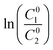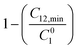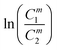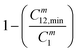Synergism and aggregation behaviour in an aqueous binary mixture of cationic–zwitterionic surfactants: physico-chemical characterization with molecular simulation approach
Bharatkumar
Kanoje
a,
Shailesh
Padshala
b,
Jigisha
Parikh
c,
Suban K
Sahoo
a,
Ketan
Kuperkar
 *a and
Pratap
Bahadur
b
*a and
Pratap
Bahadur
b
aApplied Chemistry Department, Sardar Vallabhbhai National Institute of Technology (SVNIT), Ichchhanath, Surat-395 007, Gujarat, India. E-mail: ketankuperkar@gmail.com
bDepartment of Chemistry, Veer Narmad South Gujarat University (VNSGU), Udhana-Magdalla road, Surat-395 007, Gujarat, India
cChemical Engineering Department, Sardar Vallabhbhai National Institute of Technology (SVNIT), Ichchhanath, Surat-395 007, Gujarat, India
First published on 28th November 2017
Abstract
Aqueous interactions between a cationic surfactant benzyl dimethylhexadecylammonium chloride (BDHAC) and alkyldimethylammoniopropane sulfonates (CnDAPS) based three zwitterionic surfactants n = 10, 12, and 14 (abbreviated as C10DAPS, C12DAPS and C14DAPS, respectively) were studied using tensiometry, and fluorescence spectrophotometry techniques. The critical micelle concentration degree of synergism and various other parameters such as interaction parameter (β), activity coefficients (fm) and interfacial parameters such as surface pressure (πCMC), packing parameter (P), surface excess concentration (Γmax), surface tension at CMC (γCMC), and minimum area per molecule (Amin) were evaluated using the Regular Solution Theory (RST) of mixed systems. The results indicate a strong dependency on the mixed system and their composition. For the quantitative prediction, the molecular architecture of the surfactants in mixed systems and their synergistic interactions were investigated by computational simulation using Spartan’14 V1.1.8. The structural optimization results obtained were found to be in good agreement with the estimations made using RST. The reduction in surface tension indicates a certain efficiency in mixed micelle formation owing to electrostatic attraction between the cationic and zwitterionic surfactants. In addition, the binary surfactant systems evaluated by Maeda's approach infer the mixed micelles are thermodynamically stable. The aggregation number (Nagg) appeared to be larger at the composition point where the efficiency of mixed micelle formation is greatest. The strength of the interaction between BDHAC and CnDAPS followed the order: C14DAPS > C12DAPS > C10DAPS indicating a greater synergism at 0.25 molar ratio of zwitterionic surfactants to cationic surfactants in the aqueous solution at 303.15 K.
Introduction
Cationic surfactants, which are frequently encountered ionic surfactants, consist at a minimum of a nonpolar, hydrophobic long alkyl chain and a positively charged hydrophilic head group.1–3 These groups could be modified further to tune their solution behaviour for various industrial and commercial applications.4,5 One common surfactant with a strong antibacterial activity of this type is benzyldimethylhexadecylammonium chloride (BDHAC).6 Zwitterionic surfactants are often desirable due to their mildness and their electrically neutral nature, which clearly distinguishes them from other classes of surfactants. As solutions in pure water, their behaviour is similar to that of the ionic surfactants.7 We choose alkyldimethylammonium propane sulfonate, because these zwitterionic surfactants are not only insensitive to pH, but also remain zwitterionic at any pH in aqueous solutions.8 However, reported studies have shown that the solution behaviour and physicochemical properties of zwitterionic surfactants are greatly influenced by the length and number of hydrophobic tail chain and charged head groups.9,10 As a consequence, these zwitterionic surfactants are becoming more desirable and widely applicable because of their good solubility in water and reduced sensitivity towards temperature and salts.11,12Generally, the interfacial characteristics and colloidal properties of a mixture of two or more surfactants are unique and significantly better than their individual components. Such surfactant blends are less expensive and have therefore been applied widely in detergency, enhanced oil recovery, cosmetics, textiles, and pharmaceuticals.13–15 Recent studies on mixed surfactant systems pay special attention to the synergism observed in micellization upon mixing of various ionic/non-ionic surfactants. Several models and theories have been proposed to describe the micellization phenomenon in mixed surfactant systems.16,17 Studies have reported that if the mixture shows a greater surface activity, for example, low critical micelle concentration (CMC) compared to the individual components, then the mixture exhibits synergism.18,19 Synergism is often modelled in the literature by employing the regular solution theory (RST) with a negative interaction parameter.20,21 Reports on such binary surfactant systems have revealed a strong interaction. Therefore, it is interesting and useful to design a mixed ionic-zwitterionic surfactant system in which the advantages of the surfactant can be exploited.22–25
In line with this interest, herein, we report the interfacial and bulk properties in a mixed system of a cationic surfactant (benzyldimethylhexadecylammonium chloride, BDHA+Cl−) with three zwitterionic surfactants, 3-(N,N-dimethyl decylammonium) propane sulfonate (C10DAPS), 3-(N,N-dimethyldodecylammonio) propane sulfonate (C12DAPS), 3-(N,N-dimethyltetradecyl-ammonio) propane sulfonate (C14DAPS). In this study, the head group–head group and chain–chain interactions are considered important because both the surfactants in mixed systems are ionic, which could underline the synergistic mechanism. Several mixed systems have been well discussed and presented in terms of CMC, surface excess concentration (Γmax), minimum area per molecule (Amin), surface pressure at CMC (πCMC), aggregation number (Nagg), interaction parameters in the monolayer (β0) and in the micelle (βm), standard Gibbs free energy of adsorption (ΔGads), excess free energy of micellization (ΔGex), and standard Gibbs free energy of micellization (ΔGM) for the mixtures of CnDAPS + BDHAC. Compared with the experimental methods, it is important to design the molecular architecture of surfactants for the purpose of optimizing the synergistic performance of the mixtures of zwitterionic–cationic surfactants in which we are interested, as such study is very limited. Thus, the experimental observations were rationalized with a computer based quantum mechanical study to provide atomistic or molecular details and to understand the behaviour of mixed surfactants at the interfaces, which could prove highly beneficial from a commercial and industrial point of view.
Experimental
Materials
Benzyldimethylhexadecylammonium chloride (BDHAC), and alkyldimethylammoniopropane sulfonate (CnDAPS) (n = 10, 12, 14), the fluorescent probe: pyrene, and N-cetylpyridinium chloride (CPC) were purchased from Sigma-Aldrich (purity > 99.5%) and used without further purification. To ensure removal of surface active contaminants, all glassware was cleaned in chromic acid and rinsed with double distilled water. All solutions were prepared in water with a specific conductance and pH in the range of 1–2 μS cm−1 and ∼6.5, respectively. The entire experimental study was performed at 303.15 K.Methods
The surface parameters such as πCMC, Γmax, Amin and P were calculated using an appropriate form of the Gibb's adsorption equation:
| πCMC = γwater − γCMC | (1) |
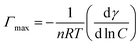 | (2) |
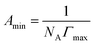 | (3) |
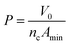 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is the maximum slope, R is the gas constant (8.314 J mol−1 K−1); T is the absolute temperature in Kelvin, C is surfactant concentration in mol L−1, NA is the Avogadro number, Γmax represents the maximum surface excess concentration at the CMC, V0 is volume of hydrocarbon chain and nc is the number of carbon atoms in hydrocarbon chains, given by Tanford formula. Here, Amin is the cross-sectional area occupied by the hydrophilic head group.26–30
C is the maximum slope, R is the gas constant (8.314 J mol−1 K−1); T is the absolute temperature in Kelvin, C is surfactant concentration in mol L−1, NA is the Avogadro number, Γmax represents the maximum surface excess concentration at the CMC, V0 is volume of hydrocarbon chain and nc is the number of carbon atoms in hydrocarbon chains, given by Tanford formula. Here, Amin is the cross-sectional area occupied by the hydrophilic head group.26–30
The fluorescence intensities of the obtained peaks decreased with an increase in the quencher concentration without the appearance of any new peaks.
According to the theory proposed by Turro and Yekta,31 the relationship between the steady state fluorescence intensities with (I0) and without (I1) quencher is determined by the total surfactant concentration (C) and the quencher concentration (Q) in micelles as:
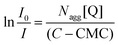 | (5) |
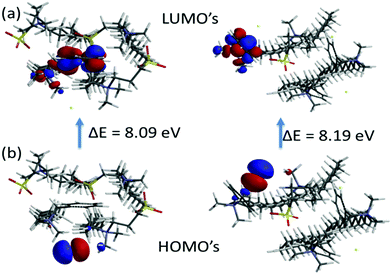
Thus, using the slope of the straight line from the plot of ln(I0/I) versus [Q] shown in Fig. 3 for the C14DAPS + BDHAC system in various ratios the aggregation number, Nagg can be obtained. The fluorescence intensities I0 and I also can be used to calculate the Stern–Volmer binding constants (Ksv) by using the following equation:
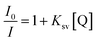 | (6) |
I(t) = A0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−A − A1t − A2(1 − exp(−A3t))) exp(−A − A1t − A2(1 − exp(−A3t))) | (7) |
Results and discussion
Synergism in the mixed surfactant system
Reported studies have shown that there is correlation between the concentration and activity for binary mixtures at a given temperature, considering their mole fractions in mixed solution system. Rubingh was apparently the first to use this correlation to fit the mixture CMC data for binary surfactant systems.7 Thus it was more profound to employ Rubingh's activity coefficient relationship for our investigation, which earlier was addressed as a one-parameter Margules equation for describing the nonideality in aqueous mixed surfactant systems.32,33According to Rubingh's nonideal solution theory, the mixed CMC, when more than one surfactant is mixed, is evaluated using eqn (8):
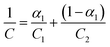 | (8) |
After measuring the CMC of the aqueous solution of mixed surfactants and the CMC of the individual surfactants, the value of the interfacial composition (Xm1) in the aqueous phase can be calculated from the equation:
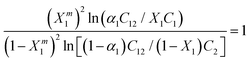 | (9) |
After determining Xm1 from eqn (9), we can obtain the interaction parameter (βm) according to the equation:
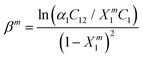 | (10) |
The interaction parameter (βm) is related to the activity coefficient (fm) of surfactant within the micelle by the following relations:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fm1 = βn(1 − X1)2 fm1 = βn(1 − X1)2 | (11) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fm2 = βm(X1)2 fm2 = βm(X1)2 | (12) |
Studies have reported the chain–chain and head group–head group interactions as a driving force in mixed micellar systems depicting the stability of mixed micelles.35,36 The free energy of micellization (ΔGMaeda) in the mixed micelle is given by:
| ΔGMaeda = RT(B0 + B1X1 + B2X12) | (13) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2 (C2 is the CMC of CnDAPS); B1 + B2 = ln
C2 (C2 is the CMC of CnDAPS); B1 + B2 = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (C1/C2) (C1 is the CMC of surfactant BDHAC) and B2 = −βm.
(C1/C2) (C1 is the CMC of surfactant BDHAC) and B2 = −βm.
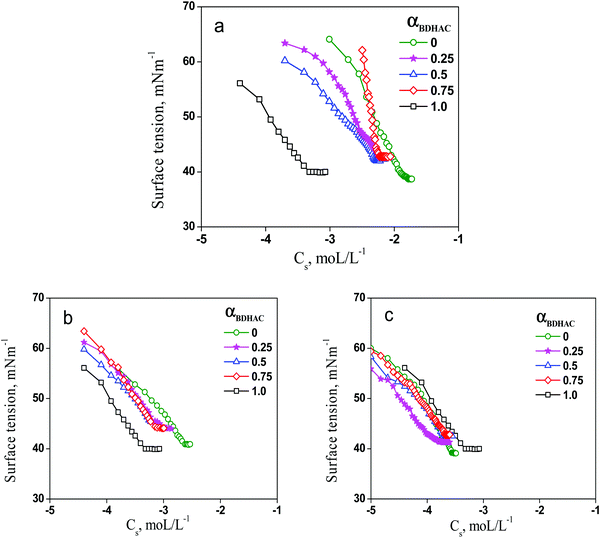
For the air–water interface study, solutions of both the surfactants (CnDAPS + BDHAC) were prepared in varying mole fractions of 0.25, 0.50 and 0.75. Plots of the surface tension versus logarithm Cs for pure surfactants and their mixtures depict the CMC with sharp break points (Fig. 1). The evaluated CMC of BDHAC and CnDAPS is in harmony with the reported values from the literature.37 Mixed CMC data along with the computed results for the mixed micellar composition, interaction parameter (β) and activity coefficients (fm) are presented in Table 1.
| α BDHAC | CMC (mM) | X m 1 | β m | f m 1 | f m 2 | |
|---|---|---|---|---|---|---|
| Ideal | Experimental | |||||
| BDHAC + C10DAPS | ||||||
| 0 | 19.00 | 16.80 | — | — | — | — |
| 0.25 | 1.73 | 6.20 | 0.35 | 5.25 | 9.33 | 1.89 |
| 0.50 | 0.91 | 5.31 | 0.40 | 7.24 | 14.33 | 3.06 |
| 0.75 | 0.62 | 3.12 | 0.46 | 8.17 | 10.73 | 5.67 |
| 1.0 | 0.50 | 0.47 | — | — | — | — |
| BDHAC + C12DAPS | ||||||
| 0 | 2.50 | 2.40 | — | — | — | — |
| 0.25 | 1.18 | 0.93 | 0.58 | −1.01 | 0.84 | 0.71 |
| 0.50 | 0.73 | 0.86 | 0.89 | 0.68 | 1.01 | 1.71 |
| 0.75 | 0.52 | 0.70 | 0.98 | 1.81 | 1.00 | 5.63 |
| 1.0 | 0.50 | 0.47 | — | — | — | — |
| BDHAC + C14DAPS | ||||||
| 0 | 0.31 | 0.27 | — | — | — | — |
| 0.25 | 0.30 | 0.16 | 0.35 | −3.26 | 0.25 | 0.67 |
| 0.50 | 0.34 | 0.22 | 0.43 | −1.84 | 0.55 | 0.71 |
| 0.75 | 0.40 | 0.24 | 0.56 | −2.08 | 0.67 | 0.52 |
| 1.0 | 0.50 | 0.47 | — | — | — | — |
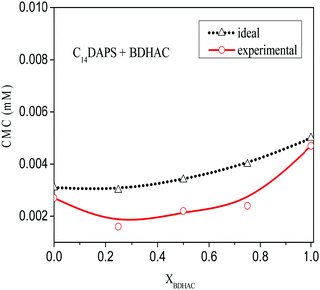
An almost linear decrease in surface tension was observed in the pre-micellar region until CMC; however, beyond CMC in the post-micellar region, the surface tension appeared more or less constant. The CMCs for the mixed systems presented revealed the CMC values lie between those of the pure single conventional components. It can be seen from our findings that the micellization behaviour of zwitterionic–cationic mixed surfactants significantly differs from their pure surfactants. The CMC values decrease correspondingly with the addition of the zwitterionic to a cationic surfactant with varying ratios. The interaction parameter (β) value appeared to be positive for the mix systems containing C10DAPS and C12DAPS, thereby indicating antagonism while it appeared negative for C14DAPS indicating synergism. It can be seen from Fig. 2 that out of the three different mix systems only one, C14DAPS + BDHAC, has a β value as more negative, indicating the plausible interaction between them.
Fig. 2 show a comparison between the ideal and experimental CMCs for the C14DAPS + BDHAC system, where the three mix ratios exhibit a negative deviation from its ideal behaviour, thereby indicating a synergistic interaction between the binary surfactant system. The negative deviation is also supported by a negative value of βm which also indicates an attractive interaction. Such behaviour could be attributed to the electrostatic interaction between C14DAPS and BDHAC.
Adsorption of mixed surfactants at the air–water interface
The adsorption efficiency at the interface is an important criterion for the surfactant in order to understand its solution.38 The value of surface pressure at CMC (πCMC) of the binary surfactant mixtures is presented in Table 2. It can be observed that in all surfactant mixtures, the (πCMC) values are lower than their respective pure surfactants. While (πCMC) of the binary mixtures decreases gradually with an increase in the mole fraction of the cationic surfactant, the formation of mixed micelles is due to the favoured hydrophobic effect.| α BDHAC | X 01 | β 0 | f 01 | f 02 | γ CMC mN m−1 | Γ max (× 106 mol m−2) | A min (Å2) | P | π CMC |
|---|---|---|---|---|---|---|---|---|---|
| BDHAC + C10DAPS | |||||||||
| 0 | — | — | — | — | 38.92 | 1.88 | 88.5 | 0.48 | 32.48 |
| 0.25 | 0.35 | 5.20 | 8.94 | 1.90 | 42.94 | 1.41 | 117.6 | 0.36 | 28.46 |
| 0.50 | 0.40 | 7.15 | 12.90 | 3.18 | 41.85 | 1.12 | 148.0 | 0.29 | 29.55 |
| 0.75 | 0.46 | 8.10 | 10.62 | 5.56 | 42.63 | 1.42 | 116.6 | 0.36 | 28.77 |
| 1.0 | — | — | — | — | 39.79 | 1.14 | 145.5 | 0.29 | 31.61 |
| BDHAC + C12DAPS | |||||||||
| 0 | — | — | — | — | 40.88 | 1.13 | 146.6 | 0.15 | 30.32 |
| 0.25 | 0.58 | −1.25 | 0.80 | 0.66 | 44.4 | 1.12 | 148.6 | 0.14 | 26.80 |
| 0.50 | 0.75 | −0.81 | 0.95 | 0.63 | 44.79 | 1.03 | 161.5 | 0.13 | 26.41 |
| 0.75 | 0.72 | 0.4 | 1.00 | 1.45 | 44.01 | 1.28 | 129.3 | 0.16 | 27.19 |
| 1.0 | — | — | — | — | 39.99 | 1.14 | 145.5 | 0.15 | 31.21 |
| BDHAC + C14DAPS | |||||||||
| 0 | — | — | — | — | 39.31 | 1.40 | 118.4 | 0.18 | 31.89 |
| 0.25 | 0.35 | −3.54 | 0.22 | 0.65 | 41.56 | 0.92 | 179.8 | 0.12 | 29.64 |
| 0.50 | 0.44 | −2.66 | 0.43 | 0.60 | 43.02 | 1.03 | 161.5 | 0.13 | 28.18 |
| 0.75 | 0.56 | −2.88 | 0.57 | 0.40 | 43.01 | 1.08 | 153.9 | 0.14 | 28.19 |
| 1.0 | — | — | — | — | 39.99 | 1.14 | 145.5 | 0.15 | 31.21 |
The minimum area per molecule (Amin) suggests the assembly of the surfactant molecule at the air–water interface was observed to be higher for pure BDHAC as compared to CnDAPS, which may be ascribed to the greater electrostatic repulsions between similarly charged head groups at the surface monolayer. The entire mole fraction, except 0.5 mole fraction, of the three systems shows low Amin values due to strong electrostatic interactions between cationic and zwitterionic surfactants, while in the 0.5 mole fraction the value appears higher which indicates the packing expansion at the air–water interface. However, the correct reason for the packing expansion at the 0.5 mole fraction is not known. This expansion may be due to the dissimilarity in the nature of the interaction amongst the hydrophobic and hydrophilic groups in the mixed adsorbed layer. The packing parameter P which gives information about the size of the micelle was found to be low, where the Amin was more.39,40
Thermodynamic and interfacial adsorption study of surfactants in aqueous binary mixtures
The thermodynamic parameter for the evolution of synergism in the mixed system at equilibrium is described as follows:41| ΔGmin = AminγCMCNA | (14) |
The excess free energy of micellization, ΔGex:
ΔGex = [X1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f1 + (1 − X1)ln f1 + (1 − X1)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f2]RT f2]RT | (15) |
The free energy of micellization can be given by:
ΔGM = [X1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X1f1 + X2 X1f1 + X2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X2f2]RT X2f2]RT | (16) |
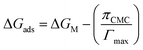 | (17) |
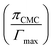 in eqn (17) expresses the work involved in transferring the surfactant molecule from a monolayer at a zero surface pressure to the micelle. Here, for all the binary mixtures, the last term of eqn (16) is very small as compared to ΔGM, which suggests that work involved in transferring the surfactant molecule from the monolayer at zero surface pressure to the micelle is negligible.
in eqn (17) expresses the work involved in transferring the surfactant molecule from a monolayer at a zero surface pressure to the micelle. Here, for all the binary mixtures, the last term of eqn (16) is very small as compared to ΔGM, which suggests that work involved in transferring the surfactant molecule from the monolayer at zero surface pressure to the micelle is negligible.
RST assumes that the excess entropy of mixing and ideal enthalpy of mixing are zero. According to RST, the relation between the excess free energy of micellization, excess enthalpy and the enthalpy of micellization can be written as:
ΔGex = ΔHex = ΔHM = [X1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f1 + (1 − X1)ln f1 + (1 − X1)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f2]RT f2]RT | (18) |
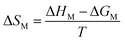 | (19) |
In Table 3(a) the value of B1 appears to be negative in only the 0.25 mole ratio, indicating that the chain–chain interaction plays an important role in mixed micellization. When a hydrocarbon chain is dissimilar, the chain–chain interaction gives a more negative value of B1.42
| (a) | |||
|---|---|---|---|
| α BDHAC | B 0 | B 1 | B 2 |
| BDHAC + C10DAPS | |||
| 0 | — | — | — |
| 0.25 | 2.82 | 1.67 | −5.25 |
| 0.50 | 2.82 | 3.66 | −7.24 |
| 0.75 | 2.82 | 4.59 | −8.17 |
| 1.0 | — | — | — |
| BDHAC + C12DAPS | |||
| 0 | — | — | — |
| 0.25 | 0.87 | −2.64 | 1.01 |
| 0.50 | 0.87 | −0.95 | −0.68 |
| 0.75 | 0.87 | 0.16 | −1.80 |
| 1.0 | — | — | — |
| BDHAC + C14DAPS | |||
| 0 | — | — | — |
| 0.25 | −1.30 | −2.70 | 3.26 |
| 0.50 | −1.30 | −1.24 | 1.84 |
| 0.75 | −1.30 | −1.53 | 2.08 |
| 1.0 | — | — | — |
| (b) | ||||||
|---|---|---|---|---|---|---|
| α BDHAC | ΔGMaeda (KJ mol−1) | ΔGmin (KJ mol−1) | ΔGM (KJ mol−1) | ΔGads (KJ mol−1) | ΔGex = ΔHM (KJ mol−1) | ΔSM (J mol−1 K−1) |
| BDHAC + C10DAPS | ||||||
| 0 | — | 20.73 | — | — | — | — |
| 0.25 | 6.97 | 30.40 | 1.37 | −18.81 | 2.99 | 5.37 |
| 0.50 | 7.91 | 37.31 | 2.66 | −23.72 | 4.35 | 5.57 |
| 0.75 | 8.07 | 29.93 | 3.37 | −16.89 | 5.11 | 5.74 |
| 1.0 | — | 34.88 | — | — | — | — |
| BDHAC + C12DAPS | ||||||
| 0 | — | 36.10 | — | — | — | — |
| 0.25 | −0.80 | 39.73 | −2.33 | −26.31 | −0.62 | 5.65 |
| 0.50 | −1.23 | 43.57 | −0.705 | −26.40 | 0.17 | 2.88 |
| 0.75 | −1.73 | 34.28 | −0.158 | −21.33 | 0.08 | 0.81 |
| 1.0 | — | 35.06 | — | — | — | |
| BDHAC + C14DAPS | ||||||
| 0 | — | 28.03 | — | — | — | |
| 0.25 | −4.68 | 45.00 | −3.45 | −35.59 | −1.86 | 5.38 |
| 0.50 | −3.83 | 41.86 | −2.86 | −30.28 | −1.14 | 5.68 |
| 0.75 | −3.81 | 39.87 | −3.02 | −29.15 | −1.30 | 5.70 |
| 1.0 | — | 35.05 | — | — | — | |
Another thermodynamic parameter presented in Table 3(b) describes the transition of a solution component from the bulk phase to the surface in the mixed study at equilibrium.43,44 It is known that the lower the value of Gibb's free energy (ΔGMaeda, ΔGM, and ΔGads) the surface is more thermodynamically stable or more surface active. In our case, C14DAPS + BDHAC attain clear surface activity thereby indicating good synergism. Similarly, the extent of synergism in our mixed surfactant system depends on the lowering of the measured free energy. ΔGM and ΔGads values are negative and reveal that the latter is more spontaneous; implying that the adsorption of surfactants and their mixtures at the air/solution interface is more favourable than that of micelle formation. The negative value of excess free energy (ΔGex) of micellization indicates the energetic stabilization accompanied by the mixed micelle formation. The positive values for entropy (ΔSM) contribution must be the driving force of micellization. This means that the mixed micelle formation is an entropically favourable process.45–47
Synergism
The order of interaction between the individual surfactants in the mixture and CMC plays a vital role in predicting synergism. However, it obeys certain conditions: (a) β0 and βm must be negative, (b) |β0| > |ln(C01/C02)|, where C01 and C02 are the molar concentrations of the individual surfactants in a binary mixture and (c) |βm| > |ln(Cm1/Cm2)|, where Cm1 and Cm2 are the CMCs of the individual surfactants. The terms: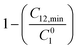 and
and 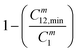 were evaluated using eqn (20) proposed by Liu and Rosen48 where the value must be a maximum of 1 and which determines the degree of synergism in reducing the surface tension efficiently.
were evaluated using eqn (20) proposed by Liu and Rosen48 where the value must be a maximum of 1 and which determines the degree of synergism in reducing the surface tension efficiently.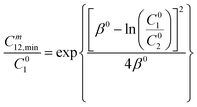 | (20) |
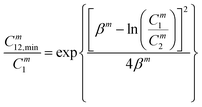 | (21) |
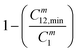 (maximum = 1), the greater the degree of synergism. Values are given in Table 4. All the values are close to 1 for all three systems indicating synergism.
(maximum = 1), the greater the degree of synergism. Values are given in Table 4. All the values are close to 1 for all three systems indicating synergism.
The zwitterionic surfactants studied here, alkyldimethylammoniopropane sulfonates, are not sensitive to pH in the solution. Although there is no net charge, many physical properties of these surfactants are quite different from those of non-ionic surfactants and very similar to those of ionic surfactants and they can have a synergistic effect with BDHAC by means of a electrostatic attraction between the N+(CH3)2 group in the zwitterionic surfactants and the aryl chloride group in the BDHAC.
Computational study
Recent studies have shown the intensive applications of the computational approach can offer an insight into the atomic studies of structure and dynamics phenomena in surfactants, inorganic/organic complexes and biomolecules etc. at different interfaces.49–51 Based on our experimental findings for the binary BDHAC + CnDAPS systems in aqueous solution, the quantum molecular simulations were performed by applying the Spartan’14 V1.1.8 program in the gas phase to understand the interactions in the formation of various stable adducts (synergism) between the two surfactants in the mixed surfactant system. It was reported that the interfacial structure of the mixed surfactant complex depends on the surfactant concentration at the interface. Furthermore, it was determined that the electrostatic interaction and hydrophobic interaction are the driving forces between surfactants that exhibit the degree of synergism. In the case of homogeneous mix surfactant systems (BDHAC and CnDAPS) with no phase separation, it could be depicted that an adsorption monolayer of BDHAC and CnDAPS at the air/water surface is formed, with their headgroups extending into the water solvate (i.e. the head groups are hydrated in the water phase), and the carbon chains are stretching towards air. However, a small portion of the carbon chains penetrates into the water. Based on our findings, it could be concluded that mixed BDHAC + CnDAPS surfactant systems display adequate surface activity in mix molar ratios with n = 14 as the maximum, compare to 10 and 12.The optimized structure obtained by the Spartan’14 V1.1.8 for the individual surfactants, as well as for the surfactant mixtures in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio is shown in Fig. 3. As can be seen in Fig. 3c, the hydrophobic carbon tails stretch into the plane with a length of 19.71 Å and 17.77 Å in the case of BDHAC and C14DAPS, respectively. The headgroups of these two surfactants are found to be localized in such a way that the negative O− atom of the sulphonic group in C14DAPS becomes bonded with the H atom of the BDHAC head group leading to a hydrogen bond formation of length ∼1.912–1.940 Å. Here the tapering tail ends of both surfactants appear a little closer due to hydrophobic interactions. Furthermore, the significant fractions of the counterions are found to exist in the interfacial area, near to the headgroups of these surfactants. The binding forces of BDHAC and C14DAPS molecules at the air/water interface could be attributed to two types of interactions: the electrostatic interaction between the ionic headgroups, and the hydrophobic interaction between the carbon chains. The top view of the 1
1 molar ratio is shown in Fig. 3. As can be seen in Fig. 3c, the hydrophobic carbon tails stretch into the plane with a length of 19.71 Å and 17.77 Å in the case of BDHAC and C14DAPS, respectively. The headgroups of these two surfactants are found to be localized in such a way that the negative O− atom of the sulphonic group in C14DAPS becomes bonded with the H atom of the BDHAC head group leading to a hydrogen bond formation of length ∼1.912–1.940 Å. Here the tapering tail ends of both surfactants appear a little closer due to hydrophobic interactions. Furthermore, the significant fractions of the counterions are found to exist in the interfacial area, near to the headgroups of these surfactants. The binding forces of BDHAC and C14DAPS molecules at the air/water interface could be attributed to two types of interactions: the electrostatic interaction between the ionic headgroups, and the hydrophobic interaction between the carbon chains. The top view of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts of BDHAC and C14DAPS clearly indicates the slight conformational changes due to the hydrophobic interaction between the carbon chains. The association process between the two surfactants: BDHAC (area = 520.52 Å2; volume = 483.63 Å3) and C14DAPS (area = 453.10 Å2; volume = 414.63 Å3) resulted in the decrease in the area and volume upon close contact between the two surfactants in the BDHAC:C14DAPS adduct (area = 904.99 Å2; volume = 890.04 Å3).
1 adducts of BDHAC and C14DAPS clearly indicates the slight conformational changes due to the hydrophobic interaction between the carbon chains. The association process between the two surfactants: BDHAC (area = 520.52 Å2; volume = 483.63 Å3) and C14DAPS (area = 453.10 Å2; volume = 414.63 Å3) resulted in the decrease in the area and volume upon close contact between the two surfactants in the BDHAC:C14DAPS adduct (area = 904.99 Å2; volume = 890.04 Å3).
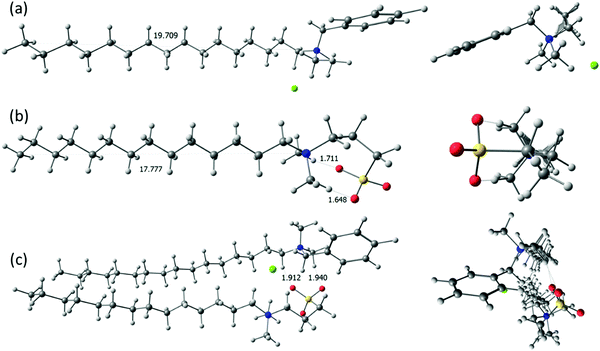 | ||
Fig. 3 The two views (side and top) of the optimized structure of (a) BDHAC, (b) C14DAPS and (c) the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of BDHAC + C14DAPS. 1 adduct of BDHAC + C14DAPS. | ||
Considering the experimental data, the structural simulations were performed exclusively for the surfactants BDHAC and C14DAPS by keeping the molar ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 4a) and 3
3 (Fig. 4a) and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4b). The results showed that the ratio of 1
1 (Fig. 4b). The results showed that the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 exhibited better synergistic behaviour. The bond position of these two headgroups at a molar ratio of 1
3 exhibited better synergistic behaviour. The bond position of these two headgroups at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is nearly overlapping resulting in a compact spatial arrangement, thereby indicating a large interaction between these two surfactants, as could be seen in the optimized space-filling model structure. The plausible explanation for such behaviour could be that the head groups of C14DAPS have more sulphonic groups, for example an O− atom on the surface which is responsible for forming a H-bond with the ammonium head group of BDHAC, as a result all the surfactant molecules are packed closely to each other thereby remaining intact or compact with a surface area of 1669.89 Å2 and volume of 1697.83 Å3. The molecules of both surfactants exhibit an electrostatic interaction between the ionic head groups of BDHAC and C14DAPS instead of repelling each other which rationalizes the high surface activity obtained by the surface tension and other results. In contrast, the optimized structure of the mixed surfactants BDHAC + C14DAPS for the ratio 3
3 is nearly overlapping resulting in a compact spatial arrangement, thereby indicating a large interaction between these two surfactants, as could be seen in the optimized space-filling model structure. The plausible explanation for such behaviour could be that the head groups of C14DAPS have more sulphonic groups, for example an O− atom on the surface which is responsible for forming a H-bond with the ammonium head group of BDHAC, as a result all the surfactant molecules are packed closely to each other thereby remaining intact or compact with a surface area of 1669.89 Å2 and volume of 1697.83 Å3. The molecules of both surfactants exhibit an electrostatic interaction between the ionic head groups of BDHAC and C14DAPS instead of repelling each other which rationalizes the high surface activity obtained by the surface tension and other results. In contrast, the optimized structure of the mixed surfactants BDHAC + C14DAPS for the ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with several ammonium groups on the surface with a single sulphonic group leads to the formation of the limited non-covalent interactions between the head groups of the adjacent surfactant. As a result, the head group of one of the BDHAC molecules is free from the 3
1 with several ammonium groups on the surface with a single sulphonic group leads to the formation of the limited non-covalent interactions between the head groups of the adjacent surfactant. As a result, the head group of one of the BDHAC molecules is free from the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct, and therefore the compactness is found to be less than the 1
1 adduct, and therefore the compactness is found to be less than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct, as shown in the 3D space-filling model. In addition to the interactions with BDHAC, the C14DAPS molecule yields a repulsive force with its head group, therefore, the cluster shows a depurative formation that leads to an increased surface area of 1786.48 Å2 and volume of 1836.22 Å3. Thus our findings suggest that the presence of a lower number of O− ions in the adduct plays a decisive role for synergism in mixed surfactants at the surface.
3 adduct, as shown in the 3D space-filling model. In addition to the interactions with BDHAC, the C14DAPS molecule yields a repulsive force with its head group, therefore, the cluster shows a depurative formation that leads to an increased surface area of 1786.48 Å2 and volume of 1836.22 Å3. Thus our findings suggest that the presence of a lower number of O− ions in the adduct plays a decisive role for synergism in mixed surfactants at the surface.
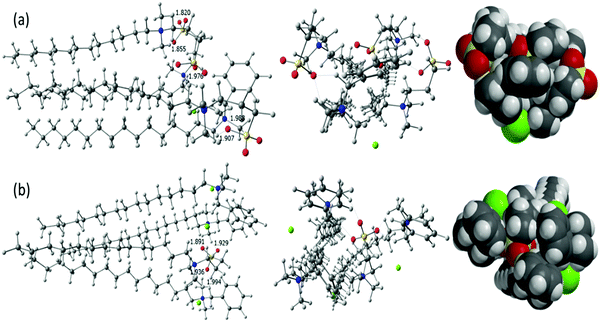 | ||
Fig. 4 Three views (side, top and 3D ball) of the optimized structure of BDHAC + C14DAPS in varying ratios (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and (b) 3 3 and (b) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The synergism of the mixed surfactants in the adducts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 3
3 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) was also analysed by comparing the band gap (ΔE) between the highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbital energies (Fig. 5). The band gap of BDHAC + C14DAPS (1
1 ratio) was also analysed by comparing the band gap (ΔE) between the highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbital energies (Fig. 5). The band gap of BDHAC + C14DAPS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was ∼8.09 eV which is lower than BDHAC + C14DAPS (3
3) was ∼8.09 eV which is lower than BDHAC + C14DAPS (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ∼ 8.19 eV due to the better compactness that promotes the charge transfer within the four surfactants in the respective set of the mixture. In addition, the larger dipole moment μ = 17.58 Debye of BDHAC + C14DAPS (1
1) ∼ 8.19 eV due to the better compactness that promotes the charge transfer within the four surfactants in the respective set of the mixture. In addition, the larger dipole moment μ = 17.58 Debye of BDHAC + C14DAPS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) compared to μ = 10.14 Debye of the BDHAC + (3
3) compared to μ = 10.14 Debye of the BDHAC + (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system indicates the former showed a higher polarity that facilitates the interaction of the surfactant with water molecules leading to a thermodynamic stable system.
1) system indicates the former showed a higher polarity that facilitates the interaction of the surfactant with water molecules leading to a thermodynamic stable system.
Aggregation (Nagg) in mixed micellar systems
Micelle aggregation number (Nagg) is determined by using fluorescence spectroscopy. The representative Stern–Volmer plot of ln(I0/I1) versus quencher concentration [Q] shown in Fig. 6a depicts the linear characteristics indicating that the individual surfactant in the mixture is accessible to [Q] and the slope value gives Nagg, as shown in Table 5. The Nagg values for the pure zwitterionic surfactant have been found to be close to the literature values.52,53 The decay profile for pyrene quenching by CPC is presented in Fig. 6b for the mix ratio of the binary surfactants in aqueous solution. It can be seen that the fluorescence decay was observed to be parallel for a long time thereby implying that the quencher could move freely between the micelles and the water pseudo-phase.It was observed that the Nagg values for the mixed surfactant ratio are larger than for pure BDHAC, but lower than pure C14DAPS. The decrease in Nagg is observed in the mixture when the ratio of BDHAC was 0.25 and 0.75. Such behaviour is associated with an increase in the average repulsive interaction between the head groups with decreasing C14DAPS; as zwitterionic surfactant molecules are progressively replaced by the cationic surfactant. The smallest aggregation number corresponds to the highest surface charge density, when the ratio of pure zwitterionic surfactant is more than the cationic BDHAC surfactant. However, for the molar ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 the Nagg was found to be higher because the head group–head group charge density increases between the cationic and zwitterionic surfactant. Also, in terms of the electrostatic charges, the negative charge of the sulfonate of C14DAPS faces towards the positive charge of the ammonium head group of BDHAC, since the micelles are stabilized, resulting in a high aggregation number.54,55 It was also observed that the high Ksv values suggest that an increase in quenching is due to the presence of both the pyrene and quencher in the strong hydrophobic environment.
50 the Nagg was found to be higher because the head group–head group charge density increases between the cationic and zwitterionic surfactant. Also, in terms of the electrostatic charges, the negative charge of the sulfonate of C14DAPS faces towards the positive charge of the ammonium head group of BDHAC, since the micelles are stabilized, resulting in a high aggregation number.54,55 It was also observed that the high Ksv values suggest that an increase in quenching is due to the presence of both the pyrene and quencher in the strong hydrophobic environment.
Conclusions
Our work offers a better understanding of the adsorption at the air–water interface, the thermodynamics of micellization, and the aggregation behaviour obtained using tensiometry and fluorescence spectroscopy techniques for conventional surfactants and their binary mixtures at different mole fractions in aqueous solution. The results obtained are well explained and provide an insight into the nature of the individual surfactants alone and also in mixtures of zwitterionic surfactants Cn (n = 10, 12 and 14) with the cationic surfactant benzyldimethylhexadecylammonium chloride (BDHAC). The mixed surfactant system, BDHAC + C14DAPS exhibited better solution properties as compared to C10DAPS + BDHAC and BDHAC + C12DAPS. The CMC's in the mixed surfactant systems at any mole ratio lies between those of their single surfactants, thereby indicating the values of CMC strongly depend on the composition of the mixture. Using RST, deviations from ideal behaviour were observed which implied the molecular interaction parameter (β), activity coefficients, and the excess Gibbs energy of the mixed micelle formation are close to the ideal behaviour. The chain–chain interactions indicated a fair level of the stability of mixed micelles. The interactions at micelles (βm) are more attractive and reflect more synergistic behaviour than at the monolayer (β0) in mixed systems. Aggregation data obtained using fluorescence measurements highlighted the accumulation of compact packing of monomers in the micelle, which was found to be clearly delineated with the optimization results. Such behaviour could be attributed to the electrostatic interactions, for example hydrogen bond formation in BDHAC + C14DAPS leading to the fair adsorption and thereby resulting in a strong affinity in the mixed surfactants. The addition of BDHAC leads to a great increase in the total charge and generally makes the surfactants arrange themselves more loosely, resulting in a decrease of the micellar aggregation number in the mixture. Furthermore, it was also found that the values of the standard Gibb's energy of micellization for the mixture of these two surfactants confirms the synergetic effect, thereby predicting the mixed micellar system to be more spontaneous. Considering these findings, we anticipate that such a study may prove more beneficial to design a mixed ionic-zwitterionic surfactant system and tune the interfacial and micellar properties in which the advantages of either surfactant can be exploited.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge Technical Education Quality Improvement Programme-II (TEQIP-II) Credit No.: 4685-0IN for financial aid and Sardar Vallabhbhai National Institute of Technology (SVNIT), Surat, Gujarat-INDIA for providing the central instrumentation facility for analysis.References
- R. Atkin, V. Craig, S. J. Wanless and E. J. Biggs, Mechanism of cationic surfactant adsorption at the solid–aqueous interface, Adv. Colloid Interface Sci., 2003, 103, 219–304 CrossRef CAS PubMed.
- P. Somasundaran and U. L. Huang, adsorption/aggregation of surfactants and their mixtures at solid/liquid interfaces, Adv. Colloid Interface Sci., 2000, 88, 179–208 CrossRef CAS PubMed.
- K. Lunkenheimer, A. Lind and M. Jost, Surface tension of surfactant solutions, J. Phys. Chem. B, 2003, 107, 7527–7531 CrossRef CAS.
- T. Farías, L. C. de Ménorval, J. Zajac and A. Rivera, Solubilization of drugs by cationic surfactants micelles: conductivity and 1H-NMR experiments, Colloids Surf., A, 2009, 345, 51–57 CrossRef.
- J. L. Trompette, J. Zajac, E. Keh and S. Partyka, Scanning of the cationic surfactant adsorption on a hydrophilic silica Surface at low surface coverages, Langmuir, 1994, 10, 812–818 CrossRef CAS.
- Z. Grenoble and S. Baldelli, Adsorption of benzyldimethylhexadecylammonium chloride at the hydrophobic silica-water interface studied by total internal reflection Raman spectroscopy: effects of silica surface properties and metal salt addition, J. Phys. Chem. B, 2013, 117, 9882–9894 CrossRef CAS PubMed.
- D. López-Díaz, I. García-Mateos and M. M. Velazquez, Synergism in mixtures of zwitterionic and ionic surfactants, Colloids Surf., A, 2005, 270, 153–162 CrossRef.
- K. Singh and D. G. Marangoni, Synergistic interactions in the mixed micelles of cationic gemini with zwitterionic surfactants: the pH and spacer effect, J. Colloid Interface Sci., 2007, 315, 620–626 CrossRef CAS PubMed.
- M. J. Rosen, Synergism in mixtures containing zwitterionic surfactants, Langmuir, 1991, 7, 885–888 CrossRef CAS.
- T. Iwasaki, M. Ogawa, K. Esumi and K. Meguro, Interactions between betaine-type zwitterionic and anionic surfactants in mixed micelles, Langmuir, 1991, 7, 30–35 CrossRef CAS.
- Y. Konodo, H. Uchiyama, N. Yoshino, K. Nishiyama and M. Abe, Spontaneous vesicle formation from aqueous solutions of didodecyldimethylammonium bromide and sodium dodecyl sulfate mixtures, Langmuir, 1995, 11, 2380–2384 CrossRef.
- J. Marignan, F. Gauthier-Fournier, J. Appell and F. Akoum, Size and shape of micelles in the ternary system n-dodecylbetaine/water/1-pentanol, J. Phys. Chem., 1988, 92, 440–445 CrossRef CAS.
- G. Ran, Y. Zhang, Q. Song and D. C. Wang, The adsorption behavior of cationic surfactants onto human hair fibers, Colloids Surf., B, 2009, 68, 106–110 CrossRef CAS PubMed.
- Z. Grenoble and S. Baldelli, Adsorption of Benzyldimethylhexadecylammonium Chloride at the Hydrophobic Silica–Water Interface Studied by Total Internal Reflection Raman Spectroscopy: Effects of Silica Surface Properties and Metal Salt Addition, J. Phys. Chem. B, 2013, 117, 9882–9894 CrossRef CAS PubMed.
- (a) P. Somasundaran and L. Zhang, Adsorption of surfactants on minerals for wettability control in improved oil recovery processes, J. Pet. Sci. Eng., 2006, 52, 198–212 CrossRef CAS; (b) B. Adibhatla, K. K. Mohanty, P. Berger and C. Lee, Effect of surfactants on wettability of near-wellbore regions of gas reservoirs, J. Pet. Sci. Eng., 2006, 52, 227–236 CrossRef CAS.
- T. R. Desai and S. G. Dixit, Interaction and viscous properties of aqueous solutions of mixed cationic and nonionic surfactants, J. Colloid Interface Sci., 1996, 177, 471–477 CrossRef CAS.
- M. Prasad, S. P. Moulik and R. Palepu, Self-aggregation of binary mixtures of alkyltriphenylphosphonium bromides: a critical assessment in favor of more than one kind of micelle formation, J. Colloid Interface Sci., 2005, 284, 658–666 CrossRef CAS PubMed.
- N. El Kadi, F. Martins and D. Clausse, Critical micelle concentration of aqueous hexadecyltrimethylammoniumbromide-sodium oleate mixtures, Colloid Polym. Sci., 1998, 281, 353–362 Search PubMed.
- (a) O. Owoyomi, J. Ige and O. Soriyan, Mixed micelles of tetradecyltrimethylammonium and N-alkyltriphenylphosphonium bromides, J. Dispersion Sci. Technol., 2014, 35, 826–831 CrossRef CAS; (b) T. Joshi, B. Bharatiya and K. Kuperkar, Micellization and Interaction Properties of Aqueous Solutions of Mixed Cationic and Nonionic Surfactants, J. Dispersion Sci. Technol., 2008, 29, 1–7 CrossRef.
- M. S. Bakshi, I. Kaur, R. Sood, J. Singh, K. Singh, S. Sachar, K. J. Singh and G. Kaur, Mixed micelles of benzyldimethyltetradecylammonium chloride with tetradecyltrimethylammonium and tetradecyltriphenylphosphonium bromides: a head group contribution, J. Colloid Interface Sci., 2004, 271, 227–231 CrossRef CAS PubMed.
- S. Ghosh, Surface chemical, and micellar properties of binary and ternary surfactant mixtures (cetyl pyridinium chloride, Tween-40, and Brij-56) in an aqueous medium, J. Colloid Interface Sci., 2001, 244, 128–138 CrossRef CAS.
- A. Avranas, E. Malasidou and E. I. Mandrazidou, Adsorption of cetyl dimethyl benzyl ammonium chloride on octane emulsions droplets: the effect of the presence of Tween 80, J. Colloid Interface Sci., 1998, 207, 363–370 CrossRef CAS PubMed.
- R. K. Mahajan and R. Sharma, Analysis of interfacial and micellar behavior of sodium dioctyl sulphosuccinate salt (AOT) with zwitterionic surfactants in aqueous media, J. Colloid Interface Sci., 2011, 363, 275–283 CrossRef CAS PubMed.
- T. R. Desai and S. G. Dixit, Interaction and viscous properties of aqueous solutions of mixed cationic and nonionic surfactants, J. Colloid Interface Sci., 1996, 177, 471–477 CrossRef CAS.
- (a) M. Prasad, S. P. Moulik and R. Palepu, Self-aggregation of binary mixtures of alkyltriphenylphosphonium bromides: a critical assessment in favor of more than one kind of micelle formation, J. Colloid Interface Sci., 2005, 284, 658–666 CrossRef CAS PubMed; (b) K. Glenn, A. van Bommel, S. C. Bhattacharya and R. Palepu, Self aggregation of binary mixtures of sodium dodecyl sulfate and polyoxyethylene alkyl ethers in aqueous solution, Colloid Polym. Sci., 2005, 283, 845–853 CrossRef CAS; (c) A. Al-Wardian, K. Glenn and R. Palepu, Thermodynamic and interfacial properties of binary cationic mixed systems, Colloids Surf., A, 2004, 247, 115–123 CrossRef CAS.
- C. Minero, E. Pramauro, E. Pelizzetti, V. Degiorgio and M. Corti, Micellar properties of sodium dodecylpoly(oxyethy-1-ene) sulfates, J. Phys. Chem., 1986, 90, 1620–1625 CrossRef CAS.
- Q. Zhou and M. J. Rosen, Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach, Langmuir, 2003, 19, 4555–4562 CrossRef CAS.
- G. Bai, J. Wang, H. Yan, Z. Li and R. K. Thomas, Thermodynamics of molecularself assembly of cationic gemini and related double chain surfactants in aqueous solution, J. Phys. Chem. B, 2001, 105, 3105–3108 CrossRef CAS.
- C. Tanford, The Hydrophobic Effect: Formation of Micelles and Biological Membranes, Wiley, New York, 1980 Search PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525–1568 RSC.
- N. J. Turro and A. Yekta, Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelle, J. Am. Chem. Soc., 1978, 100, 5951–5952 CrossRef CAS.
- (a) P. Letellier, A. Mayaffre and M. Turmine, Thoughts on the ideal behavior of mixed micelles and the appropriate application of regular solution theory (RST), J. Colloid Interface Sci., 2011, 354, 248–255 CrossRef CAS PubMed; (b) P. Letellier, A. Mayaffre and M. Turmine, Thermodynamics of mixed micelles: determination of aggregate composition, J. Colloid Interface Sci., 2008, 327, 186–190 CrossRef CAS PubMed.
- S. D. Christian, E. E. Tucker and J. F. Scamehorn, Terminology for Describing Thermodynamic Properties of Nonideal Mixed Micellar Systems, in Mixed Surfactant Systems, ACS Symposium Series, American Chemical Society, Washington, DC, 1992, ch. 3, pp. 46–51 Search PubMed.
- K. Kuperkar, L. Abezgauz, K. Prasad and P. Bahadur, Formation and Growth of Micelles in dilute aqueous CTAB solutions in the presence of NaNO3 and NaClO3, J. Surfact Deterg., 2010, 13, 293–303 CrossRef CAS.
- P. M. Holland and D. N. Rubingh, Mixed Surfactant Systems, Am. Chem. Soc., Washington DC, 1992 Search PubMed.
- H. Maeda, A simple thermodynamic analysis of the stability of ionic/non-ionic mixed micelles, J. Colloid Interface Sci., 1995, 172, 98–105 CrossRef CAS.
- C. C. Ruiz and J. Aquiar, Interaction, stability, and microenvironmental properties of mixed micelles of Triton X100 and n-alkyltrimethylammonium bromides: influence of alkyl chain length, Langmuir, 2000, 16, 7946–7953 CrossRef CAS.
- T. Oida, N. Nakashima, S. Nagadome, J. Ko, S. Oh and G. Sugihara, Adsorption and micelle formation of mixed surfactant systems in water. III. A comparison between cationic gemini /cationic and cationic gemini/nonionic combinations, J. Oleo Sci., 2003, 52, 509–522 CrossRef CAS.
- Z. Lin, Y. Zheng, H. T. Davis, L. E. Scriven, Y. Talmon and J. L. Zakin, Unusual effects of counterion to surfactant concentration ratio on viscoelasticity of a cationic surfactant drug reducer, J. Non-Newtonian Fluid Mech., 2000, 93, 363–373 CrossRef CAS.
- P. Parekh, D. Varade, J. Parikh and P. Bahadur, Anionic–cationic mixed surfactant systems: micellar interaction of sodium dodecyl trioxyethylene sulfate with cationic Gemini surfactants, Colloids Surf., A, 2011, 385, 111–120 CrossRef CAS.
- R. K. Mahajan and R. Sharma, Analysis of interfacial and micellar behavior of sodium dioctyl sulphosuccinate salt (AOT) with zwitterionic surfactants in aqueous media, J. Colloid Interface Sci., 2011, 363, 275–283 CrossRef CAS PubMed.
- F. Li, G. Z. Li and J. B. Chen, Synergism in mixed zwitterionic–anionic surfactant solutions and the aggregation numbers of the mixed micelles, Colloids Surf., A, 1998, 145, 167–174 CrossRef CAS.
- Y. Kadam, T. Patel, G. Ghosh and P. Bahadur, Mixed micellization of n-alkyltrimethylammonium bromides and dodecyl polyoxyethylene ethers, J. Dispersion Sci. Technol., 2009, 30, 843–851 CrossRef CAS.
- T. Oida, N. Nakashima, S. Nagadome, J. Ko, S. Oh and G. Sugihara, Adsorption and micelle formation of mixed surfactant systems in water. III. A comparison between cationic Gemini/cationic and cationic Gemini/nonionic combinations, J. Oleo Sci., 2003, 52, 509–522 CrossRef CAS.
- A. A. Dar, B. Chatterjee, G. M. Rather and A. R. Das, Mixed micellization and interfacial properties of dodecyltrimethylammonium bromide and tetraethyleneglycol mono-n-dodecyl ether in absence and presence of sodium propionate, J. Colloid Interface Sci., 2006, 298, 395–405 CrossRef CAS PubMed.
- S. Paria, The mixing behavior of n-alkylpyridinium bromide–NP-9 mixed surfactant systems, Colloids Surf., A, 2006, 281, 113–118 CrossRef CAS.
- E. Rodenas, M. Valiente and M. S. Villafruela, Different theoretical approaches for the study of the mixed tetraethylene glycol mono-n-dodecyl ether/hexadecyltrimethylammonium bromide micelles, J. Phys. Chem. B, 1999, 103, 4549–4554 CrossRef CAS.
- L. Liu and M. J. Rosen, The interaction of some novel diquaternary gemini surfactants with anionic surfactants, J. Colloid Interface Sci., 1996, 179, 454–459 CrossRef CAS.
- S. H. Chun, J. W. Kim, J. Kim, H. Zheng, C. C. Stoumpos, C. D. Malliakas, J. F. Mitchell, K. Mehlawat, Y. Singh, Y. Choi, T. Gog, A. Al-Zein, M. Moretti Sala, M. Krisch, J. Chaloupka, G. Jackeli, G. Khaliullin and B. J. Kim, Direct evidence for dominant bond-directional interactions in a honeycomb lattice iridate Na2IrO3, Nat. Phys., 2015, 11, 462–466 CrossRef CAS.
- J. Wang, X. Xu, S. Ding, J. Zeng, R. Spurr, X. Liu, K. Chance and M. Mishchenko, A numerical testbed for remote sensing of aerosols, and its demonstration for evaluating retrieval synergy from a geostationary satellite constellation of GEO-CAPE and GOES-R, J. Quant. Spectrosc. Radiat. Transfer, 2014, 146, 510–528 CrossRef CAS.
- T. Wang, K. Birsoy, N. W. Hughes, K. M. Krupczak, Y. Post, J. J. Wei, E. S. Lander and D. M. Sabatini, Identification and characterization of essential genes in the human genome, Science Express Report, 2015, 350, 1096–1101 CAS.
- G. B. Behera, B. K. Mishra, P. K. Behera and M. Panda, Fluorescent probes for structural and distance effect studies in micelles, reversed micelles and microemulsions, Adv. Colloid Interface Sci., 1999, 82, 1–42 CrossRef CAS.
- K. Thakkar, B. Bharatiya, D. O. Shah and P. Bahadur, Investigations on zwitterionic alkylsulfobetaines and nonionic Triton X-100 in mixed aqueous solutions: effect on size, phase separation and mixed micellar characteristics, J. Mol. Liq., 2015, 209, 569–577 CrossRef CAS.
- J. R. Rodríguez, A. González-Pérez, J. L. Castillo and J. Czapkiewicz, Thermodynamics of Micellization of Alkyldimethylbenzylammonium Chlorides in Aqueous Solutions, J. Colloid Interface Sci., 2002, 250, 438–443 CrossRef PubMed.
- K. S. Sharma, P. A. Hassan and A. K. Rakshit, Self-aggregation of binary surfactant mixtures of a cationic dimeric (Gemini) surfactant with nonionic surfactants in aqueous medium, Colloids Surf., A, 2006, 289, 17–24 CrossRef CAS.
| This journal is © the Owner Societies 2018 |