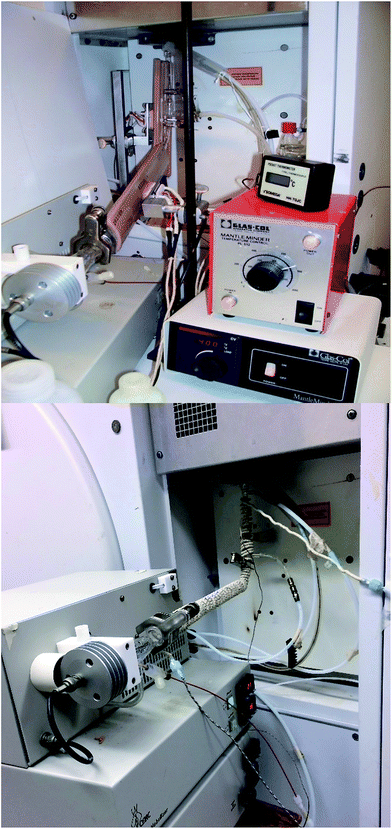
Improvement of sample introduction to inductively coupled plasma optical emission spectrometry using an ultrasonic nebulizer with an infrared heated pre-evaporation tube
Tia K.
Anderlini†
and
Diane
Beauchemin
 *
*
Department of Chemistry, Queen's University, Kingston, Ontario K7L 3N6, Canada. E-mail: diane.beauchemin@chem.queensu.ca; Fax: +1-613-533-6669; Tel: +1-613-533-2619
First published on 4th December 2017
Abstract
In the search for greater sample introduction efficiency and enhanced analytical performance with inductively coupled plasma optical emission spectrometry (ICPOES), a conventional ultrasonic nebuliser was modified to replace the heater/condenser with an infrared heated pre-evaporation tube. In continuation from previous works with pre-evaporation, the current work investigated the effects of infrared heating with ceramic block and ceramic beaded rope heaters. Heating temperatures were varied. By monitoring changes to sensitivity, detection limit, precision, and robustness, and analyzing two certified reference materials, a sample introduction system operating at 300 °C and 0.8 mL min−1 sample uptake rate was established, which provided improved sample introduction efficiency and comparable analytical performance to a previous system operating at 400 °C and 1.5 mL min−1. These conditions are robust, with a Mg 280.270 nm/285.213 nm ratio of 12.4 ± 0.3 (n = 6), which enabled the accurate analysis of two certified reference materials with a simple external calibration without internal standardization.
Introduction
Fast, robust, and sensitive multi-elemental analysis techniques that are accurate and precise are required across various disciplines including health and food sciences, geochemical exploration, pharmaceuticals, and atmospheric sciences. Two of the most common techniques for multi-elemental analysis are inductively coupled plasma (ICP) mass spectrometry (MS) and ICP optical emission spectrometry (OES).1–4 While the former is used for ultra-trace elemental analysis, the latter is typically employed for analysis of major, minor, and trace elements. Although ICPMS has a clear advantage in detection limits, ICPOES wins in the category of robustness, as its passive measurement of light is able to resist matrix effects, while the physical extraction of ions in the plasma of ICP-MS subjects it to both spectroscopic and non-spectroscopic interferences.5 If the detection abilities of ICPOES could be improved to the level of ICPMS, ICPOES could see huge success in new-found applications across many disciplines. This research will therefore investigate the potential of improving sensitivities and detection limits of ICPOES without sabotaging robustness.One of the leading sample introduction systems for nearing this goal is the ultrasonic nebuliser (USN) with desolvation system. By greatly reducing the noise caused by variable droplet size, and increasing the transport efficiency, the USN generally results in a 10-fold improvement in detection limits over conventional pneumatic nebulisation with ICPOES. Through vaporising and condensing the sample aerosol with the USN's heater and condenser, respectively, solvent is removed to pre-concentrate the analyte and increase the sample introduction efficiency to 30%.6,7 However, the matrix is concurrently pre-concentrated.8 Moreover, some elements may be lost during the process of desolvation, and memory effects may become exacerbated by the long path involved in the heater-condenser (HC) system, as well as the membrane desolvator used for organic solvents.9,10 Finally, by removing water in the HC system, the beneficial attributes of water as a load buffer in the plasma are lost. Water typically minimizes matrix effects11 and acts as the main source of hydrogen in the plasma. Having a high thermal conductivity, hydrogen facilitates the transfer of energy between the bulk and central channel of the plasma.12
By replacing the HC of a USN with a pre-evaporation tube (PET), water is preserved while the benefits of ultrasonic nebulisation are still achieved.13,14 Using heating tape to heat the PET, improvements in detection limit in comparison to a conventional PN were achieved while preserving robustness. This method also allowed for the successful determination of Hg, which otherwise would have been lost in the desolvation system.14 However, the plasma extinguished at sample uptake rates above 0.3 mL min−1, whereas insignificant improvements in detection limit and a degradation of instrumental precision were obtained compared to the standard USN system.
Alternatively, by using a ceramic block infrared (IR) heater, the benefits of a fast heating rate and uniform temperature were realized.15,16 A 10–25 fold improvement in detection limit was achieved over conventional pneumatic nebulization,13 proving that pre-evaporating the sample aerosol improves both the plasma excitation conditions and robustness by increasing the amount of water vapour entering the plasma. The objective of this work was to improve on these past USN-PET approaches using two types of infrared heaters at lower temperatures. While 400 °C had been used in the past work, it was the lowest temperature tested with the IR heater,13 which had been selected based on the fact that 400 °C was the highest temperature that could be used with the heating tape and provided the best results.14 The present goal was to see if a lower heating temperature with the IR heater would provide sufficient pre-evaporation to improve sample introduction efficiency and if a beaded rope could replace the bulkier block heater.
Experimental
Instrumentation
Method development and application was performed on an ARCOS ICPOES instrument (lateral view, SPECTRO Analytical Instruments, Kleve, Germany). A cyclonic double-pass spray chamber and pneumatic Seaspray nebuliser from Agilent Technologies (Santa Clara, California, United States) were used for reference experiments. This pneumatic nebulisation (PN) system was replaced with a CETAC U-6000 AT ultrasonic nebulization system (CETAC Technologies, Omaha, Nebraska, USA), which was further adjusted by removing the HC and inserting an IR-heated pre-evaporation glass tube and sheathing device, as shown in Fig. 1. The optimal operating conditions for all systems are summarized in Table 1. In the case of the USN-HC, the operating conditions were identical to those used previously13 to enable a comparison with the previous study.| Parameter | PN | USN-HC | USN-PET 300 °C, IR block | USN-PET 160 °C, IR block | USN-PET 250 °C, IR rope |
|---|---|---|---|---|---|
| RF power (kW) | 1.40 | ||||
| Plasma observation height (mm) | 11.0 | ||||
| Plasma gas flow rate (L min−1) | 12.00 | ||||
| Auxiliary gas flow rate (L min−1) | 1.0 | 1.0 | 2.50 | 2.50 | 2.50 |
| Sample uptake rate (mL min−1) | 2.0 | 2.0 | 0.8 | 0.5 | 0.8 |
| Drain removal rate (mL min−1) | 11.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Aerosol carrier gas flow rate (L min−1) | 1.00 | 0.75 | 0.33 | 0.33 | 0.43 |
| Sheathing gas flow rate (L min−1) | — | — | 0.40 | 0.40 | 0.40 |
| USN heater temperature (°C) | — | 140 | — | — | — |
| USN condenser temperature (°C) | — | 3 | — | — | — |
IR-heated pre-evaporation system
As in the previous work,13 the IR-heated pre-evaporation system involved a 38.1 cm long glass tube with a 28 mm/15 mm glass ball joint connected to the USN at one end, and a 12 mm/5 mm joint connected to a sheathing device at the other end. The 7 cm long sheathing device with 12 mm/5 mm ball inlet-socket ends was connected to a conventional ICP torch (SCP Science, Baie d'Urfe, Quebec, Canada). Two 60 mm wide and 245 mm long ceramic IR block heaters (Process Heaters Inc., Toronto, Ontario, Canada) were aligned parallel to the PET, sheathing device and bottom 7 cm of the torch (Fig. 1). The temperature of these heaters was controlled by two PL512 Mantle-Minder temperature controllers (GLAS-COL Apparatus Company), with a thermocouple connected on the inner surface of each heater.Reagents and certified reference materials
Standard solutions and samples were prepared with 18 MΩ cm−1 double deionized water (DDW) (Pro UV/DI, Sartorius Stedim Biotech, Gottingen, Germany) and with HNO3 (ACS grade; Fisher Scientific, Ottawa, Canada) that was purified prior to use with a DST-1000 Teflon sub-boiling distillation system (Savillex, Minnetonka, USA). Multi-elemental 100 mg L−1 stock solutions were prepared using 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 mono-elemental plasma standard solutions (SCP Science, Baie d'Urfe, Quebec, Canada) (Bi, Ga, Hg, Eu, Na, In, Se, Li, Y, Ge, Mo, Sb, Si, Ti, Zr, Al, As, Be, Cd, Co, Ce, Cr, Cu, Fe, K, La, Mg, Mn, Ni, P, Pb, S, Sr, V, Zn) and 4% v/v HNO3. From these, 10 mg L−1 stock solutions were made with 2% v/v HNO3, and more dilute multi-element solutions were prepared daily as calibration standards with corresponding matrix-matched blanks.
000 mg L−1 mono-elemental plasma standard solutions (SCP Science, Baie d'Urfe, Quebec, Canada) (Bi, Ga, Hg, Eu, Na, In, Se, Li, Y, Ge, Mo, Sb, Si, Ti, Zr, Al, As, Be, Cd, Co, Ce, Cr, Cu, Fe, K, La, Mg, Mn, Ni, P, Pb, S, Sr, V, Zn) and 4% v/v HNO3. From these, 10 mg L−1 stock solutions were made with 2% v/v HNO3, and more dilute multi-element solutions were prepared daily as calibration standards with corresponding matrix-matched blanks.
Certified reference waste water EU-L-3 (SCP Science, Baie d'Urfé, Québec, Canada) was analyzed directly for method validation, while SRM 8433 Corn Bran (National Institute of Standards and Technology, Gaithersburg, MD, USA) was digested on a hot plate prior to analysis. About 0.3 g of SRM 8433 was weighed into a Teflon decomposition vessel (Savillex, Minnetonka, USA) and 2.5 mL HNO3 and 0.5 mL H2O2 (30 m/m% in H2O, ACS reagent, Sigma-Aldrich, Steinhein, Germany) were added prior to placing the vessel on a hot plate at 50 °C for 2 h. Digested samples were diluted to 60 mL with DDW, and all standards and blanks for calibration were prepared with matching final acid concentrations.
Optimization
A 100 μg L−1 multi-elemental solution was used for multivariate optimizations, in order to find the best compromise conditions in terms of robustness and sensitivity. By monitoring the Mg II 280.270 nm/Mg I 285.213 nm ratio, operating parameters that provided the most robust conditions were chosen, where a ratio of 10 or above indicated a robust plasma. At such a robustness, changes in matrix composition and solvent loading do not significantly affect plasma excitation. Hence, improved sensitivity and detection limit can be established. A face-centered central composite design was used for optimisation of aerosol carrier gas flow rate, sheath gas flow rate, sample uptake rate, and IR temperature (Table 2). Chosen operating conditions for RF power, plasma gas flow rate, observation height and auxiliary gas flow rate were based on previous optimisations on the same instrument.13 A robust plasma is indeed achieved when the RF power is at least 1400 W,16 and higher RF power increases robustness.17 The IR temperature was kept at a maximum of 300 °C for this experiment to create a large enough separation from previous work.13 160 °C was chosen as a lower temperature cut off to investigate the effects of heating only minimally above the conventional heater temperature of USN (140 °C).| Experiment number | IR temperature (°C) | Sample uptake rate (mL min−1) | Sheath gas flow rate (L min−1) | Aerosol carrier gas flow rate (L min−1) |
|---|---|---|---|---|
| 1 | 300 | 0.75 | 0.45 | 0.3 |
| 2 | 300 | 0.5 | 0.4 | 0.4 |
| 3 | 230 | 0.75 | 0.45 | 0.3 |
| 4 | 160 | 0.5 | 0.5 | 0.2 |
| 5 | 160 | 0.5 | 0.4 | 0.4 |
| 6 | 230 | 0.75 | 0.45 | 0.3 |
| 7 | 230 | 0.75 | 0.4 | 0.3 |
| 8 | 300 | 0.5 | 0.5 | 0.2 |
| 9 | 230 | 0.75 | 0.45 | 0.3 |
| 10 | 160 | 0.75 | 0.45 | 0.3 |
| 11 | 230 | 0.75 | 0.45 | 0.4 |
| 12 | 160 | 0.5 | 0.5 | 0.4 |
| 13 | 230 | 0.75 | 0.5 | 0.3 |
| 14 | 300 | 1 | 0.5 | 0.2 |
| 15 | 160 | 1 | 0.4 | 0.2 |
| 16 | 230 | 0.75 | 0.45 | 0.3 |
| 17 | 230 | 0.75 | 0.45 | 0.3 |
| 18 | 230 | 0.75 | 0.45 | 0.3 |
| 19 | 230 | 0.75 | 0.45 | 0.3 |
| 20 | 160 | 0.5 | 0.4 | 0.2 |
| 21 | 230 | 1 | 0.45 | 0.3 |
| 22 | 230 | 0.75 | 0.45 | 0.2 |
| 23 | 160 | 1 | 0.4 | 0.4 |
| 24 | 230 | 0.5 | 0.45 | 0.3 |
| 25 | 300 | 1 | 0.4 | 0.2 |
| 26 | 160 | 1 | 0.5 | 0.4 |
| 27 | 160 | 1 | 0.5 | 0.2 |
| 28 | 300 | 1 | 0.4 | 0.4 |
| 29 | 300 | 0.5 | 0.5 | 0.4 |
| 30 | 300 | 1 | 0.5 | 0.4 |
| 31 | 300 | 0.5 | 0.4 | 0.2 |
Data analysis
Minitab 17 Software was used in creating and analyzing experimental designs for multivariate optimizations. Sensitive atomic and ionic emission lines free from possible spectroscopic interference were selected for 35 analytes. The signals were corrected using two points (one on either side of the emission peak) for polynomial background correction (Smart Analyzer Vision Software, SPECTRO Analytical Instruments, Kleve, Germany). All subsequent data treatments were processed in Microsoft Excel 2013. The signal intensity of the blank was subtracted from that of its corresponding multi-element standard solution or sample to give the net signal intensity for the specified standard or sample. Detection limits for all analytes were calculated as 3 times the standard deviation of the average signal intensity of at least 10 consecutive blanks divided by the slope of the calibration curve (i.e. the sensitivity).Results and discussion
Selection of operating conditions
As expected from the previous work by Asfaw et al.,13 using the ceramic block heater at 300 °C provided higher signal intensities and Mg II/Mg I ratio than at 160 °C, as the aerosol is vapourized more efficiently at higher temperatures, enabling a higher sample uptake rate (0.8 instead of 0.5 mL min−1), which likely increased sample introduction efficiency. Similar trends were observed for all analytes, which facilitated the selection of compromise operating conditions. RF power, along with auxiliary gas flow rate, observation height and plasma gas flow rate, were kept the same as in the previous work13 to enable an unbiased comparison of robustness. With a total central gas (aerosol carrier gas + sheath gas) flow rate of 0.73 L min−1, the results are in agreement with a report of Grotti et al.18 who found that both the Mg II/Mg I ratio and excitation temperature increase with a carrier gas flow rate increase of up to 0.7–0.85 L min−1 (depending on the RF power), but decrease past this point.According to the contour plots (not shown) that resulted from the multivariate optimization, a decreased sample uptake rate of 0.8 mL min−1 along with 0.33 L min−1 aerosol carrier gas flow rate at 300 °C provided the best compromise in terms of signal intensity and robustness versus the 1.5 mL min−1 sample uptake rate and 0.25 L min−1 aerosol carrier gas flow rate previously used at 400 °C (ref. 13) (all other operating conditions were identical). With a decreased temperature, a lower sample uptake rate is required to ensure all sample aerosol is vaporized in the PET. This trend is also observed in the 160 °C experiment, whereby a 0.5 mL min−1 sample uptake rate was selected. Increasing the sample uptake rate much higher than this would result in a buildup of un-vaporized sample and condensation at the base of the torch, which would inevitably extinguish the plasma. Because the ceramic beaded rope heater provided both convective and IR heating, the area immediately above that in direct contact with the IR heaters was found to be 30–60 °C higher, according to an IR temperature gun, than the temperature measured by the thermocouple at the base of the torch, resulting in a lower optimum temperature when compared to the block heater temperature. Hence, the vapour entering the plasma was of higher temperature than the surface of the base of the torch due to the added convective heating. This is supported by the fact that the same sample uptake rate of 0.8 mL min−1 as for the block heater at 300 °C was optimal with the beaded rope at 250 °C.
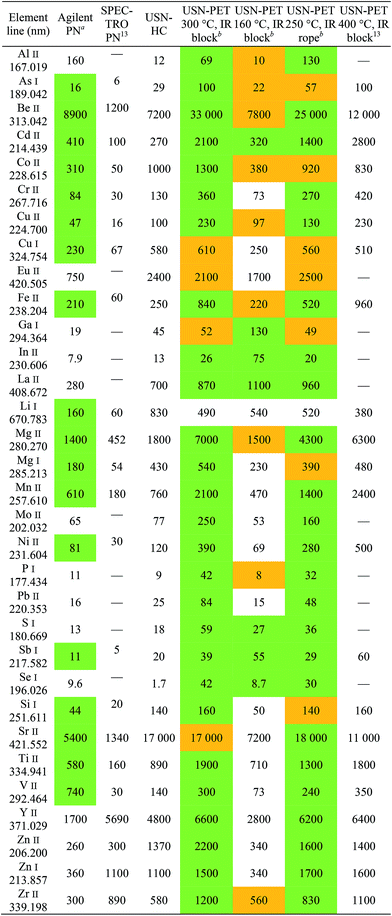
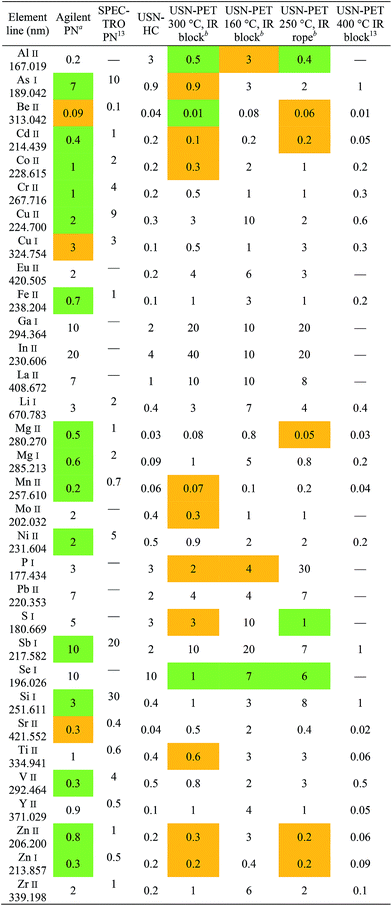
Sensitivity, detection limit and precision
Tables 3 and 4 respectively provide examples of the sensitivities and detection limits for the various sample introduction systems used in this work. The results previously obtained with the USN-PET at 400 °C were reproduced with comparable sensitivities. As expected from the reduction in average droplet size and analyte pre-concentration in the desolvation system, sensitivity and especially detection limit respectively improved by factors of 2.0 ± 1.2 and 6.0 ± 5.6 on average (n = 32 emission lines) when using USN-HC instead of PN. Paired Student's t tests indicated that only the improvement in detection limit is significant. This is less than the order of magnitude improvement reported in the previous work13 because the Agilent sample introduction system provided on average four-fold (4.3 ± 4.5) the sensitivity of the SPECTRO sample introduction system at the same sample uptake rate and three-fold (2.8 ± 3.0) better detection limit. The significant difference in sensitivities and detection limits between the two PN systems was confirmed using paired t tests.Using USN-PET with IR block at 300 °C instead of the regular USN-HC resulted in an average factor of improvement in sensitivity for 32 emission lines of 3.2 ± 4.2, which is not significant according to a paired t test. Similarly, the average factor of improvement of 1.1 ± 2.0 in detection limits against the regular USN-HC is not significant according to a paired t test. Hence, a similar performance is obtained at 0.8 mL min−1 sample uptake rate using USN-PET with IR block at 300 °C as with USN-HC operated at over twice the sample uptake rate (2.0 mL min−1). This demonstrates the greater efficiency of the IR-heated PET system.
To compare the effects of heating temperature and method of IR heating (i.e. block heaters vs. beaded rope heaters), experiments were conducted at 300 °C and 160 °C with block heaters and at 250 °C with a beaded rope heater. Heating at 300 °C instead of 160 °C resulted in average improvement factors of 3.5 ± 1.9 for sensitivity and 3.6 ± 2.6 for detection limit, which paired t tests indicated was only significant for sensitivity. Unsurprisingly, heating to 160 °C at a reduced sample uptake rate was not as efficient at vaporizing the aerosol and therefore degraded sensitivities through a concurrent reduction in transport efficiency. The fact that detection limits were not significantly degraded stems from the poorer precision observed at 300 °C than at 160 °C (Table 5). Heating at 300 °C with block heaters instead of 250 °C with the beaded rope heater at the same sample uptake rate resulted in average improvement factors of 1.3 ± 0.3 for sensitivity and 2.6 ± 3.0 for detection limit, neither of which is significant according to paired t tests. Hence, because beaded rope heaters use both convective and IR heating, a lower heating temperature may be used to attain similar performance as with block heaters. Indeed, the area downstream of the rope heater was actually 30–60 °C higher than that measured by the thermocouple, indicating that the true temperature used for pre-evaporation may in fact be the same for both heater types.
In any case, because compromise operating conditions were used for each sample introduction system, degradation in performance resulted for some elements while an enhancement occurred for others. To better assess improvements in analytical performance, atomic and ionic lines were compared element-by-element. Generally speaking, sensitivity enhancements are greater for ionic lines than atomic lines, due to lines with a higher total excitation potential being more sensitive to changes in the ICP's excitation conditions.19 For instance, the factors of improvement for detection limit when using recommended ionic lines over recommended atomic lines for Al, Be, and Mg are 120, 22, and 4.2, respectively, when comparing USN-PET at 300 °C to USN-HC. In terms of sensitivity, these factors of improvement are 7.1, 2.7, and 3.1, for Al, Be, and Mg respectively.
Finally, the USN-PET with IR block at 300 °C is compared to that from the previous work at 400 °C.13 The average sensitivity ratio (sensitivity at 300 °C divided by the one at 400 °C) of 1.1 ± 0.4 (for 22 emission lines) indicates no significant difference in sensitivity, which was confirmed by a paired t test. On the other hand, the average detection limit ratio (detection limit at 400 °C divided by the one at 300 °C) of 0.4 ± 0.3, indicates a degradation of detection limits by reducing the heating temperature to 300 °C, which was confirmed by a paired t test. However, the previous work was carried out at 1.5 mL min−1, i.e. almost twice the sample uptake rate used in this work. If the work at 400 °C had been carried out at 0.8 mL min−1, the corresponding figures of merit would have been degraded (because sensitivity increased with sample uptake rate up to 1.5 mL min−1),13 which may then have translated into no degradation or perhaps even an improvement in detection limits upon heating to 300 °C instead of 400 °C. The detection limits under the two sets of conditions (Table 4) are in fact similar for several elements (notably, As, Be and Si). Hence, the 300 °C system is more efficient, as it provides similar sensitivities and nearly the same detection limits as the 400 °C system used in the previous work, while operating at nearly half the sample uptake rate. Similar observations were made with the beaded rope heater at 250 °C. However, the range of detection limit ratios for 22 emission lines of 0.5–2.1 at 250 °C versus 0.7–2.8 at 300 °C suggests that the latter may be better.
Instrumental precisions, represented as % relative standard deviation (RSD) of 10 replicates of a 100 μg L−1 multi-element standard, are listed in Table 5. While USN-PET at 400 °C had an average RSD of 1.2 ± 0.7% (n = 22 emission lines), USN-PET at 160 °C had a greater RSD of 2.1 ± 0.7%, and USN-PET at 300 °C had an even larger RSD of 3.6 ± 1.0%, according to values listed in Table 5 (n = 32 emission lines). USN-HC provided a comparable RSD to the beaded rope heater, with average values of 2.3 ± 2.3% and 2.4 ± 1.3% for USN-HC and USN-PET rope at 250 °C, respectively. Finding the reason for the slight degradation in precision at 300 °C will be the aim of a future investigation. In any case, the higher RSD obtained with the USN-PET with IR block at 300 °C constitutes a very small sacrifice when considering that it provides similar detection limits for many elements at 0.8 mL min−1 to those with USN-HC at 2.0 mL min−1 with drastically improved robustness, as shown in the next section.
| Element line (nm) | PN | USN-HC | USN-PET 300 °C, IR block | USN-PET 160 °C, IR block | USN-PET 250 °C, IR rope | USN-PET 400 °C, IR block13 |
|---|---|---|---|---|---|---|
| Al II 167.019 | 1 | 1.1 | 6.9 | 0.9 | 3.1 | — |
| As I 189.042 | 0.5 | 0.88 | 4.9 | 1.6 | 1.1 | 1.4 |
| Be II 313.042 | 1.3 | 3.2 | 4.7 | 2 | 4.7 | 0.7 |
| Cd II 214.439 | 0.8 | 2.5 | 4.3 | 2.8 | 4.8 | 0.3 |
| Co II 228.615 | 0.53 | 1.8 | 4.1 | 3 | 2.2 | 2.4 |
| Cr II 267.716 | 0.73 | 1.8 | 3.9 | 3.4 | 2 | 1.7 |
| Cu II 224.700 | 0.63 | 1.6 | 3.4 | 1.6 | 1.5 | 2 |
| Cu I 324.754 | 0.54 | 1.5 | 3.3 | 2.2 | 1.8 | 1.4 |
| Eu II 420.505 | 0.6 | 3 | 2.6 | 1.3 | 3.6 | — |
| Fe II 238.204 | 0.73 | 1.8 | 3.6 | 2.2 | 2.3 | 2.1 |
| Ga I 294.364 | 0.61 | 0.5 | 2.2 | 2.3 | 2 | — |
| In II 230.606 | 0.42 | 0.38 | 2.7 | 2.3 | 2.3 | — |
| La II 408.672 | 0.5 | 2.1 | 2.4 | 1.7 | 2.5 | — |
| Li I 670.783 | 0.49 | 0.83 | 2.4 | 1.7 | 2 | 1.6 |
| Mg II 280.270 | 0.67 | 3.6 | 4 | 1.8 | 4.9 | 0.2 |
| Mg I 285.213 | 0.45 | 1.3 | 3 | 1.7 | 2.4 | 1.5 |
| Mn II 257.610 | 0.62 | 2.1 | 3.7 | 2.1 | 4.7 | 0.7 |
| Mo II 202.032 | 0.83 | 0.86 | 4.1 | 1.2 | 2.3 | — |
| Ni II 231.604 | 0.74 | 1.7 | 4.3 | 3.2 | 2 | 1.8 |
| P I 177.434 | 0.69 | 1.9 | 3.4 | 2.6 | 1.2 | — |
| Pb II 220.353 | 0.99 | 0.86 | 4 | 2.2 | 0.72 | — |
| S I 180.669 | 0.73 | 0.79 | 2.2 | 1.7 | 0.92 | — |
| Sb I 217.582 | 0.41 | 0.46 | 3 | 1.4 | 0.78 | 1.4 |
| Se I 196.026 | 0.55 | 1.9 | 5.6 | 1.4 | 0.62 | — |
| Si I 251.611 | 0.63 | 1.5 | 3.2 | 3.3 | 1.4 | 2 |
| Sr II 421.552 | 1.4 | 3 | 3.8 | 1.3 | 3.7 | 0.2 |
| Ti II 334.941 | 0.7 | 2 | 3.3 | 1.7 | 4.4 | 1 |
| V II 292.464 | 0.63 | 1.7 | 3.7 | 3.9 | 1.4 | 1.6 |
| Y II 371.029 | 0.81 | 3.2 | 3.2 | 1.2 | 4.4 | 0.9 |
| Zn II 206.200 | 0.6 | 11 | 4.3 | 2.9 | 2.1 | 0.8 |
| Zn I 213.857 | 0.56 | 10 | 3.6 | 2.1 | 2.1 | 0.2 |
| Zr II 339.198 | 0.55 | 2 | 2.9 | 1.8 | 2 | 0.2 |
Plasma robustness
By replacing the desolvation system with an IR-heated PET, not only are aerosol droplets vapourized, in turn reducing noise caused by large droplets, but the important benefits from water as a load buffer in the plasma are not lost from solvent removal. The previous USN-PET at 400 °C resulted in a Mg 280.270 nm/285.213 nm ratio of 13.0 ± 0.3, which is comparable to the ratio of 12.4 ± 0.3 (n = 6) obtained at 300 °C. A robust plasma is defined by Mermet as one with a Mg II/Mg I ratio of at least 10,16 where Mg is chosen as the test element due to the close excitation energies of the atomic and ionic lines, which simplifies the second exponential of the Saha equation, and because both lines are highly sensitive to parameter changes. According to this definition, only the conditions with USN-PET at 300 and 400 °C can be classified as robust. The lowest Mg ratio at 4.0 ± 0.1 was obtained with the USN-HC, while a Mg ratio of 9.4 ± 0.2 was achieved with the IR beaded rope and a similar one, 8.9 ± 0.6, resulted with the USN-PET at 160 °C. The lower robustness at 160 °C than at 300 °C with an identical sample introduction system, but a slightly reduced sample uptake rate at 160 °C, further demonstrates the detrimental impact of too low a pre-evaporation temperature.Analysis of food and water samples
The plasma robustness using the USN-PET with a block heater at 300 °C was further investigated through a comparison of measured analyte concentrations to certificate values (and their confidence limits at the 95% confidence level) for two certified reference materials in Table 6. A simple external calibration without internal standardisation was carried out. However, the corn bran digests were diluted until a 4% HNO3 concentration was reached so as to match the calibration standard solutions in terms of acid concentration. As a result, the concentration of many analytes decreased below the detection limit and could not be quantified, thereby reducing the number of elements listed for the corn bran SRM 8433. Internal standardisation with an Ar emission line, as had previously been done,13,14 was not beneficial and therefore was not carried out. The fact that results in good agreement with the reference values were obtained with a simple external calibration and without internal standardization for two completely different certified reference materials, demonstrates the robustness of USN-PET with a ceramic IR block heater at 300 °C. Interestingly, the RSD for the elements measured in the waste water CRM ranged from 0.2 to 2.1%, with an average of 1.4% (n = 15). Hence, acceptable precision was achieved at higher analyte concentration than the 100 μg L−1 used in Table 5.| Element | Waste water, low (EU-L-3) (mg L−1) | Corn bran SRM 8433 (mg kg−1) | ||
|---|---|---|---|---|
| Measured | Certified ± confidence limit | Measured | Certified ± confidence limit | |
| Al | 6.50 ± 0.04 | 6.28 ± 0.19 | ||
| As | 8.45 ± 0.10 | 8.40 ± 0.12 | ||
| Be | 1.23 ± 0.01 | 1.23 ± 0.02 | ||
| Cu | 10.58 ± 0.21 | 10.6 ± 0.2 | ||
| Cd | 2.29 ± 0.03 | 2.28 ± 0.05 | ||
| Cr | 6.15 ± 0.07 | 6.26 ± 0.15 | ||
| Fe | 5.60 ± 0.11 | 5.80 ± 0.09 | 15.47 ± 0.32 | 14.8 ± 1.8 |
| K | 202.6 ± 4.2 | 207 ± 5 | 475 ± 85 | 566 ± 75 |
| Mn | 11.83 ± 0.16 | 12.2 ± 0.2 | 2.30 ± 0.15 | 2.55 ± 0.29 |
| Mo | 3.97 ± 0.05 | 3.97 ± 0.08 | ||
| Na | 471 ± 65 | 430 ± 31 | ||
| Ni | 8.29 ± 0.06 | 8.34 ± 0.12 | ||
| Pb | 4.13 ± 0.01 | 4.18 ± 0.06 | ||
| S | 659 ± 84 | 860 ± 150 | ||
| Sb | 1.78 ± 0.03 | 1.84 ± 0.07 | ||
| Se | 2.84 ± 0.05 | 2.79 ± 0.16 | ||
| Zn | 2.90 ± 0.06 | 3.05 ± 0.21 | 17.09 ± 0.73 | 18.6 ± 2.2 |
Conclusions
As formerly found, replacing the HC of a conventional USN-HC with an IR-heated pre-evaporation tube resulted in drastically improved analytical performance in terms of detection limit, sensitivity, and robustness, as a result of the preservation of water vapour. While the uniformity of pure IR heating when using block heaters is beneficial, the ability of ceramic beaded rope heaters to produce heat via both IR and convective means enables similar performance at a lower temperature because this additional convective heating resulted in the area downstream of the heated region having higher temperatures than that measured by the thermocouple at the base of the torch. The current experiments also proved that higher temperatures provide more efficient pre-evaporation, in turn enabling a higher sample uptake rate and, consequentially, better detection capabilities through a concurrent increase in sample introduction efficiency. Although lower detection limits were previously obtained for several elements with the PET at 400 °C, IR heating of the PET at 300 °C generally resulted in similar sensitivities and robustness despite the sample uptake rate being nearly halved. This indicates that operating the USN-PET at 300 °C is more efficient than at 400 °C. This was further verified through the accurate analysis of certified reference materials of waste water and corn bran digest by a simple external calibration without internal standardization. Future work will focus on the ceramic beaded heater as the resulting system is more compact. The size of the ceramic beads will be varied to see if improved performance may result from the use of smaller beads for instance.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge Anglo American Plc for the donation of the SPECTRO ARCOS ICP-OES instrument and the Natural Sciences and Engineering Research Council of Canada (NSERC) for research funding (grant number 39487-2013). TKA thanks the School of Graduate Studies and Research of Queen's University for a graduate award.References
- E. S. Chaves, M. T. C. de Loos-Vollebregt, A. J. Curtius and F. Vanhaecke, Spectrochim. Acta, Part B, 2011, 66, 733–739 CrossRef CAS.
- P. Pohl and R. E. Sturgeon, TrAC, Trends Anal. Chem., 2010, 29, 1376–1389 CrossRef CAS.
- E. P. Nardi, F. S. Evangelista, L. Tormen, T. D. Saint'Pierre, A. J. Curtius, S. S. de Souza and J. F. Barbosa, Food Chem., 2009, 112, 727–732 CrossRef CAS.
- P. Pohl, N. Vorapalawut, B. Bouyssiere, H. Carrier and R. Lobinski, J. Anal. At. Spectrom., 2010, 25, 704–709 RSC.
- A. Martin-Esteban, B. Slowikowski and K. H. Grobecker, Talanta, 2004, 63, 667–673 CrossRef CAS PubMed.
- J. Borkowska-Burnecka, A. Lesniewicz and W. Zymicki, Spectrochim. Acta, Part B, 2006, 61, 579–587 CrossRef.
- K. V. Desboeufs, R. Losno and J. L. Colin, Anal. Bioanal. Chem., 2003, 375, 567–573 CrossRef CAS PubMed.
- J. Mora, S. Maestre, V. Hernandis and J. L. Todoli, Trends Anal. Chem., 2003, 22, 123–132 CrossRef CAS.
- I. Novotny, J. C. Farinas, J. L. Wan, E. Poussel and J. M. Mermet, Spectrochim. Acta, Part B, 1996, 51, 1517–1526 CrossRef.
- M. Bensimon, J. Bourquin and A. Parriaux, J. Anal. At. Spectrom., 2000, 15, 731–734 RSC.
- T. Prohaska, S. Hann, C. Latkoczy and G. Stingeder, J. Anal. At. Spectrom., 1999, 14, 1–8 RSC.
- S. E. Long and R. F. Browner, Spectrochim. Acta, Part B, 1988, 43, 1461–1471 CrossRef.
- A. Asfaw, W. MacFarlane and D. Beauchemin, J. Anal. At. Spectrom., 2012, 27, 1254–1263 RSC.
- A. Asfaw and D. Beauchemin, Spectrochim. Acta, Part B, 2010, 65, 376–384 CrossRef.
- A. Belhamra, R. Diabi and A. Moussaoui, J. Eng. Appl. Sci., 2007, 2, 1183–1187 Search PubMed.
- J. M. Mermet, Anal. Chim. Acta, 1991, 250, 85–94 CrossRef CAS.
- X. Romero, E. Poussel and J. M. Mermet, Spectrochim. Acta, Part B, 1997, 52, 487–493 CrossRef.
- M. Grotti, C. Lagomarsino and R. Frache, J. Anal. At. Spectrom., 2005, 20, 1365–1373 RSC.
- I. B. Brenner and A. T. Zander, Spectrochim. Acta, Part B, 2000, 55, 1195–1240 CrossRef.
Footnote |
| † Current address: Seastar Chemicals, 10005 McDonald Park Road, Sidney, BC V9L 5Y2, Canada. |
| This journal is © The Royal Society of Chemistry 2018 |