Highly stretchable polymer conductors based on as-prepared PEDOT:PSA/n-PAA hydrogels†
Xiangyan
Ma‡
 ,
Wanping
Cai‡
,
Sai
Zhang
,
Wanping
Cai‡
,
Sai
Zhang
 ,
Jiahong
Guo
,
Xing
Peng
,
Zhoutong
Qiu
,
Jie
Ying
and
Jikui
Wang
*
,
Jiahong
Guo
,
Xing
Peng
,
Zhoutong
Qiu
,
Jie
Ying
and
Jikui
Wang
*
Shanghai Key Laboratory of Advanced polymeric Materials, Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: wang326@ecust.edu.cn
First published on 21st November 2017
Abstract
Conducting polymers with inherently good flexibility and conductivity are good candidates for stretchable conductor applications, such as wearable electronics and healthcare devices. In this article, the crosslinked PAA (polyacrylic acid, n-PAA) hydrogels were fabricated via the reaction between hydrophilic groups of polymers in the presence of the water-based silicone crosslinking agent. By using the PEDOT:PSA (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate-co-acrylic acid) as a conducting component, the conductive hydrogels prepared by this method are highly conductive with a value of 2.00 S cm−1, highly stretchable with 800% strain and exceedingly stable in ambient air for a long time; also they possess superior properties to similar crosslinking methods. The resistance change with stretching is almost strain-insensitive up to 50% strain after 1000 fatigue cycles.
Introduction
Electronically conducting polymers with inherently good flexibility and conductivity are good candidates for stretchable conductor applications, including stretchable displays,1,2 electronic textiles,3 dielectric elastomer actuators,4 artificial muscles5 and electronic skin.6 Currently, stretchable conductors are almost always prepared by combining conductive polymers, such as polyaniline (PANI), polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), with hydrated matrix polymers, which have been applied to functional devices, for instance, bioelectrodes, supercapacitors, sensors and organic solar cells.7–11 Moreover, a variety of materials have been developed for flexible and stretchable conductors, including metal-based materials, such as nanowires,12,13 flakes,14,15 composites,16–19 carbon nanotubes, and graphene, into elastomers.20–24 Unfortunately, stretchable conductors also have apparent drawbacks such as poor processability and unsatisfactory performance in terms of conductivity, flexibility and strength. The popular method for developing stretchable conductors is admixing conducting polymer composites with flexible thin films or gels. Therefore, combining the physical and mechanical properties of hydrogels with the electrical activity of an electroactive/conductive component can create unique opportunities for the next generation of materials.Conducting hydrogels have potential applications due to certain synthetic hydrogels that exhibit exceptional mechanical behaviour and conductive polymers with electrical conductivity.25,26 In general, the preparation of polymer conductive hydrogels is carried out in two ways: (1) Conductive hydrogel is fabricated by the crosslinking polymerization of gel monomers in the conductive polymer solution.27–30 These conductive hydrogels comprise insulating gels that sustain the conductive polymers to be stretchable and deformable, but also constrain the flexibility and stretchability of the conductive polymers, which restrict the variety of the conductivity. (2) The conductive hydrogel was synthesized by adding conductive polymer monomers in crosslinked hydrogels, followed by oxidative polymerization. The conductivity of the hydrogels is usually not high and should be improved by further treatment or reaction.31–35 Lim et al. reported the fabrication of polymeric nanocomposite hydrogels from polyacrylamide by incorporation of graphene–silver–poly(3,4-ethylenedioxythiophene):polystyrene sulfonate via the photopolymerization method. The compressive strength of the hydrogels reaches 1.71 MPa and the electrical conductivity is 3.91 × 10−5 S cm−1.36 Wu et al. reported the supramolecular conducting hydrogel in situ by doping poly(N-acryloyl glycinamide-co-2-acrylamide-2-methylpropanesulfonic) hydrogels with poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS). The tensile strength and breaking strain is 0.22–0.58 MPa and 817–1709%, respectively.28 However, conducting hydrogels were still mechanically weak and exhibit low conductivity, which sufficiently hinders their potential applications.37–39 Therefore, developing a facile and solution-processable method to fabricate highly stretchable hydrogels with both high conductivity and tensile strength is very important in this area.
In this study, a novel construction of hydrogel is developed that can balance conductivity and high mechanical properties. A one-step method is proposed to prepare stretchable electronic conductors utilizing linear structure polymers instead of monomers to fabricate polymer hydrogels with high strength structures. PAA serves not only as the backbone of conducting hydrogels but also promotes the interaction between PAA and PEDOT:PSA. In addition, PAA is an anionic polymer and its chain segments are more flexible and uniformly dispersed in solution, which preserves its doping performance for synthesizing PEDOT, so that the PEDOT:PSA dispersion possesses a highly original conductivity.40 Intriguingly, by using PEDOT:PSA as a conducting component instead of PEDOT:PSS, which possesses the structure of closely packed and similarly crosslinked, an electrical mechanical crosslinking was effectively formed inside the PAA hydrogels. On the premise of improving the electrical conductivity, the conductive hydrogels exhibit excellent mechanical properties in the presence of the crosslinking structure of PAA. Moreover, the n-PAA possesses a network structure, which would endow the PEDOT:PSA/n-PAA hydrogels with a high electrical conductivity even under a highly stretching deformation of 800%, which is higher than that of most conductive hydrogels prepared using the crosslinking method (Fig. S1, ESI†).
Experimental
Preparation of PEDOT:PSA/n-PAA hydrogels
The PSA copolymer was synthesized from AA (acrylic acid) and SS (p-styrene sulfonate) monomer as we had previously reported40 (Fig. S2, ESI†). Briefly, 1.0 g EDOT monomers (HEROchem) and 1.676 g Na2S2O8 (Sigma-Aldrich) were added dropwise within 25 min into an aqueous solution of PSA, which was prepared by mixing 1.5 g PSA (HEROchem) with 100 mL DI H2O. Then, the mixture was reacted for 24 h to synthesize PEDOT:PSA aqueous solution.The prepared PEDOT:PSA aqueous solution, dopant, crosslinking agent (XR-501, XiRun), ethanol (EtOH) and deionized (DI) water were blended to form a mixed solution. Then, PAA (Greagent) was slowly added into the mixed solution with ultrasonic stirring simultaneously for 2 h to receive PEDOT:PSA/PAA hydrated solution, which was subsequently poured into a glass mould with dimensions of 100 mm × 75 mm × 50 mm (w × l × t). The concentration of PEDOT:PSA to the PEDOT:PSA/PAA hydrated solution was varied to 5.84%, 7.04%, 8.16%, 9.21% and 10.19%. Following by standing for 6 h, the PEDOT:PSA/n-PAA hydrogels were obtained after the hydrated solution was gelled at 70 °C in an oven for 48 h. The synthesized hydrogels were washed in DI water mixed with ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 3 days to remove the additional ions, impurities and PSA chains, changing the solution every day, and then drying in a vacuum oven.
1) for 3 days to remove the additional ions, impurities and PSA chains, changing the solution every day, and then drying in a vacuum oven.
Preparation of PEDOT:PSS/n-PAA hydrogels
The PEDOT:PSS/n-PAA hydrogels were fabricated in the same manner, except that the PSA was replaced with PSS. The concentration of PEDOT:PSS to the PEDOT:PSS/PAA hydrated solution was varied to 5.84%, 7.04% and 8.16%.Characterization
The internal structures of hydrogels were observed using a scanning electron microscope (SEM S-3400) under an electron beam with an accelerating voltage of 15 keV and a working distance of 4 mm. For the mechanical strength test, the stress–strain curve was obtained using a microtensile tester (CMT2203). The dimensions of the hydrogels were 30 mm × 10 mm (w × l) rectangles with thicknesses of 1 mm. Tensile fatigue tests were also conducted from 50% strain to 0% strain for the 1st, 500th, 1000th cycles. The displacement rate was 10 mm min−1. Moreover, the resistance was recorded by a digital multimeter (MY61) when the hydrogels were stressed 1000 times. The sheet resistance of the hydrogels was measured using a four-point probe method (RTS-9). The changes in the electrical resistances of the hydrogels during stretching were measured in situ by a digital precision multimeter (Tektronix, DMM 4050) and a RST electrochemical workstation. The LED demonstration of the hydrogels was intuitively observed using two LEDs and a 6 V battery. The hydrogel was cut into a rectangle with dimensions of 27 mm × 5 mm × 1 mm (w × l × t), interconnected between the LEDs and wired to a battery. The moisture rate and cycle test of hydrogels were investigated by weighing the hydrogels after obtaining constant weight (m0) after drying at room temperature. Hydrogel samples were then placed in 50% relative humidity to reach the constant weight (m). The moisture absorption rate (Q) of hydrogels was determined at room temperature according to the following equation:As such, the cyclic moisture absorption curves were obtained by repeating the aforementioned procedure.
Result and discussion
Fig. 1a shows the synthetic procedure of the PEDOT:PSA/n-PAA hydrogels. A stable suspension of PEDOT:PSS/n-PAA and PEDOT:PSA/n-PAA was generated after sonication. The suspension was poured into a glass mould and was left undisturbed to eliminate bubbles. It can be observed that the replacement of PSS with PSA can prevent and minimize serious agglomeration of the PEDOT:PSS due to the flexibility of PSA. Accordingly, the synergetic effects from the flexibility of PEDOT:PSA chains and the functional group –COOH of the PAA result in a more uniform and higher crosslinking degree in PEDOT:PSA/n-PAA hydrogels. The co-crosslinking reaction between the PAA and PSA can promote the high dispersity, suggesting that conductive components are firmly bound to the network. The PEDOT:PSA/n-PAA hydrogels present outstanding conducting properties via the crosslinking of the carboxyl group. Fig. 1b illustrates a stretchable LED circuit, in which two LED lights are interconnected with conductive PEDOT:PSA (8.16%)/n-PAA hydrogels. The two LED lights remain turned on during stretching. Furthermore, it is elastically recovered after being released.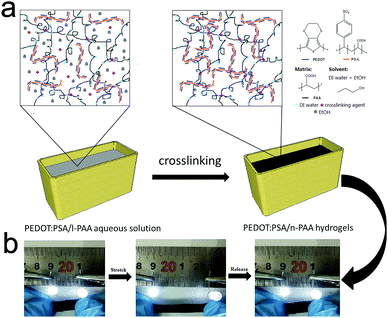 | ||
| Fig. 1 (a) Schematic illustration of PEDOT:PSA/n-PAA hydrogels. (b) Optical images of the circuit before and after stretching up to 200% strain. | ||
The SEM images of PEDOT:PSS/n-PAA and PEDOT:PSA/n-PAA hydrogels are shown in Fig. 2. The changes observed in the microstructures provide important information to reveal the crosslinking mechanism of the hydrogels. It is observed that the PEDOT:PSS/n-PAA hydrogels display numerous protruding morphologies with irregular shapes of small granule islands (Fig. S3, ESI†). As shown in Fig. 2a, the excess amount of PEDOT:PSS would inevitably prevent crosslinking of the PAA chains owing to the stiffness of PSS, resulting in club shapes in the hydrogel internal structure. Comparing to the PEDOT:PSS/n-PAA hydrogels, a more uniform and close folding texture are presented in the PEDOT:PSA/n-PAA hydrogels. The regular shape suggests that PEDOT was successfully embedded in the PEDOT:PSA/n-PAA hydrogel and formed a fibrous part. This indicates that the flexible structure of PSA and the similarity to the structure of PAA should enhance the interfacial interactions between the PEDOT:PSA and PAA chains. This promotes the PEDOT:PSA chains highly to disperse in the PAA hydrogels, which is an important factor for increasing the conduction of the hydrogels. Furthermore, the structure becomes more uniform and closely packed in the PEDOT:PSA/n-PAA hydrogels on increasing the PEDOT:PSA weight ratio. A large amount of PEDOT clusters appear and are distributed in the hydrogel. The changes of microstructures of the hydrogels could be associated with the mechanical properties.
The fabricated PEDOT:PSA/n-PAA hydrogels are mechanically soft and highly stretchable. As presented in Fig. 3a, the hydrogels were fixed to two acryl plates installed in the tensile machine and then stretched until mechanical rupture occurred. To investigate the effect of PEDOT formation on the mechanical behaviour of the hydrogels, the tensile tests were conducted for different PEDOT weights. Fig. 3b shows the stress–strain curves of PEDOT:PSA(S)/n-PAA hydrogels. The strain and mechanical strength are dependent on the weight ratio of PEDOT:PSA in the hydrogels. The tensile strength and strain of the PEDOT:PSA/n-PAA hydrogels presents are similar even on increasing the weight ratio of PEDOT. Interestingly, the mechanical properties show different trends with an increasing PEDOT ratio in the two types of hydrogels. For PEDOT:PSS/n-PAA hydrogels, the carboxyl group of PAA chains could crosslink better at lower weight ratios making the hydrogel exhibit a higher tensile strength. The hydrogels show lower tensile strength and higher rupture strain as the weight ratio increases (Fig. S4, ESI†). This is because PAA cannot completely crosslink due to a hard segment. Furthermore, compared to the PEDOT:PSS/n-PAA hydrogels, the crosslinking structures resulting from the carboxyl groups of PSA and PAA chains at higher weight ratios in the PEDOT:PSA/n-PAA hydrogels make the hydrogels show higher stiffness than the PEDOT:PSS/n-PAA hydrogels. As shown in Fig. 3c, with increasing PEDOT:PSA weight ratio, the Young's modulus of the hydrogels is enhanced, but the rupture strain of the hydrogels decreases sharply at 8.16%. In other words, these hydrogels are gradually stretched to greater than 400% strain; the tensile strength of the hydrogels is in the range of 0.5–4.2 MPa. These phenomena suggest that there are some different crosslink types in the PEDOT:PSA(S)/n-PAA hydrogels with increasing weight ratio. In other words, the microstructural difference between the two types of hydrogels critically influences the mechanical properties. Thus, the crosslinking between the PAA structure and the PEDOT:PSA is stronger for the PEDOT:PSA/n-PAA hydrogels. The mechanical strength measured by a stress–strain curve shows 4-fold enhancement over the single PAA hydrogel.9 Hence, the PEDOT:PSA/n-PAA hydrogels totally possess excellent tensile strength.
To investigate the tensile fatigue of the PEDOT:PSA/n-PAA hydrogels, highly repeated tensile deformation up to 50% strain for 1000 cycles was applied. Every cycle of stress and strain was carried out during the tensile fatigue of the PEDOT:PSA (8.16%)/n-PAA hydrogels. As shown in Fig. 3d, it is observed that the stress–strain curves display a stable and similar trend at the 1st, 500th, and 1000th cycle as representative cycles, suggesting that the hydrogels could be recoverable after tensile fatigue. This behaviour is in accordance with the microstructure of hydrogels after tensile stress cycles (Fig. S5, ESI†). This is further evidence that the hydrogels are highly stretchable. Fig. 3e displays the resistance changes of the PEDOT:PSA (8.16%)/n-PAA hydrogels through the cycle test. The resistance of the hydrogel is typically fluctuating before the 200 cycles and tends to keep nearly steady on subsequent tests. This resistance undulation inversely suggests that the conductive path is influenced by the tensile deformation for the initial cycle up to the 200th cycle. However, as the number of fatigue tests increased, the resistance change was almost invariable and stable. These behaviours can indicate that the repeat tests preserve the network of conductive paths within hydrogels.
The fabricated PEDOT:PSA/n-PAA hydrogels are electrically conductive and highly stretchable (Fig. S6 and Movie S1, ESI†). It shows that the conductivity of the hydrogel is not influenced under repeated load-unloading. As shown in Fig. 4a, the electrical conductivity of the PEDOT:PSA/n-PAA hydrogels increases dramatically with the increase in the weight ratio of PEDOT:PSA. The results obtained here for conductivity are slightly higher than that of the same weight percent PEDOT:PSS/n-PAA hydrogels. On the premise of a small increase the electrical conductivity, the PEDOT:PSA/n-PAA hydrogels shows well mechanical properties through using PEDOT:PSA instead of PEDOT:PSS as a conductive component. The electrical conductivity of the hydrogels is ascribed to crosslinking hydrogel-conjugated polymer systems, while conventional conductive hydrogels are mostly composed of water containing extra ions inside the hydrogels. In addition, these conductivities are significantly higher than those of other conductive hydrogels, whose conductivities are almost below 2.00 S cm−1 (Fig. S1, ESI†). As shown in Fig. 4b, we also studied the changes in the electrical resistance in the PEDOT:PSA/n-PAA hydrogels during stretching up to 800% strain. The internal percolation path of PEDOT:PSA is well preserved inside the PEDOT:PSA/n-PAA hydrogels, even under tensile deformation. The PEDOT:PSA/n-PAA hydrogels show remarkably low increases of resistance during uniaxial stretching. Interestingly, almost all hydrogels show strain insensitivity for larger strains except the PEDOT:PSS/n-PAA hydrogels. The resistance change clearly increased during stretching. Therefore, the PEDOT:PSA/n-PAA hydrogels are still conductive under a large strain compared to PEDOT:PSS/n-PAA hydrogel. This phenomenon is based on the conductive components dispersing effectively in the hydrogel and the better crosslinking with carboxyl groups.
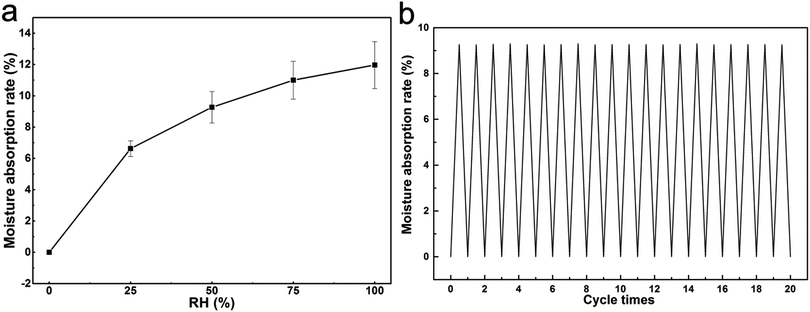
To further prove the PEDOT:PSA/n-PAA hydrogels have extensive applications, the moisture rate and cycle test were conducted to demonstrate the environment stability of hydrogels. Fig. 5a presents the moisture absorption rate (Q) of PEDOT:PSA (8.16%)/n-PAA hydrogels as a function of relative humidity (RH) at 25 °C. It is observed that the water absorption of the hydrogels tends to increase as the relative humidity increases. The hydrogels present good water absorption after the carboxyl group crosslinking of PSA and PAA. As shown in Fig. 5b, it is also proved that the hydrogels display excellent environment stability when the cycle test was conducted. In addition, the PEDOT:PSS/n-PAA hydrogels easily dried out and became a hard bulk polymer after one day during the experimental process. In contrast, the PEDOT:PSA/n-PAA hydrogel almost maintained its initial state and also can repeatedly stretch compared to a pure n-PAA hydrogel (Fig. S7 and Movie S2, ESI†). Thus, based on these results, we proved ensure the good long-term stability of the conductive hydrogels compared with other PEDOT:PSS/n-PAA hydrogels.
Conclusions
In this study, we proposed a facile method to construct highly stretchable conductive hydrogels using the linear PAA hydrogels co-crosslinked by the hydrophilic groups between PSA and PAA. Electronic conductivity of the PAA hydrogels investigated herein was significantly enhanced by sequential introduction of PEDOT:PSA. The addition of PSA copolymers is shown to significantly increase the strain of the PEDOT:PSA/n-PAA hydrogels. The mechanical strength measured by a stress–strain curve shows 4-fold enhancement over the single PAA hydrogel. Moreover, the elastic polymer conductors prepared by this method are highly conductive with a value of 2.00 S cm−1 and exceedingly stable in ambient conditions for a long time, which have potential application for flexible devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely acknowledge ‘Shanghai university-industry collaboration program (CXY-2014-023)’ and ‘Scientific and technological achievements transformation program of Jiangsu Province (SBA2014010034)’.References
- M. Y. Teo, N. Kim, S. Kee, B. S. Kim, G. Kim, S. Hong, S. Jung and K. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 819 CAS.
- S. K. Lee, B. J. Kim, H. Jang, S. C. Yoon, C. Lee, B. H. Hong, J. A. Rogers, J. H. Cho and J. H. Ahn, Nano Lett., 2011, 11, 4642 CrossRef CAS PubMed.
- C. Wu, T. W. Kim, F. Li and T. Guo, ACS Nano, 2016, 10, 6449 CrossRef CAS PubMed.
- S. Liu, M. Tian, B. Yan, Y. Yang, L. Zhang, T. Nishi and N. Ning, Polymer, 2015, 56, 375–384 CrossRef CAS.
- D. Pattavarakorn, P. Youngta, S. Jaesrichai, S. Thongbor and P. Chaimongkol, Energy Procedia, 2013, 34, 673–681 CrossRef CAS.
- A. Chortos, J. Liu and Z. Bao, Nat. Mater., 2016, 15, 937 CrossRef CAS PubMed.
- D. Mawad, E. Stewart, D. L. Officer, T. Romeo, P. Wagner, K. Wagner and G. G. Wallace, Adv. Funct. Mater., 2012, 22, 2691 CrossRef.
- H. Ding, M. Zhong, Y. J. Kim, P. Pholpabu, A. Balasubramanian, C. M. Hui, H. He, H. Yang, K. Matyjaszewski and C. J. Bettinger, ACS Nano, 2014, 8, 4348–4357 CrossRef CAS PubMed.
- S. Naficy, J. M. Razal, G. M. Spinks, G. G. Wallace and P. G. Whitten, Chem. Mater., 2012, 24, 3425–3433 CrossRef CAS.
- L. Li, Y. Wang, L. Pan, Y. Shi, W. Cheng, Y. Shi and G. Yu, Nano Lett., 2015, 15, 1146 CrossRef CAS PubMed.
- Y. Y. Lee, H. Y. Kang, S. H. Gwon, G. M. Choi, S. M. Lim, J. Y. Sun and Y. C. Joo, Adv. Mater., 2016, 28, 1636 CrossRef CAS PubMed.
- Y. Ko, S. K. Song, N. H. Kim and S. T. Chang, Langmuir, 2016, 32, 366 CrossRef CAS PubMed.
- G. Jin, H. B. Yao, W. Xu, Y. D. Ye, J. L. Wang, Z. Y. Wu, J. W. Liu, F. J. Fan, H. L. Gao and C. L. Zhang, Angew. Chem., 2013, 52, 1654 CrossRef PubMed.
- Y. Huang, X. Bai, Z. Ming, S. Liao, Z. Yu, Y. Wang and W. Hui, Nano Lett., 2016, 16, 5846 CrossRef CAS PubMed.
- Z. Li, Z. Liu, H. Sun and C. Gao, Chem. Rev., 2015, 15, 7046–7117 CrossRef PubMed.
- M. S. Lee, K. Lee, S. Y. Kim, H. Lee, J. Park, K. H. Choi, H. K. Kim, D. G. Kim, D. Y. Lee and S. Nam, Nano Lett., 2013, 13, 2814 CrossRef CAS PubMed.
- S. Yun, X. Niu, Z. Yu, W. Hu, P. Brochu and Q. Pei, Adv. Mater., 2012, 24, 1321 CrossRef CAS PubMed.
- M. Park, J. Im, M. Shin, Y. Min, J. Park, H. Cho, S. Park, M. B. Shim, S. Jeon and D. Y. Chung, Nat. Nanotechnol., 2012, 7, 803–809 CrossRef CAS PubMed.
- B. Chen, J. J. Lu, C. H. Yang, J. H. Yang, J. Zhou, Y. M. Chen and Z. Suo, ACS Appl. Mater. Interfaces, 2014, 6, 7840–7845 CAS.
- Y. Sun, W. M. Choi, H. Jiang, Y. Y. Huang and J. A. Rogers, Nat. Nanotechnol., 2006, 1, 201–207 CrossRef CAS PubMed.
- J. Zang, S. Ryu, N. Pugno, Q. Wang, Q. Tu, M. J. Buehler and X. Zhao, Nat. Mater., 2013, 12, 321 CrossRef CAS PubMed.
- F. Xu, X. Wang, Y. Zhu and Y. Zhu, Adv. Funct. Mater., 2012, 22, 1279–1283 CrossRef CAS.
- C. Wu, L. Fang, X. Huang and P. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 21026–21034 CAS.
- Y. Liu, G. Q. Qi, C. L. Liang, R. Y. Bao, W. Yang, B. H. Xie and M. B. Yang, J. Mater. Chem. C, 2014, 2, 3846–3854 RSC.
- Y. Xie, S. Xu, Z. Xu, H. Wu, C. Deng and X. Wang, Carbon, 2016, 98, 381–390 CrossRef CAS.
- K. J. Henderson, T. C. Zhou, K. J. Otim and K. R. Shull, Macromolecules, 2010, 43, 6193–6201 CrossRef CAS.
- X. Yu, H. Yang, H. Meng, Y. Sun, J. Zheng, D. Ma and X. Xu, ACS Appl. Mater. Interfaces, 2015, 7, 15961–15967 CAS.
- W. Qian, J. Wei, X. Bing, X. Liu, H. Wang, W. Wei, Q. Wang and W. Liu, Sci. Rep., 2017, 7, 41566 CrossRef PubMed.
- H. Yu, Y. Guo, C. Yao, D. Perepichka and H. Meng, J. Mater. Chem. C, 2016, 4, 11055 RSC.
- Y. Wang, C. Zhu, R. Pfattner, H. Yan, L. Jin, S. Chen, F. Molinalopez, F. Lissel, J. Liu and N. I. Rabiah, Sci. Adv., 2017, 3, e1602076 CrossRef PubMed.
- B. Pépin-Donat, A. Viallat, J.-F. Blachot and C. Lombard, Adv. Mater., 2010, 18, 1401–1405 CrossRef.
- I. González, M. Vecino, M. E. Muñoz, A. Santamaría and J. A. Pomposo, Macromol. Chem. Phys., 2004, 205, 1379–1384 CrossRef.
- W. Yang, Y. Zhao, X. He, Y. Chen, J. Xu, S. Li, Y. Yang and Y. Jiang, Nanoscale Res. Lett., 2015, 10, 1–7 CrossRef PubMed.
- X. Wu, J. Liu, D. Wu, Y. Zhao, X. Shi, J. Wang, S. Huang and G. He, J. Mater. Chem. C, 2014, 2, 4044–4050 RSC.
- R. Kishi, K. Kubota, T. Miura, T. Yamaguchi, H. Okuzaki and Y. Osada, J. Mater. Chem. C, 2013, 2, 736–743 RSC.
- B. C. Lim, B. S. Singu, S. E. Hong, Y. H. Na and K. R. Yoon, Polym. Adv. Technol., 2016, 27, 366–373 CrossRef CAS.
- T. Ni, L. Xu, Y. Sun, W. Yao, T. Dai and Y. Lu, ACS Sustainable Chem. Eng., 2015, 3, 862–870 CrossRef CAS.
- T. Dai, X. Qing, H. Zhou, C. Shen, J. Wang and Y. Lu, Synth. Met., 2010, 160, 791–796 CrossRef CAS.
- H. Warren and M. I. H. Panhuis, Synth. Met., 2015, 206, 61–65 CrossRef CAS.
- W. Cai, X. Ma, J. Guo, X. Peng, S. Zhang, Z. Qiu, J. Ying and J. Wang, J. Appl. Polym. Sci., 2017, 134 DOI:10.1002/app.45163.
Footnotes |
| † Electronic supplementary information (ESI) available: Comparison of comprehensive properties with other recently reported conducting hydrogels; synthesis of the PSA copolymers; microstructure demonstration of PEDOT:PSS/n-PAA hydrogels; mechanical properties of PEDOT:PSS/n-PAA hydrogels; demonstration of PEDOT:PSA/n-PAA hydrogels for stretchable interconnects; comparison of PEDOT:PSA(S)/n-PAA hydrogels for environmental stability the movie caption of PEDOT:PSA/n-PAA hydrogels. See DOI: 10.1039/c7nj03103c |
| ‡ Xiangyan Ma and Wanping Cai contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |