Photoredox catalysed synthesis of amino alcohol†
Vishal
Srivastava
a,
Pravin K.
Singh
b,
Sudhanshu
Kanaujia
c and
Praveen P.
Singh
 *c
*c
aDepartment of Chemistry, United College of Engineering & Management, Naini, Allahabad – 211010, India
bFood Analysis and Research Lab, Centre of Food Technology, University of Allahabad, Allahabad – 211002, India
cDepartment of Chemistry, United College of Engineering & Research, Naini, Allahabad – 211010, India. E-mail: ppsingh23@gmail.com
First published on 29th November 2017
Abstract
A mild and efficient one-pot visible light-induced method has been developed for the synthesis of amino alcohols. Amino alcohols are important structural elements that are frequently found in pharmaceutical agents and biologically active natural products. The routine procedure in the drug discovery and development process to prepare and fully characterize amino alcohols makes them a drug candidate for biological evaluation. This protocol utilizes visible light as the greenest reagent and eosin Y as an organophotoredox catalyst to synthesise bioactive amino alcohols. Gram scale reactions demonstrated the potential synthetic applications of this protocol.
Introduction
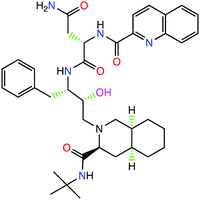
β-Amino alcohols are found in a large variety of biologically important compounds, like natural products and peptides. They play an important role in medicinal and organic chemistry.1,2 This functional group is often found in many bioactive compounds. Cyclic amino alcohols form a large group of biologically active natural products; for example, quinine that is used for malaria treatment. One important class of cyclic amino alcohols is the polyhydroxylated alkaloids, also known as aza-sugars, e.g. castanospermine3 that was found to be a potent inhibitor of α- and β-glucosidases. Peptidomimetics constitute a large group of synthetically produced pharmacologically active amino alcohols, most commonly Renin and HIV-1 protease inhibitors; for example, saquinavir (Fig. 1).4,5β-Amino alcohols are used as intermediates in the synthesis of a wide range of biologically active natural and synthetic products6–8 as well as in unnatural amino acids.9,10 The β-amino alcohols also play an important role as chiral ligands, most commonly derived from natural sources. Chiral 1,2-amino alcohols are common structural motifs found in a vast array of natural and biologically active molecules. The amino alcohols are generally derivatized to improve their chelating ability or to increase their steric directing effect11 and also used in asymmetric synthesis.12–15
The potential of developing new synthetic methodologies using visible light has recently received much attention from a number of research groups.16,17 This is because solar energy (visible light) is clean, easy to handle and an unlimited energy source having great prospects for the development of sustainable and eco-friendly protocols for organic synthesis.18 Visible light is increasingly appreciated as an abundant, renewable, and clean energy source for chemical reactions. Photoredox catalysis is an attractive approach to activating organic molecules by translating visible light energy via single-electron transfer (SET). Some pioneering researchers have dedicated their work to converting solar energy into chemical energy for chemical transformations19,20 which includes the development of a promising strategy for the application of photoredox catalysts to initiate single electron transfer processes.21,22 A surge of interest from the synthetic community has brought photoredox manifolds to the forefront of catalysis. In this sequence visible light photoredox catalysis has recently received much attention in organic synthesis owing to the readily availability, sustainability, non-toxicity and ease of handling of visible light.23–31
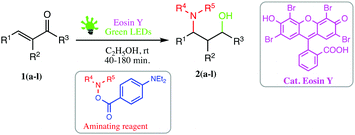
However, transition metal based photocatalysts disadvantageously exhibit high cost, low sustainability and potential toxicity. Recently, superior alternatives to transition metal photoredox catalysts, especially metal-free organic dyes such as eosin Y, fluorescein, rose bengal, nile red, perylene and rhodamine B, have been used as economically and ecologically superior surrogates for Ru(II) and Ir(II) complexes in visible-light promoted organic transformations involving SET32–35 (single electron transfer). These organic dyes have attracted much more attention in the last few years also due to their ease of handling, eco-friendliness and great potential for applications in visible-light-mediated organic synthesis36–39 which fulfils the basic principle of green chemistry. Encouraged by organocatalytic visible light-mediated transformations and in continuation of our work on the development of novel environmentally benign synthesis40–46 herein we report a simple, visible light irradiated, efficient and green protocol for the synthesis of amino alcohols.
Results and discussion
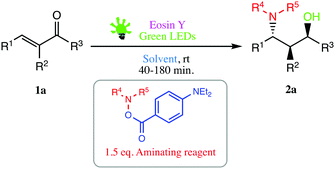
In order to realise our idea and optimise the reaction conditions, the key reaction of α,β-unsaturated aldehydes 1a with a catalytic amount of eosin Y in a solvent under irradiation with green LEDs [2.50 W, λ = 535 nm] was carried out (Table 1).| Entry | Catalyst (mol%) | Solvent | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), catalyst (mol%), in 3 mL solvent irradiated using Luxeon Rebel high power green LEDs [2.50 W, λ = 535 nm] at rt for 40–180 min. b Isolated yield of the pure product 2a. c Reaction was performed in the dark. d Reaction was carried out without the catalyst. e 18 W CFL (compact fluorescent lamp, Philips) was used. | ||||
| 1 | Eosin Y (2) | CH3OH | 40 | 92 |
| 2 | Rose bengal (2) | CH3OH | 40 | 72 |
| 3 | Fluorescein (2) | CH3OH | 40 | 70 |
| 4 | Nile red (2) | CH3OH | 40 | 74 |
| 5 | Perylene (2) | CH3OH | 40 | 75 |
| 6 | Rhodamine B (2) | CH3OH | 40 | 76 |
| 7 | Eosin Y (2) | AcOH | 40 | 33 |
| 8 | Eosin Y (2) | iPrOH | 40 | 78 |
| 9 | Eosin Y (2) | EtOH | 40 | 86 |
| 10 | Eosin Y (1) | CH3OH | 40 | 67 |
| 11 | Eosin Y (3) | CH3OH | 40 | 92 |
| 12 | Eosin Y (2) | CH3OH | 180 | Tracesc |
| 13 | — | CH3OH | 180 | n.d.d |
| 14 | Eosin Y (2) | CH3OH | 180 | 58e |
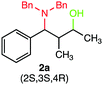
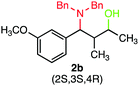
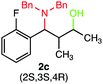
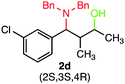
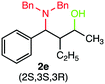
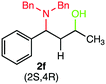
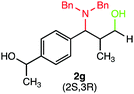
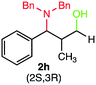
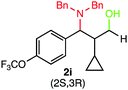
We were delighted to obtain the desired substituted amino alcohol product, 2a, in 92% yield (Table 1, entry 1). Then, the control experiments were carried out, which show that eosin Y and visible light are essential for the reaction, because in the absence of any of the reagents/reaction parameters either the product was not detected (n.d.) or was formed in traces (Table 1, entries 1 versus 12 and 13). The scope of the present protocol across a range of α,β-unsaturated aldehydes incorporating various substituents was studied. It was found that α,β-unsaturated aldehydes with an electron-donating group on the aromatic ring appear to react faster and afford marginally higher yields in comparison to those bearing an electron-withdrawing group (Table 2).
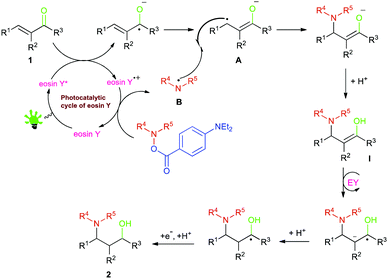
On the basis of our observations and the literature reports,47–52 a plausible mechanistic pathway is depicted in Scheme 1.
On absorption of visible light, the organophotoredox catalyst eosin Y (EY) is excited to its singlet state 1EY* which through inter system crossing (ISC) comes to its more stable triplet state 3EY* and undergoes a single electron transfer (SET). 3EY* may undergo both reductive and oxidative quenching.53–57 A SET from substituted α,β-unsaturated aldehydes 1 generates an allyl free radical A, which combines with a di benzyl amine radical B, generated from the aminating reagent to form an intermediate compound I, which is followed by SET as well as protonation and acceptance of an electron giving the desired product 2.
A broad singlet at δ 4.70 (exchangeable with D2O) was attributed to the –OH group in the desired product. Similarly, the presence of IR absorption bands in the region of 3600–3200 cm−1 in the IR spectra indicates the presence of –OH groups in the desired amino alcohol product. These observed data supported that all synthesized compounds have –OH groups, obtained by the reduction of carbonyl groups.
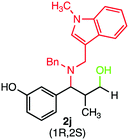
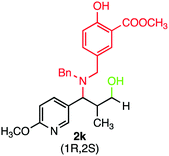
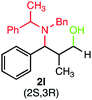
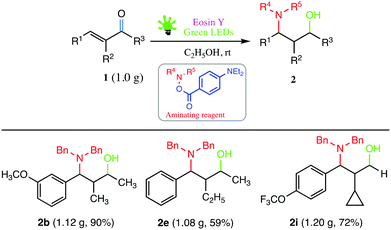
During our investigation, several gram-scale reactions were carried out under standard reaction conditions and the desired products were obtained in good yield (Scheme 2). The results might suggest the potential application of the developed protocol in the library synthesis as well as in screening of biologically active compounds.
Conclusions
In conclusion, we have developed a novel, one-pot procedure for the synthesis of substituted amino alcohols from substituted α,β-unsaturated aldehydes via photocatalysed reduction. This protocol involves the use of an aminating reagent and visible light as the cheapest and eco-sustainable reagents as well as eosin Y as an organophotoredox catalyst at room temperature. Thus, it is a superior alternative to the existing method with respect to green and sustainable chemistry (better atom- and step-economy) for reduction reactions. In addition, the gram scale reactions demonstrated the potential synthetic applications of the developed procedure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely thank the Emerging Science Society for financial support as well as SAIF, CDRI, Lucknow, and IISc, Bangalore, for providing microanalyses and spectra.Notes and references
- G. Cardillo and C. Tomasini, Chem. Soc. Rev., 1996, 25, 117 RSC.
- D. C. Cole, Tetrahedron, 1994, 50, 9517 CrossRef CAS.
- (a) J. P. Michael, Nat. Prod. Rep., 1999, 16, 675 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2001, 18, 520 RSC.
- S. C. Bergmeier, Tetrahedron, 2000, 56, 2561 CrossRef CAS.
- W. R. Baker and S. L. Condon, J. Org. Chem., 1993, 58, 3277 CrossRef CAS.
- B. M. Teresa, J. C. M. Adilia, C. D. Maycockd and T. Michaud, Tetrahedron, 2005, 61(33), 7960 CrossRef.
- E. J. Corey and F. Zhang, Angew. Chem., Int. Ed., 1999, 38(13–14), 1931 CrossRef CAS.
- C. W. Johannes, M. S. Visser, G. S. Weatherhead and A. H. Hoveyda, J. Am. Chem. Soc., 1998, 120(33), 8340 CrossRef CAS.
- P. O’Brien, Angew. Chem., Int. Ed., 1999, 38(3), 326 CrossRef.
- G. Li, H. T. Chang and K. B. Sharpless, Angew. Chem., Int. Ed., 1996, 35(4), 451 CrossRef CAS.
- D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS PubMed.
- D. E. Frantz, R. Fässler and E. M. Carriera, J. Am. Chem. Soc., 2000, 122, 1806 CrossRef CAS.
- S. Kobayashi, M. Sugiura, H. Kitagawa and W. W.-L. Lam, Chem. Rev., 2002, 102, 2227 CrossRef CAS PubMed.
- S. L. Shi, Z. L. Wong and S. L. Buchwald, Nature, 2016, 532, 353 CrossRef CAS PubMed.
- W. Ding, L. Q. Lu, J. Liu, D. Liu, H. T. Song and W. J. Xiao, J. Org. Chem., 2016, 81, 7237 CrossRef CAS PubMed.
- J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed.
- D. R. Heitz, K. Rizwan and G. A. Molander, J. Org. Chem., 2016, 81, 7308 CrossRef CAS PubMed.
- X. Sala, I. Romero, M. Rodriguez, L. Escriche and A. Llobet, Angew. Chem., Int. Ed., 2009, 48, 2842 CrossRef CAS PubMed.
- D. Mandler and I. Willner, J. Am. Chem. Soc., 1984, 106, 5352 CrossRef CAS.
- O. Ishitani, S. Yanagida, S. Takamuku and C. Pac, J. Org. Chem., 1987, 52, 2790 CrossRef CAS.
- A. Inagaki and M. Akita, Coord. Chem. Rev., 2010, 254, 1220 CrossRef CAS.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355 CrossRef CAS.
- J. Xie, H. Jina, P. Xu and C. Zhu, Tetrahedron Lett., 2014, 55, 36 CrossRef CAS.
- X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2014, 43, 473 RSC.
- J. Hu, J. Wang, T. H. Nguyen and N. Zheng, Beilstein J. Org. Chem., 2013, 9, 1977 CrossRef PubMed.
- D. Rovelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97 RSC.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed.
- J. Xuan and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed.
- J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed.
- J. R. Chen, D. M. Yan, Q. Wei and W. J. Xiao, ChemPhotoChem, 2017, 1, 148 CrossRef CAS.
- X. J. Yang, B. Chen, L. Q. Zheng, L. Z. Wu and C. H. Tung, Green Chem., 2014, 16, 1082 RSC.
- Y. C. Teo, Y. Pan and C. H. Tan, ChemCatChem, 2013, 5, 235 CrossRef CAS.
- K. Fidaly, C. Ceballos, A. Falguières, M. S. I. Veitia, A. Guy and C. Ferroud, Green Chem., 2012, 14, 1293 RSC.
- D. T. Yang, Q. Y. Meng, J. J. Zhong, M. Xiang, Q. Liu and L. Z. Wu, Eur. J. Org. Chem., 2013, 7528 CrossRef CAS.
- Y. Q. Zou, J. R. Chen, X. P. Liu, L. Q. Lu, R. L. Davis, K. A. Jørgense and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784 CrossRef CAS PubMed.
- D. P. Hari and B. König, Org. Lett., 2011, 13, 3852 CrossRef CAS PubMed.
- M. Neumann, S. Füldner, B. König and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951 CrossRef CAS PubMed.
- V. Rey, S. M. Soria-Catro, J. E. Arguello and A. B. Peñéñory, Tetrahedron Lett., 2009, 50, 4720 CrossRef CAS.
- V. Srivastava, P. K. Singh and P. P. Singh, Chem. Heterocycl. Compd., 2014, 50, 573 CrossRef CAS.
- V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2014, 87, 91 CrossRef.
- V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2015, 88, 59 CrossRef CAS.
- V. Srivastava, P. K. Singh and P. P. Singh, Croat. Chem. Acta, 2015, 88, 227 CrossRef CAS.
- V. Srivastava, P. K. Singh and P. P. Singh, Asian J. Chem., 2016, 28(10), 2159 CrossRef CAS.
- V. Srivastava, P. K. Singh, S. Sinha and P. P. Singh, Rev. Roum. Chim., 2016, 61(10), 755 Search PubMed.
- V. Srivastava and P. P. Singh, RSC Adv., 2017, 7, 31377 RSC.
- F. R. Bisogno, A. Cuetos, I. Lavandera and V. Gotor, Green Chem., 2009, 11, 452 RSC.
- D. Menigaux, P. Belmont and E. Brachet, Eur. J. Org. Chem., 2017, 2008 CrossRef CAS.
- D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688 RSC.
- M. D. Kärkäs, ACS Catal., 2017, 7, 4999 CrossRef.
- T. Xiong and Q. Zhang, Chem. Soc. Rev., 2016, 45, 3069 RSC.
- J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC.
- D. C. Neckers and O. M. Valdes-Aguilera, Adv. Photochem., 1993, 18, 315 CrossRef CAS.
- M. V. Encinas, A. M. Rufs, S. G. Bertolotti and C. M. Previtali, Polymer, 2009, 50, 2762 CrossRef CAS.
- T. Lizarides, T. McCormick, P. Du, G. Luo, B. Lindley and R. Eisenberg, J. Am. Chem. Soc., 2009, 131, 9192 CrossRef PubMed.
- S. H. Lee, D. H. Nam and C. B. Park, Adv. Synth. Catal., 2009, 351, 2589 CrossRef CAS.
- T. Xiao, X. Dong, Y. Tang and L. Zhou, Adv. Synth. Catal., 2012, 354, 3195 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nj03068a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |