-22 are 96.3%, 95.6%, 94.3% and 88.7% after 0.05, 0.07, 0.1 and 0.2 M NaOH etching, respectively (Table 1), indicating a weight loss of less than 12%, which is much lower than those20 for zeolites with Si/Al > 30 after alkaline treatment. Fig. 2 shows the FT-IR spectra of the parent and alkaline-treated ZSM-22 samples. All of the samples display bands at 808, 640 and 551 cm−1, which are typical characteristic peaks of the TON structure.11,25 The FT-IR results imply that alkaline treatment has no obvious effect on the chemical bonds of the zeolite, which agrees well with the XRD results.| Sample | Alkali conc. (M) | Zeolite yield (%) | Si/Al (bulk) | Si/Al (surface) | Pore volume/cm3 g−1 | S BET/m2 g−1 |
S
meso/m2 g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|---|---|---|---|---|---|---|---|
| a S meso = SBET − Smicro, and Smicro was obtained using the t-plot method. | |||||||
| HZSM-22 | — | — | 19.8 | 13.7 | 0.17 | 53 | 32 |
| HZAT-0.05 | 0.05 | 96.3 | 18.2 | 12.2 | 0.17 | 60 | 40 |
| HZAT-0.07 | 0.07 | 95.6 | 17.9 | 13.2 | 0.17 | 63 | 36 |
| HZAT-0.1 | 0.1 | 94.3 | 17.7 | 11.2 | 0.17 | 64 | 41 |
| HZAT-0.2 | 0.2 | 88.7 | 16.7 | 9.2 | 0.16 | 57 | 35 |
Fig. 3 shows the SEM images of ZSM-22 crystals before and after alkaline treatment. All of the samples represent typical needle-shaped crystals with a size of 50 × 50 × 500 nm. The treated samples almost keep the original morphology (Fig. 2a–e) due to the mild alkali media, and the low Si/Al ratio of the parent zeolite may also help in keeping the morphology. Fig. 3f shows a schematic drawing of the process of the alkaline treatment of ZSM-22.
 | ||
| Fig. 3 SEM images of (a) the parent HZSM-22, (b) HZAT-0.05, (c) HZAT-0.07, (d) HZAT-0.1 and (e) HZAT-0.2; and (f) a schematic drawing of the alkaline-treatment process. | ||
The TEM images in Fig. 4 also show the typical needle-like agglomerates and confirm that the microporous channels run along the (001) direction of the individual ZSM-22 crystals (Fig. 4d). The images present that the Pt nanoparticles cover the surface of ZSM-22 uniformly. The average size of the Pt nanoparticles is about 2.1 nm for both Pt/ZSM-22 and Pt/ZAT-0.07, from calculating the size of more than 50 Pt nanoparticles. The TEM images also reveal that the interplanar spacing of the Pt nanoparticles is about 0.217 nm (Fig. 4c and f), which can be assigned to the crystallographic (110) plane.
 | ||
| Fig. 4 TEM images of (a–c) the parent sample (Pt/ZSM-22) and (d–f) the alkaline-treated ZSM-22 (Pt/ZAT-0.07); inset are PSDs of the Pt nanoparticles. | ||
As shown in Fig. S1 and Table S1 (ESI†), there is no significant change in the Pt nanoparticles before and after the hydroisomerization reaction. Also, there is no aggregation of the Pt particles after the reaction as seen from Fig. S1b (ESI†), which is also reported by the ref. 15 and 26.
Fig. S2 (ESI†) shows the N2 adsorption–desorption isotherms of the parent and alkaline-treated samples. All of the samples display the characteristics of Type I isotherms, and the increased uptake in the low pressure region at P/P0 < 0.1 is related to the micropores typical for TON frameworks.27
In addition, these samples present a modest uptake at mid-to-high relative pressures (P/P0 from 0.5 to 1.0) indicating the intercrystalline voids of the agglomerated ZSM-22 needles. Table 1 shows the textual data as well as the Si/Al ratio both in the bulk and on the surface, detected using XRF and XPS respectively.28Table 1 exhibits that the bulk Si/Al ratio decreases typically in the order of HZSM-22 > HZAT-0.05 > HZAT-0.07 > HZAT-0.1 > HZAT-0.2, which may stem from the enhanced desilication process when the concentration of the solutions increased. The difference in the Si/Al ratio between the bulk and the surface may come from the fact that the rim of the zeolite has a higher aluminium concentration than the inside because of the desilication process, which is the same as in previous reports by Liu, Martens, and Hayasaka.11,19,27 Interestingly, no obvious difference between the pore volume and surface area of these samples can be found after NaOH etching, due to the mild alkaline condition and the lower Si/Al ratio of these zeolites (<25). Taking into account that the zeolite yield after alkali treatment decreases to less than 90% when the NaOH concentration reaches 0.2 M, NaOH solution with a concentration less than 0.2 M is recommended for alkaline-treatment under these experimental conditions (90 °C, 2 h) to obtain a high zeolite yield and almost unchanged pore structures. The acidic property of the parent and alkaline-treated samples was determined using NH3-TPD and FT-IR spectra with adsorbed pyridine (Py-IR).
Fig. 5 shows the NH3-TPD profiles of all of the samples with two desorption peaks observed at ∼210 °C and ∼460 °C, which can be ascribed to weak acid sites and medium-strength acid sites, respectively. However, the peak areas of these two peaks for the treated samples decrease with the NaOH concentration, indicating a decreased amount of acid sites, which may stem from the extraction of partial silica species or the blocking of some acid sites by Al-rich species from the frameworks after alkali etching. It was reported that the ideal structure of ZSM-22 as an efficient support for the isomerization reaction should contain partially blocked acid sites in the micropores.29 FT-IR spectra with adsorbed pyridine (Py-IR) were used to investigate the types of acid site of the prepared samples. As shown in Fig. 6, the bands at 1540–1548 cm−1 and 1445–1460 cm−1 correspond to pyridine molecules chemisorbed on Brønsted acid (B acid) and Lewis acid (L acid) sites, respectively.23,30 The bands at 1490 cm−1 are ascribed to the combination of adsorption on both B acid sites and L acid sites. Table 2 lists the total amount of (B + L) acid sites that can be calculated from the Py-IR results at 200 °C, and the amount of medium and strong B and L acid sites that can be obtained from the Py-IR spectra at 350 °C. Table 2 shows that the total amount of (B + L) acid sites decreases with a trend of HZSM-22 > HZAT-0.05 > HZAT-0.07 > HZAT-0.1 > HZAT-0.2, which is exactly the same trend for the increase in NaOH concentration, showing that the acid sites were successfully partially removed after the alkaline treatment. For example, there is a large decline in the total (B + L) acid sites in HZSM-22 from 182 μmol g−1 to 132 μmol g−1 in HZAT-0.05. Importantly, the amount of strong B acid sites, which is responsible for excessive cracking product selectivity, decreased significantly from 109 μmol g−1 for HZSM-22 to 53 μmol g−1 for HZAT-0.07 after alkaline treatment. The amount of strong B acid sites decreases following the trend of HZSM-22 > HZAT-0.05 > HZAT-0.07 > HZAT-0.1 > HZAT-0.2. The ratio of Bs/Bw in Table 2, defined as the ratio of strong Brønsted acid sites over weak Brønsted acid sites, decreases after the alkaline treatment of the parent sample. In addition, the Bs/Bw ratios of HZAT-0.07 and HZAT-0.1 are relative lower among these samples, implying that these two samples have higher proportions of weak Brønsted acid sites.
 | ||
| Fig. 6 FT-IR spectra of the parent and alkaline-treated samples after the adsorption of pyridine at (a) 200 °C and (b) 350 °C. | ||
| Samples | B | Bs | L | Ls | B + L | Bs/Bwa | |
|---|---|---|---|---|---|---|---|
| 200 °C | 350 °C | 200 °C | 350 °C | 200 °C | 350 °C | ||
| a Bs/Bw: ratio of strong and weak Brønsted acid sites; the latter was calculated from the difference in value of B acid sites and Bs acid sites. | |||||||
| HZSM-22 | 147 | 109 | 35 | 23 | 182 | 132 | 2.9 |
| HZAT-0.05 | 97 | 58 | 35 | 30 | 132 | 88 | 1.5 |
| HZAT-0.07 | 98 | 53 | 28 | 24 | 126 | 77 | 1.2 |
| HZAT-0.1 | 101 | 52 | 20 | 12 | 121 | 64 | 1.1 |
| HZAT-0.2 | 73 | 44 | 24 | 12 | 97 | 56 | 1.5 |
Fig. 7a shows the total (B + L) acid sites against the Al content in the prepared samples. With the increase of NaOH concentration, the samples have a higher Al content, since more Si would be removed by NaOH, and also the total amount of acid sites (B + L) decreases under the alkaline treatment. The value of dividing the total amount of acid sites by the amount of Al (eqn (S1) (ESI†)) in the samples is noted as the corresponding accessibility index (ACIPy).31 The ACIPy decreases with the increased concentration of NaOH, following the order of HZSM-22 (0.22) > HZAT-0.05 (0.15) > HZAT-0.07 (0.14) > HZAT-0.1 (0.13) > HZAT-0.2 (0.10), which may be related to the fact that more Al-rich species (weak acid sites) were produced instead of acidic sites in the HZSM-22 framework after the alkaline treatment.
 | ||
| Fig. 7 The relationship of (a) the total acid sites and Al content of the parent and alkaline-treated samples, and (b) the ACIPy values and the concentration of NaOH. | ||
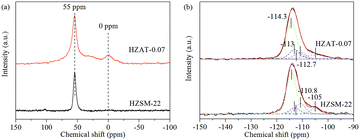
27Al and 29Si NMR spectra of the parent and alkaline-treated ZSM-22 samples are given in Fig. 8. Fig. 8a shows that the parent ZSM-22 exhibits one strong peak at 54.5 ppm while the alkaline-treated sample (HZAT-0.07) has an additional peak at around 0 ppm. The resonance peak at 54.5 ppm can be assigned to tetrahedrally coordinated framework Al atoms while the signal at 0 ppm corresponds to the octahedral extra-framework Al3+ species,20 confirming that the extra-framework Al3+ species exist in HZAT-0.07.
It is further reported that the Al-rich species (weak acid sites) will be produced after the alkaline-treatment.18 In the 29Si NMR spectra (Fig. 8b), four nonequivalent tetrahedral sites with shifts of −110.8, −112.7, −113.0 and −114.3 ppm can be found on both HZSM-22 and HZAT-0.07, which can be ascribed to the siliceous form of ZSM-22.11,29 The peaks at −114.3 ppm and −105 ppm for HZSM-22 are assigned to Si(0Al) and Si(1Al) respectively, and the resonance of Si(1Al) at −105 ppm decreases for HZAT-0.07 compared with that for the parent HZSM-22 under the alkaline treatment.
3.2 Hydroisomerization over Pt/H-ZSM-22 catalysts
The hydroisomerization of n-dodecane on the parent and alkaline-treated catalysts was tested in a fixed-bed reactor at 280 °C and 2.0 MPa with a WHSV of 2.0 h−1. Fig. S3 (ESI†) shows the conversion of n-dodecane, and the selectivity and yield of the obtained isomers over various catalysts. The Pt loading of all of the catalysts is at the same level (0.5 wt%). The conversion of n-dodecane increases with the trend following Pt/ZSM-22-0.2(50.5%) < Pt/ZSM-22 (72.8%) < Pt/ZAT-0.05 (75.6%) < Pt/ZAT-0.07 (80.0%) < Pt/ZAT-0.1 (83.5%). However, the selectivity to iso-dodecane for the alkaline-treated samples is much higher than that for the parent Pt/ZSM-22 catalyst. This may come from the fact that the acid sites were partially removed after alkali etching and both the strength and type of acid site were optimized, thus enhancing the hydroisomerization performance. Interestingly, the selectivity to iso-dodecane increases from 59.5% on Pt/ZSM-22 to 87.3% on Pt/ZSM-0.07 and then decreases down to 72.5% on Pt/ZAT-0.2, which indicates that the best hydroisomerization catalyst should contain a suitable amount of acid sites in the zeolite support. The total yield of the isomers displays a volcanic type profile and the highest yield of 69.8% appears for Pt/ZAT-0.07, which is much higher than that for the parent Pt/ZSM-22 (43.3%). In fact, an excellent hydroisomerization product for lube oil requires not only high conversion and high isomer yield, but also high mono-branched selectivity to keep its high viscosity index as well as a low pour point. Table 3 shows the selectivity of mono-branched isomers and di-branched isomers in the process of hydroisomerization, and also the ratio of mono-/di-branched isomers. It can be observed that the mono-branched isomers predominated in the products among all of the samples. The selectivity of isomers is related to the shape selectivity of the TON structure and carbenium is thought to be the transient state for the isomers during the hydroisomerization process. The carbenium for the mono-branched isomer is smaller in size than that of the di-branched carbenium, and thus is more favoured in the narrow channels of ZSM-22. As listed in Table 1, the BET surface area for mesopores seemed to increase from 32 m2 g−1 for HZSM-22 to 35–41 m2 g−1 for the alkaline treated samples, which may accommodate many more di-branched carbenium and increase the selectivity for di-branched isomers. Meanwhile, the acid sites also influence the selectivity for mono- or di-branched isomers (Table 3). The selectivity for mono-branched isomers also increases with the increase of total selectivity after the alkaline treatment, however, the selectivity for di-branched isomers increases faster than that of the mono-branched isomers. As a result, the Smo/Sdi ratio for Pt/ZSM-22 is the highest and the Smo/Sdi ratio decreases after NaOH treatment, which agrees well with Liu's work.11,15,32 Among all of the alkaline treated catalysts, Pt/ZAT-0.07 shows the highest Smo/Sdi ratio of 7.2 which is much higher than that of the other alkaline treated catalysts and is close to the value for Pt/ZSM-22 (8.2). Thus, Pt/ZAT-0.07, with higher n-dodecane conversion, higher isomer yield and much higher selectivity for mono-branched isomers, is the best catalyst. Furthermore, we investigate the temperature effect on Pt/ZAT-0.07, as shown in Fig. 9. Temperature plays an important role in n-dodecane hydroisomerization performance. Fig. 9 and Table S2 (ESI†) clearly illustrate that the conversion and cracking yield of n-dodecane increase with the increase of the temperature, which is consistent with previous reports.4,11,15,17,33,34 The isomerization selectivity over Pt/ZAT-0.07 decreases with the increase of temperature, from 90.0% at 260 °C to 87.3% at 280 °C, and then rapidly drops down to 37.3% at 300 °C, which is commonly observed in the hydroisomerization of n-paraffins.15 Table S2 (ESI†) illustrates the Pt/ZAT-0.07 catalytic performance at different temperatures from 260 °C to 300 °C. It can be seen that the total isomer-selectivity decreases from 89.9% at 260 °C to 37.3% at 300 °C, as well as the mono-branched isomer-selectivity decreasing from 84.3% at 260 °C to 22.4% at 300 °C. In contrast, the di-branched isomer-selectivity shows a steady increase from 5.6% at 260 °C to 14.9% at 300 °C. In summary, the SMo/SDi ratios decreased with the increase of the temperature from 260 °C to 300 °C. The isomer yield increases with the increase of the temperature and passes through a maximum (69.8%) owing to the consumption of the isomeric products in consecutive hydrocracking reactions at higher temperature. Only a low cracking yield is achieved at low temperature (less than 5% below 270 °C), but it increases hugely from 10.2% at 280 °C to 59.1% at 300 °C. Based on the above experimental results, the acidity of the catalysts and the reaction temperature should be properly controlled in order to obtain a high hydroisomerization performance. Most importantly, hydroisomerization reactions of long chain n-paraffin require acidic sites with a certain acidic strength.| Catalysts | Pt/ZSM-22 | Pt/ZAT-0.05 | Pt/ZAT-0.07 | Pt/ZAT-0.1 | Pt/ZAT-0.2 |
|---|---|---|---|---|---|
S
T, SMo and SDi correspond to the selectivity of total isomers, mono-branched isomers, and di-branched isomers respectively, at 280 °C, 2.0 MPa, VH2/Vn-C12 = 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and WHSV = 2.0 h−1. 1 and WHSV = 2.0 h−1. |
|||||
| Conversion (%) | 72.8 | 75.6 | 80.0 | 83.5 | 50.5 |
| S T (%) | 59.5 | 78.7 | 87.3 | 78.0 | 72.5 |
| S Mo (%) | 53.0 | 68.0 | 76.6 | 58.8 | 57.4 |
| S Di (%) | 6.5 | 10.7 | 10.7 | 19.2 | 15.1 |
| S Mo/SDi | 8.2 | 6.4 | 7.2 | 3.1 | 3.8 |
 | ||
Fig. 9 The performance of Pt/ZAT-0.07 in the hydroisomerization of n-dodecane at various temperatures. (P = 2.0 MPa, VH2/Vn-C12 = 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and WHSV = 2.0 h−1.) 1, and WHSV = 2.0 h−1.) | ||
4. Conclusions
The acidity of ZSM-22 could be simply tailored using alkaline-treatment with NaOH at different concentrations. It was found that the total acid sites, along with Brønsted acid sites, decreased with the increase of NaOH concentration since some of the acid sites were blocked by the Al-rich depositing species. However, both the crystalline and textural structure of ZSM-22 with a low Si/Al ratio were well preserved after the alkaline treatment. The subsequent loading of 0.5 wt% Pt to the resultant ZSM-22 led to an excellent catalyst for the hydroisomerization of n-dodecane, and a high n-dodecane conversion of 80.0% as well as an iso-dodecane selectivity of 87.3% were obtained. Most of all, the mono-branched isomers predominated in the products among other isomers. Suitable acid sites and acid type in the catalysts were generated by blocking some of the Brønsted acid sites after alkaline treatment, which may be responsible for the superior catalytic performance. This sheds light onto a simple and efficient route to develop an acidity controlled Pt/ZSM-22 catalyst with high hydroisomerization performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the NSFC (No. 21507141 and 21776295), the Youth Innovation Promotion Association, CAS (No. 2017355) and the Science and Technology Commission of Shanghai Municipality (14DZ1207602 and 16DZ1206900).References
- V. M. Akhmedov and S. H. Al-Khowaiter, Catal. Rev., 2007, 49, 33–139 CAS.
- A. Primo and H. Garcia, Chem. Soc. Rev., 2014, 43, 7548–7561 RSC.
- F. Alvarez, F. Ribeiro, G. Perot, C. Thomazeau and M. Guisnet, J. Catal., 1996, 162, 179–189 CrossRef CAS.
- S. Ernst, J. Weitkamp, J. A. Martens and P. A. Jacobs, Appl. Catal., 1989, 48, 137–148 CrossRef CAS.
- G. E. Giannetto, G. R. Perot and M. R. Guisnet, Ind. Eng. Chem. Prod. Res. Dev., 1986, 25, 481–490 CrossRef CAS.
- H. F. Schulz and J. H. Weitkamp, Ind. Eng. Chem. Prod. Res. Dev., 1972, 11, 46–53 CAS.
- T. Pinto, P. Arquillière, G. P. Niccolai, F. Lefebvre and V. Dufaud, New J. Chem., 2015, 39, 5300–5308 RSC.
- F. Alvarez, F. R. Ribeiro, G. Perot, C. Thomazeau and M. Guisnet, J. Catal., 1996, 162, 179–189 CrossRef CAS.
- S. Tian and J. Chen, Fuel Process. Technol., 2014, 122, 120–128 CrossRef CAS.
- Y. Shi, Y. Cao, Y. Duan, H. Chen, Y. Chen, M. Yang and Y. Wu, Green Chem., 2016, 18, 4633–4648 RSC.
- S. Y. Liu, J. Ren, H. K. Zhang, E. J. Lv, Y. Yang and Y. W. Li, J. Catal., 2016, 335, 11–23 CrossRef CAS.
- S. W. Lee and S. K. Ihm, Ind. Eng. Chem. Res., 2013, 52, 15359–15365 CrossRef CAS.
- M. Y. Kim, K. Lee and M. Choi, J. Catal., 2014, 319, 232–238 CrossRef CAS.
- I. R. Choudhury, K. Hayasaka, J. W. Thybaut, C. S. L. Narasimhan, J. F. Denayer, J. A. Martens and G. B. Marin, J. Catal., 2012, 290, 165–176 CrossRef CAS.
- S. Liu, J. Ren, S. Zhu, H. Zhang, E. Lv, J. Xu and Y. W. Li, J. Catal., 2015, 330, 485–496 CrossRef CAS.
- G. Wang, Q. Liu, W. Su, X. Li, Z. Jiang, X. Fang, C. Han and C. Li, Appl. Catal., A, 2008, 335, 20–27 CrossRef CAS.
- S. Parmar, K. K. Pant, M. John, K. Kumar, S. M. Pai and B. L. Newalkar, Energy Fuels, 2015, 29, 332 CrossRef.
- D. Verboekend, A. M. Chabaneix, K. Thomas, J. P. Gilson and J. Pérez-Ramírez, CrystEngComm, 2011, 13, 3408–3416 RSC.
- J. A. Martens, D. Verboekend, K. Thomas, G. Vanbutsele, J. P. Gilson and J. Pérez-Ramírez, ChemSusChem, 2013, 6, 421–425 CrossRef CAS PubMed.
- X. Liu, S. Ren, G. Zeng, G. Liu, P. Wu, G. Wang, X. Chen, Z. Liu and Y. Sun, RSC Adv., 2016, 6, 28787–28791 RSC.
- J. C. Groen, J. C. Jansen, J. A. Moulijn and J. Pérez-Ramírez, J. Phys. Chem. B, 2004, 108, 13062–13065 CrossRef CAS.
- J. C. Groen and J. A. Moulijn, J. Mater. Chem., 2006, 16, 2121–2131 RSC.
- C. Emeis, J. Catal., 1993, 141, 347–354 CrossRef CAS.
- G. T. Kokotailo, J. L. Schlenker, F. G. Dwyer and E. W. Valyocsik, Zeolites, 1985, 5, 349–351 CrossRef CAS.
- H. Wen, Y. Zhou, J. Xie, Z. Long, W. Zhang and J. Wang, RSC Adv., 2014, 4, 49647–49654 RSC.
- G. L. Haller, J. Catal., 2003, 216, 12–22 CrossRef CAS.
- K. Hayasaka, D. Liang, W. Huybrechts, B. R. De Waele, K. J. Houthoofd, P. Eloy, E. M. Gaigneaux, G. van Tendeloo, J. W. Thybaut and G. B. Marin, Chem. – Eur. J., 2007, 13, 10070–10077 CrossRef CAS PubMed.
- A. Astafan, Y. Pouilloux, J. Patarin, N. Bats, C. Bouchy, T. J. Daou and L. Pinard, New J. Chem., 2016, 40, 4335–4343 RSC.
- F. Zhang, Y. Liu, Q. Sun, Z. Dai, H. Gies, Q. Wu, S. Pan, C. Bian, Z. Tian and X. Meng, Chem. Commun., 2017, 53, 4942–4945 RSC.
- L. Guo, X. Bao, Y. Fan, G. Shi, H. Liu and D. Bai, J. Catal., 2012, 294, 161–170 CrossRef CAS.
- D. Verboekend, K. Thomas, M. Milina, S. Mitchell, J. Pérez-Ramírez and J. P. Gilson, Catal. Sci. Technol., 2011, 1, 1331–1335 CAS.
- Y. Wang, Z. Tao, B. Wu, J. Xu, C. Huo, K. Li, H. Chen, Y. Yang and Y. Li, J. Catal., 2015, 322, 1–13 CrossRef CAS.
- M. Y. Kim, K. Lee and M. Choi, J. Catal., 2014, 319, 232–238 CrossRef CAS.
- P. S. F. Mendes, G. Lapisardi, C. Bouchy, M. Rivallan, J. M. Silva and M. F. Ribeiro, Appl. Catal., A, 2015, 504, 17–28 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj03417b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |