Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbocation catalysed ring closing aldehyde–olefin metathesis†
Shengjun
Ni
 and
Johan
Franzén
and
Johan
Franzén
 *
*
Department of Chemistry, Organic Chemistry, Royal Institute of Technology (KTH), Teknikringen 30, SE-100 44 Stockholm, Sweden. E-mail: jfranze@kth.se
First published on 25th October 2018
Abstract
A highly efficient aldehyde–olefin metathesis catalysed by the carbocation, 4-phenylphenyl-diphenylmethylium ion, has been developed. This protocol is characterized by high yields, low catalyst loading (down to 2 mol%), good functional group compatibility and mild reaction conditions.
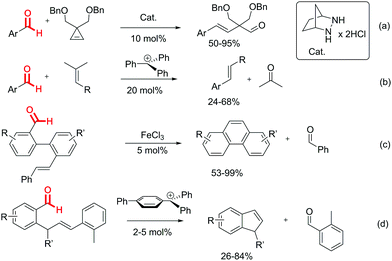
In contradiction to the well established transition metal catalysed alkene–alkene metathesis,1 the direct catalytic carbonyl–olefin metathesis is vastly underdeveloped despite its great potential with respect to atom economy and substrate scope. In the forefront of current research, a series of successful iron(III)-catalysed ring closing ketone–olefin metathesis strategies have been reported.2–6 However, the catalytic aldehyde–olefin metathesis still remains elusive.7 As one of the few exceptions, Lambert et al. reported the first aldehyde–olefin metathesis, where they showed that a bicyclic hydrazine derivate could catalyse the ring opening metathesis of cyclopropene derivatives and aldehydes (Scheme 1, equiv. (a)).8 Unfortunately, this work was limited to cyclopropene derivatives as the olefinic component. Our group reported intermolecular aldehyde–olefin metathesis by carbocation catalysis as an extension of the earlier report by Bickelhaupt (Scheme 1, equiv. (b)).9–11 Although an important proof of concept, from the synthetic point of view, this protocol suffered from high catalyst loading and a rather limited substrate scope. The main problem associated with aldehyde–olefin metathesis is mainly due to the decomposition of both the starting materials and the products in the presence of the Lewis acid catalysts required for the reaction. Schindler et al. reported a few specific examples of intramolecular aldehyde–olefin metathesis in the formation of highly stable polycyclic aromatic compounds (Scheme 1, equiv. (c)).3b
Here we show that 4-phenylphenyl-diphenylmethylium tetrafluoroborate efficiently catalyses the intramolecular aldehyde–olefin metathesis of enals under mild reaction conditions and low catalyst loading with high yields (Scheme 1, equiv. (d)). It is shown that the substituents on the olefin moiety as well as the carbocation Lewis acidity are of crucial importance for minimizing starting material and product decomposition.
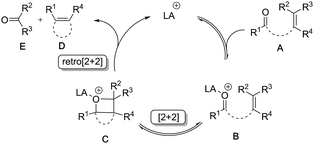
The general mechanism for the Lewis acid mediated metathesis is proposed to involve Lewis acid (LA) induced LUMO activation of enal A through initial formation of oxonium ion B (Scheme 2). The latter is now activated toward nucleophilic attack from the pendant alkene moiety resulting in the formation of oxetane C. The formation of oxetane C can occur either through a stepwise [2+2] mechanism involving a carbocationic intermediate, or through a concerted [2+2] cycloaddition. Recent mechanistic studies by Schindler et al. for FeCl3 catalysed ketone–olefin metathesis show strong support for a Lewis acid induced concerted, asynchronous [2+2]-cycloaddition.3c A subsequent fragmentation of oxetane C, either a stepwise or concerted retro-[2+2] cycloaddition, leads to the formation of the cycloalkene adduct D and the carbonyl by-product E.
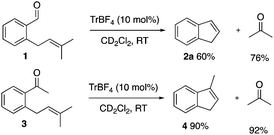
In line with our previous work9,11a–e and inspired by the elegant work of Schindler3 and Li,4 we sought to overcome the difficulties associated with the catalytic aldehyde–olefin metathesis and turned our attention towards the trityl ion catalysed ring closing metathesis of enal 1. After extensive optimization, the best yield of indene 2a was 60% after full conversion of 1a in only 5 minutes (Scheme 3). This indicates that enal 1 decomposes or undergoes side reactions in the presence of TrBF4. Furthermore, as outlined in the mechanistic proposal of the carbonyl–olefin metathesis (see Scheme 2), the metathesis adduct D and a new carbonyl compound E are formed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Thus, a comparison of the yield of 2a with the yield of the formed acetone (60% and 76%, respectively) indicates that product 2a decomposes under these reaction conditions, most likely through Lewis acid initiated polymerization. In comparison, the metathesis of the corresponding ketone 3 under the same reaction conditions gave a very high yield, almost the same as that of 4 and acetone, indicating minor decomposition/side reactions of starting material 1b and negligible trityl ion induced decomposition of methyl-indene 4 (Scheme 3).
1 ratio. Thus, a comparison of the yield of 2a with the yield of the formed acetone (60% and 76%, respectively) indicates that product 2a decomposes under these reaction conditions, most likely through Lewis acid initiated polymerization. In comparison, the metathesis of the corresponding ketone 3 under the same reaction conditions gave a very high yield, almost the same as that of 4 and acetone, indicating minor decomposition/side reactions of starting material 1b and negligible trityl ion induced decomposition of methyl-indene 4 (Scheme 3).
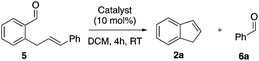
Inspired by Li's work4 we instead investigated the trityl ion catalysed ring closing metathesis of enals. Gratifyingly, indene 2a and benzaldehyde 6a were obtained in 71% and 86% yields, respectively (Table 1, entry 1). Surprisingly, FeCl3, which was successfully used for intramolecular ketone–olefin metathesis both by Schindler and Li,3,4 rapidly consumed enal 5 without any observable formation of indene 2a (Table 1, entry 2). However, the formation of benzaldehyde 6a in 74% yield shows that the metathesis do occur although indene 2a rapidly decomposes in the presence of FeCl3. The same outcome was observed for InCl3, BF3·Et2O and HBF4·Et2O that gave benzaldehyde 6a in high yields and with low or no observable formation of indene 2a (Table 1, entries 3–5). AlCl3 was the least efficient catalyst with only 36% conversion and 3% yield (Table 1, entry 6).12
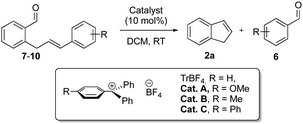
In order to gain further insight into the metathesis process, in terms of reactivity and Lewis acid induced decomposition, we turned our attention to the styryl moiety. Decreasing the electron density/nucleophilicity through an electron withdrawing para-nitro group (enal 7) completely stopped both enal decomposition and metathesis and only starting materials could be recovered (Table 2, entry 1). In contrast, increasing the electron density/nucleophilicity of the alkene by introducing an electron donating para-methoxy-group greatly accelerated the decomposition of enal 8 in the presence of TrBF4, enabling full conversion in only 5 minutes with a low yield of indene 2a (Table 2, entry 2). Additional tuning of the electronic properties of the alkene moiety revealed that the weak electron donating groups o-methyl 9a and p-methyl 10 shorten the reaction time, leading to a somewhat reduced product decomposition and only a minor increased enal decomposition (Table 2, entries 3 and 4). Among the latter, o-methyl substituted enal 9a was the most efficient, affording indene 2a in 75% yield (Table 2, entry 3).13
| Entry | S. M. | R | Catalyst | t (h) | Yieldb (%) | ||
|---|---|---|---|---|---|---|---|
| S. M. | 2a | 6 | |||||
| a Reaction conditions: TrBF4 (10 mol%) was added to 7–10 in DCM (0.01 M) for the indicated time at room temperature. b Yields were determined by 1H NMR spectroscopy. c 5 mol% of Cat C was used. | |||||||
| 1 | 7 | p-NO2 | TrBF4 | 2.5 | 100 | 0 | 0 |
| 2 | 8 | p-MeO | TrBF4 | 5 min | 0 | 22 | 26 |
| 3 | 9a | o-Me | TrBF 4 | 40 min | 0 | 75 | 84 |
| 4 | 10 | p-Me | TrBF4 | 15 min | 0 | 60 | 74 |
| 5 | 9a | o-Me | Cat A | 29 | 8 | 17 | 78 |
| 6 | 9a | o-Me | Cat B | 4 | 0 | 76 | 85 |
| 7 | 9a | o-Me | Cat C | 3 | 0 | 80 | 86 |
| 8 | 9a | o-Me | Cat C | 8 | 0 | 80 | 86 |
After identifying the o-tolyl-group in enal 9a as the best alkene-leaving group, we investigated the influence of carbocation Lewis acidity on the aldehyde–olefin metathesis. The trityl ion constitutes a rather unique mode of carbon-centered Lewis acidity with extensive possibilities for relatively easy tuning through variation of the electronic properties of the surrounding aromatic groups.11a–e,14 The trityl ions Cat A–C were screened as catalysts for the metathesis of enal 9a. The mono-methoxy substituted trityl ion, Cat A, is the least Lewis acidic carbocation screened, due to the strong electron donating properties of the p-methoxy-phenyl group to the carbocationic center. Unfortunately, the lower Lewis acidity of Cat A resulted in a drastically prolonged reaction time and low yield of indene 2a and did not prevent starting material/product decomposition (Table 2, entry 5). The mono-methyl substituted trityl ion, Cat B, is a considerably stronger Lewis acid compared to Cat A although it is less Lewis acidic compared to TrBF4 and gave full conversion after 4 h (Table 2, entry 6). Interestingly, despite the increased reaction time, the yield was virtually the same as for TrBF4, indicating that product decomposition was considerably slower with Cat B (Table 2, entry 6). However, after extensive screening, we found that with the mono-p-phenyl substituted trityl ion, Cat C, the yield of indene 2a could be increased up to 80% within 3 h (Table 2, entry 7). Notably, reducing the catalyst loading to only 5 mol% prolonged the reaction time to 8 h without any loss in the yield (Table 2, entry 8). Thus, the p-phenyl substituted trityl ion, Cat C, almost completely diminished product decomposition, giving essentially the same yields of metathesis adduct 2a and 2-methylbenzaldehyde 6.
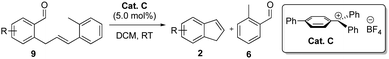
With these optimal conditions in hand (Cat C (5 mol%), DCM, RT), the aldehyde–olefin metathesis of enal 9a gave indene 2a in 81% isolated yield (Table 3, entry 1). Introducing a methyl-substituent in the 5-position of enal 9b gave the corresponding 7-methyl-indene 2b in 71% yield (Table 3, entry 2). In contrast, the 3-methyl-group in enal 9c greatly accelerated the metathesis and afforded 7-methyl-indene 2c in 78% yield within 30 minutes of reaction time (Table 3, entry 3). The increased reactivity is most likely a result of 1,3-allylic strain induced by the 3-methyl-group that locks the conformation with the olefin side chain in closer proximity to the aldehyde moiety. The 5-methoxy-substituted enal 9d also had an accelerating effect on the metathesis and gave 5-methoxy-indene 2d in 68% isolated yield (Table 3, entry 4). In contrast, the 4-methyl-group in enal 9e and the 2-benzyloxy-group in 9f gave the corresponding indenes 2e and 2f in low yields (Table 3, entries 5 and 6). This is most likely due to the increased product decomposition as a result of the higher reactivity of 6-methyl-indene 2e and 4-benzyloxy-indene 2f caused by the electron donating properties of the methyl- and benzyloxy-groups in the para- and ortho-positions, respectively, to the indene double bond. Different halogenated enals were also screened and fluorinated enals 9g–i gave indenes 2g–i in good yields. However, the 5-chloro-substituent in enal 9j had a negative effect on the metathesis and 5-chloro-indene 2j was isolated in 45% yield.
| Entry | S. M. | R | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Cat C (5 mol%) was added to 9 in DCM (0.01 M) for the indicated time at room temperature. b Isolated yield. | |||||
| 1 | 9a | H | 2a | 8 | 81 |
| 2 | 9b | 5-Methyl | 2b | 4 | 71 |
| 3 | 9c | 3-Methyl | 2c | 0.5 | 78 |
| 4 | 9d | 5-Methoxyl | 2d | 0.75 | 68 |
| 5 | 9e | 4-Methyl | 2e | 6 | 40 |
| 6 | 9f | 2-BnO | 2f | 1.75 | 26 |
| 7 | 9g | 4-Fluro | 2g | 4 | 80 |
| 8 | 9h | 6-Fluro | 2h | 0.5 | 60 |
| 9 | 9i | 4,5-Difluro | 2i | 4 | 73 |
| 10 | 9j | 5-Chloro | 2j | 5 | 45 |
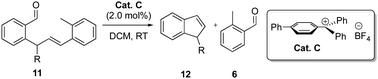
We next evaluated functionalization at the benzylic position of enals 11 that would allow for easy access to 1-functionalized indenes 12 (Table 4). To our delight, functionalization at this position had a remarkable effect on the reactivity and for the Cat C catalysed metathesis of enals 11a–h, the catalyst loading could be reduced down to 2.0 mol% without any loss in the efficiency. Under these conditions, the corresponding products 12a–h were isolated in 78–84% yields within less then one hour. This drastic increase in the reactivity is most likely due to the Thorpe–Ingold effect favouring cyclization. However, the 3-benzyloxypropyl functionalized enal 11i reacted considerably slower and required 5 mol% catalyst loading to give indene 12i in 80% yield. The terminal alkene moiety in enal 11j had a negative influence on the metathesis with an increased reaction time and side reactions to give 12j in only 37% yield.
| Entry | S. M. | R | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Cat C (2 mol%) was added to 11 in DCM (0.01 M) for the indicated time at RT. b Isolated yield. c 5 mol% catalyst loading was used. | |||||
| 1 | 11a | Me | 12a | 0.5 | 78 |
| 2 | 11b | n-Butyl | 12b | 1 | 83 |
| 3 | 11c | Cyclopentyl | 12c | 0.5 | 84 |
| 4 | 11d | Phenylethyl | 12d | 1 | 84 |
| 5 | 11e | Phenylpropyl | 12e | 0.75 | 80 |
| 6 | 11f | 3-Chlorophenylethyl | 12f | 1 | 83 |
| 7 | 11g | 2-Fluorophenylethyl | 12g | 1 | 81 |
| 8 | 11h | 4-Methylphenylethyl | 12h | 1 | 81 |
| 9c | 11i | 3-Benzyloxypropyl | 12i | 1 | 80 |
| 10c | 11j | 3-Butenyl | 12j | 4 | 37 |
In conclusion, we have developed a direct organocatalytic aldehyde–olefin ring closing metathesis. The reaction is operationally simple and enables direct coupling of aldehydes and pendant olefins in the presence of the easily available 4-phenylphenyl-diphenylmethylium ion as the Lewis acid catalyst. The catalyst loadings can be reduced as low as 2 mol% and the products are isolated in good yields, often in a very clean and selective manner. With the developed procedure, a variety of functionalized indene derivatives could be easily prepared. This protocol represents a rare example of a catalytic aldehyde–olefin metathesis and efforts to extend this process to a broader array of substrates are currently on going in our laboratory. These results will be reported in due course.
S. N. gratefully acknowledges the Chinese Scholarship Council (CSC) for a grant.
Conflicts of interest
The authors confirm the absence of conflicts of interest.Notes and references
- (a) Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons, Inc., 2014 Search PubMed; (b) A. H. Hoveyda and A. R. Zhugralin, Nature, 2007, 450, 243 CrossRef CAS PubMed.
- For recent reviews see: (a) C. Schindler and J. Ludwig, Synlett, 2017, 1501 CrossRef PubMed; (b) L. Ravindar, R. Lekkala, K. Rakesh, A. M. Asiri, H. M. Marwani and H.-L. Qin, Org. Chem. Front., 2018, 5, 1381 RSC , and references therein.
- (a) J. R. Ludwig, P. M. Zimmerman, J. B. Gianino and C. S. Schindler, Nature, 2016, 533, 374 CrossRef CAS PubMed; (b) C. C. McAtee, P. S. Riehl and C. S. Schindler, J. Am. Chem. Soc., 2017, 139, 2960 CrossRef CAS PubMed; (c) J. R. Ludwig, S. Phan, C. C. McAtee, P. M. Zimmerman, J. J. Devery 3rd and C. S. Schindler, J. Am. Chem. Soc., 2017, 139, 10832 CrossRef CAS PubMed; (d) E. J. Groso, A. N. Golonka, R. A. Harding, B. W. Alexander, T. M. Sodano and C. S. Schindler, ACS Catal., 2018, 8, 2006 CrossRef CAS PubMed; (e) J. R. Ludwig, R. B. Watson, D. J. Nasrallah, J. B. Gianino, P. M. Zimmerman, R. A. Wiscons and C. S. Schindler, Science, 2018, 361, 1363 CrossRef CAS PubMed; (f) H. Albright, P. S. Riehl, C. C. McAtee, J. P. Reid, J. R. Ludwig, L. A. Karp, P. M. Zimmerman, M. S. Sigman and C. S. Schindler, ChemRxiv DOI:10.26434/chemrxiv.7130801.
- L. Ma, W. Li, H. Xi, X. Bai, E. Ma, X. Yan and Z. Li, Angew. Chem., Int. Ed., 2016, 55, 10410 CrossRef CAS PubMed.
- L. Catti and K. Tiefenbacher, Angew. Chem., Int. Ed., 2018, 57, 14589 CrossRef CAS PubMed.
- U. P. N. Tran, G. Oss, D. P. Pace, J. Ho and T. V. Nguyen, Chem. Sci., 2018, 9, 5145 RSC.
- During the preparation of this manuscript, Schindler et al. reported on the GaCl3 catalyzed aldehyde–olefin ring opening metathesis with low to moderate yields. H. Albright, H. L. Vonesh, M. R. Becker, B. W. Alexander, J. R. Ludwig, R. A. Wiscons and C. S. Schindler, Org. Lett., 2018, 20, 4954 CrossRef CAS PubMed.
- (a) A. K. Griffith, C. M. Vanos and T. H. Lambert, J. Am. Chem. Soc., 2012, 134, 18581 CrossRef CAS PubMed; (b) A.-L. Lee, Angew. Chem., Int. Ed., 2013, 52, 4524 CrossRef CAS PubMed; (c) X. Hong, Y. Liang, A. K. Griffith, T. H. Lambert and K. N. Houk, Chem. Sci., 2014, 5, 471 RSC.
- V. R. Naidu, J. Bah and J. Franzén, Eur. J. Org. Chem., 2015, 1834 CrossRef.
- H. P. van Schaik, R. J. Vijn and F. Bickelhaupt, Angew. Chem., Int. Ed., 1994, 33, 1611 CrossRef.
- For selected examples of carbocation catalysis see; (a) V. R. Naidu, S. Ni and J. Franzén, ChemCatChem, 2015, 7, 13 CrossRef; (b) J. Bah, V. R. Naidu, J. Teske and J. Franzén, Adv. Synth. Catal., 2015, 357, 148 CrossRef CAS; (c) J. Bah and J. Franzén, Chem. – Eur. J., 2014, 20, 1066 CrossRef CAS PubMed; (d) M. A. A. El Remaily, V. R. Naidu, S. Ni and J. Franzén, Eur. J. Org. Chem., 2015, 6610 CrossRef; (e) S. Ni, V. R. Naidu and J. Franzén, Eur. J. Org. Chem., 2016, 1708 CrossRef CAS; (f) S. Ni, M. A. A. El Remaily and J. Franzén, Adv. Synth. Catal., 2018 DOI:10.1002/adsc.201800788; (g) Y. Huang, C. Qiu, Z. Li, W. Feng, H. Gan, J. Liu and K. Guo, ACS Sustainable Chem. Eng., 2016, 4, 47 CrossRef CAS; (h) J. Liu, J. Xu, Z. Li, Y. Huang, H. Wang, Y. Gao, T. Guo, P. Ouyang and K. Guo, Eur. J. Org. Chem., 2017, 3996 CrossRef CAS; (i) S. R. Roy, A. Nijamudheen, A. Pariyar, A. Ghosh, P. K. Vardhanapu, P. K. Mandal, A. Datta and S. K. Mandal, ACS Catal., 2014, 4, 4307 CrossRef CAS; (j) J. Lv, Q. Zhang, X. Zhong and S. Luo, J. Am. Chem. Soc., 2015, 137, 15576 CrossRef CAS PubMed; (k) Q. Zhang, J. Lv and S. Luo, Acta Chim. Sin., 2016, 74, 61 CrossRef CAS; (l) C. Nicolas, C. Herse and J. Lacour, Tetrahedron Lett., 2005, 46, 4605 CrossRef CAS; (m) C. Nicolas and J. Lacour, Org. Lett., 2006, 8, 4343 CrossRef CAS PubMed; (n) P. Pommerening, J. Mohr, J. Friebel and M. Oestreich, Eur. J. Org. Chem., 2017, 2312 CrossRef CAS; (o) Y. A. Rulev, Z. T. Gugkaeva, A. V. Lokutova, V. I. Maleev, A. S. Peregudov, X. Wu, M. North and Y. N. Belokon, ChemSusChem, 2017, 10, 1152 CrossRef CAS PubMed; (p) L. Wan, W. Zhu, K. Qiao, X. Sun, Z. Fang and K. Guo, Asian J. Org. Chem., 2016, 5, 920 CrossRef CAS; (q) Q. Zhang, J. Lv, S. Li and S. Luo, Org. Lett., 2018, 20, 2269 CrossRef CAS PubMed.
- For additional screening of reaction conditions see the ESI†.
- For additional screening of the substitution effect of the styryl-group see the ESI†.
- (a) E. Follet, P. Mayer and B. Berionni, Chem. – Eur. J., 2017, 23, 623 CrossRef CAS PubMed; (b) M. Horn and M. Mayr, J. Phys. Org. Chem., 2012, 25, 979 CrossRef CAS and references therein; (c) M. Horn and H. Mayr, Eur. J. Org. Chem., 2011, 6470 CrossRef CAS; (d) M. Horn, C. Metz and H. Mayr, Eur. J. Org. Chem., 2011, 6476 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc06734a |
| This journal is © The Royal Society of Chemistry 2018 |