Heterojunctions of silver–iron oxide on graphene for laser-coupled oxygen reduction reactions†
Wei-Quan
Chen
a,
Min-Chuan
Chung
a,
Joey Andrew A.
Valinton
 a,
David P.
Penaloza
Jr
a,
David P.
Penaloza
Jr
 b,
Shiow-Huey
Chuang
c and
Chun-Hu
Chen
b,
Shiow-Huey
Chuang
c and
Chun-Hu
Chen
 *a
*a
aDepartment of Chemistry, National Sun Yat-sen University, Kaohsiung, 80424, Taiwan. E-mail: chunhu.chen@mail.nsysu.edu.tw
bDepartment of Chemistry, College of Science, De La Salle University, Manila 1004, Philippines
cDepartment of Applied Chemistry, National University of Kaohsiung, Kaohsiung, Taiwan
First published on 21st May 2018
Abstract
We report a two-step hybridization of N-doped graphene and Ag-decorated Fe2O3 hematite to realize a balanced oxygen adsorption/desorption equilibrium and a laser-coupled ORR (LORR). The stable plateau currents with n values of 3.9 in a wide potential range (0.2–0.7 V) and 7.5% peroxide inhibition of the LORR are found to be directly associated with the Ag/Fe2O3 heterojunction, where interactions of semiconductor band gap excitation and plasmonic resonance-induced hot electrons are proposed to occur.
Great efforts in searching for substitutes of Pt/C as inexpensive oxygen reduction reaction (ORR) electrocatalysts have been extensively applied for modern fuel cells and metal–air batteries.1 Iron oxide catalysts exhibiting high abundance, excellent stability, and low environmental impact are promising alternatives to Pt/C. However, like other low-cost metal oxides, they suffer from weak energy densities and a two-electron transfer ORR, which generates corrosive peroxide species.2 Apparently, combining them with mesoporous carbon materials has been reported to successfully improve the number of transferred electrons during reduction,3 although achieving a stable limiting current remains elusive due to sluggish kinetics produced by a low electrocatalytic conversion.4 Efficient kinetics of both oxygen adsorption/desorption needs to be achieved to address this issue.
According to the volcano curves of ORR activity, the function of oxygen binding energy over various species,5 iron can facilitate catalytic O–O bond breaking due to strong oxygen adsorption, but this property hampers the ORR catalytic cycle because the desorption of the as-yielded intermediates/products from the occupied sites becomes difficult. Silver, on the other hand, possesses a relatively weak oxygen adsorption affinity,6 which benefits intermediate/product elimination, but O2 immobilization for effective catalysis becomes difficult. Our rational design aims to establish a well-balanced ORR system by establishing the interfacial combination of Ag and Fe, which promotes complementary and synergistic activities. Compared to platinum, the abundance and cost of silver are reasonable for commercial usage despite its low electron-transfer (n) values and high initial voltage losses.7
Recent studies demonstrated that photo-assisted ORRs are critical to realize light-driven fuel cells.8 Chen and coworkers further revealed the mechanisms via which Ag–Pt nanocages can inhibit peroxides through plasmon-induced hot electrons.9 Strong surface plasmons can only be observed in noble metals and are commonly absent in metal oxide materials. Hence, noble metals become the usual materials considered for light-assisted ORR studies.10 Exploration of the usage of alternative light-excitation routes on low-cost metal oxide materials towards enhancing the ORR is highly desirable but less established. In addition, white, continuous light sources were commonly used in the reported photo-assisted ORR;9,10 however, achieving specific excitation remains difficult as the identification of active sites remained uncertain. We thus adopted a laser as the light source with a very narrow bandwidth to realize specific excitation, and a smaller required power input due to the beam-concentrated nature of the laser.
In this work, we report a two-step synthesis to produce Ag/Fe2O3/N-doped graphene nanocomposites (Ag/FNG) with the controlled formation of a Ag/Fe2O3 heterojunction. Significantly improved ORR activity (n = 3.9) and efficient kinetics with a stable limiting current can be obtained, both of which were established to originate from the heterojunction sites. For the first time, these composite catalysts demonstrated their photoactivities towards peroxide suppression through a laser-coupled ORR (LORR), a light-electricity co-activation process on the electrocatalysts.
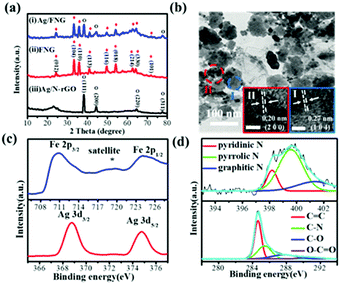
Ag/FNG was prepared by refluxing a mixture of iron(II) acetate and graphene oxide (GO) in dimethylformamide (DMF), followed by the addition of silver nitrate (10% of the moles of iron) and a subsequent hydrothermal treatment at 150 °C. Subsequently, the control samples of Fe2O3/N-doped graphene (FNG) and Ag/N-rGO were prepared similarly without the Ag and Fe precursors, respectively. The XRD patterns of Ag/FNG correspond to hematite Fe2O3 and metallic silver (Fig. 1a), confirming the deposition of metallic Ag rather than Ag+ doping in the iron oxide frameworks.11 The XRD results of the control samples FNG and Ag/N-rGO agree with their hematite and Ag components, respectively. The morphology of Ag/FNG according to the acquired TEM image resembles the deposition of finer particles (diameter 15.3 ± 0.7 nm) onto the surface of larger, distinct ones (diameter 79.3 ± 11.6 nm) supported by the graphene sheets (Fig. 1b). These particles were confirmed to be iron and silver by EDXS data (Fig. S1, ESI†), where the corresponding molar ratio according to ICP-MS is 0.139, a slightly higher value than that of the precursor mixture. The HR-TEM characterization of the large particles (Fig. 1b-I) shows 0.27 nm lattice fringes corresponding to d104 of hematite Fe2O3. Meanwhile, the observed 0.20 nm lattice fringes on the finer particles (Fig. 1b-II) agree with d200 of Ag. These results clearly demonstrate the successful formation of the discrete Ag/Fe2O3 heterojunction in Ag/FNG, highly preferred for the effective exposure of Ag-to-Fe interfaces. The XPS studies (Fig. 1c) show the binding energies of Fe 2p3/2 and 2p1/2 to be 711.5 and 724.5 eV, respectively, together with the presence of a satellite peak, corresponding to Fe(III).4c Also, the binding energies of Ag 3d5/2 and 3d3/2 at 368.8 and 374.8 eV, respectively, further verify the presence of metallic Ag.12 The C 1s spectra indicate the reduction of graphene oxide (rGO) in Ag/FNG, while N 1s deconvolution indicates nitrogen doping in rGO with graphitic (∼401.6 eV), pyrrolic (∼399.5 eV), and pyridinic (398.2 eV) forms (Fig. 1d), which is known to greatly enhance the ORR activity.13 We selected DMF, an N-containing organic solvent, for the possible formation of iron–DMF coordination complexes capable of achieving higher degrees of N doping in GO (N-rGO) which cannot be accomplished using the conventional ammonia addition approach.13b
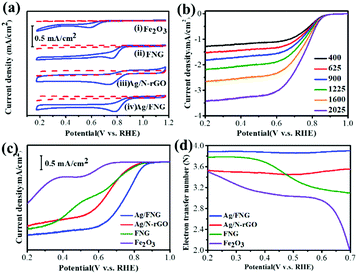
The ORR performance of Ag/FNG and the control samples was evaluated through cyclic voltammetry (CV) plots under O2- and N2-saturated alkaline solutions (Fig. 2a). The results reveal that Ag/FNG possesses the largest current and most positive onset potential at 0.92 V (vs. RHE) as compared to Ag/N-rGO (0.87 V), FNG (0.86 V), and Fe2O3 (0.70 V). Fe2O3 has a much more negative onset potential than FNG supporting the effective ORR enhancement due to N-rGO hybridization. In Fig. 2b, the linear scan voltammogram (LSV) curves of Ag/FNG demonstrate plateau currents starting at 0.6 V. The curve shapes of Ag/FNG, as well as the control samples (Fig. S2, ESI†), remain consistent as the rotation speeds are increased (400–2025 rpm), which enables more O2 to reach the electrode surface. Ag/FNG provides a greater increase in the current density as compared to others while maintaining a plateau-like diffusion limiting curve. This means that the catalyst is active enough to achieve higher conversion and faster diffusion kinetics. Ag/FNG demonstrated superior ORR activity and mass transport compared to the ones without the Ag/Fe2O3 heterojunction.
The comparison of the LSV and the number of electron transfer curves among the control samples is shown in Fig. 2c and d, respectively. The two-step LSV curves of Fe2O3 suggest two different ORR stages in the range of 0.4–0.7 V, where the two-electron transfer pathway governs the ORR at potentials above 0.6 V (Fig. 2d). Compared to Fe2O3, FNG shows a similar two-step LSV curve but, at 0.3 V, it produces higher n values (3.78) and currents. N-rGO hybridization with Fe2O3 enhances the current densities and decreases the onset potential barrier giving higher n values, suggesting the formation of Fe–N–C sites,14 as well as the quasi-four-electron ORR occurring from the combination of electrochemical two-electron reduction and chemical disproportionation with rapid mass transfer.15 However, the two-step-like LSV and the absence of limiting currents are still observed, similar to the reported graphene/iron oxide composites.3,4,16 In contrast, the single-step LSV with the plateau current of Ag/FNG indicates a direct four-electron transfer similar to that observed in benchmark Pt/C. This similarity suggests that the plateau current can be presumably associated with the balanced adsorption/desorption equilibrium exhibited by Ag. Electrochemical Impedance Spectroscopy (Fig. S3, ESI†) shows that an increase in conductivity has been exhibited by Ag/FNG due to the presence of the Ag-decorated interface with Fe2O3. The lowest charge transfer resistance of Ag/FNG among Ag/rGO and FNG further corresponds to the improved kinetics (Table S1, ESI†).
Ag/FNG exhibits the greatest n value of 3.9, which remains stable over a wide potential range (0.2–0.7 V), while Ag/N-rGO exhibits relatively low n values and more negative onset potentials. Note that both hematite and FNG also show much lower n values particularly at the high potential regions (0.6–0.7 V), indicating the essential role of the Ag/Fe2O3 heterojunction in requiring only small potential bias to realize high n values. Also, Ag/FNG only requires around 10% (i.e. 11.88%) Ag to accomplish a significant ORR enhancement, which is smaller than prescribed in the literature.9 Moreover, Ag/FNG outperforms similar electrocatalysts (Table S2, ESI†), emphasizing the formation of Ag/Fe2O3 interfaces on graphene as an effective strategy to achieve a low-cost Pt/C replacement.
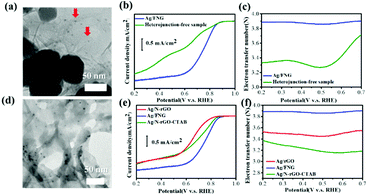
To study the role of the Ag/Fe2O3 heterojunction, an analogous control sample without Ag/Fe2O3 interfaces was synthesized to compare its behavior with Ag/FNG (see the ESI†). The acquired TEM image shows that the Fe2O3 particles have no attachment with the fine Ag dots (∼5 nm, labeled by red arrows in Fig. 3a) on graphene. The XRD data confirm the presence of both metallic Ag and hematite (Fig. S4, ESI†). The LSV results (Fig. 3b) are comparable to the two-step ORR observed in FNG, while the n values are similar to that of Ag/N-rGO (at 0.5–0.7 V, see Fig. 3c). Therefore, the electrocatalytic properties in this heterojunction-free catalyst sufficiently prove that the plateau current of Ag/FNG is due to the presence of the Ag–Fe2O3 heterojunction. Also, the selective removal of the Ag nanoparticles from Ag/FNG (named as Ag/FNG-Ag etched in Fig. S5, ESI†) replaces the features of plateau currents, most positive onset potentials, and high n values as observed in Ag/FNG with those of FNG. These results clearly confirm that the Ag–Fe2O3 heterojunction is responsible for the greatly enhanced ORR performance.
Smaller Ag particles are generally known to exhibit greater ORR activity.17 To investigate whether the Ag nanoparticles may solely act as the main active sites responsible for the enhanced ORR, we further narrowed down the Ag particle size in Ag/N-rGO for comparison. As shown in Fig. 3d, the Ag/N-rGO prepared under surfactant cetyltrimethylammonium bromide (CTAB) conditions, denoted as Ag/N-rGO-CTAB, yields a smaller Ag particle size (5.43 ± 2.25 nm) than Ag/N-rGO. Ag/N-rGO-CTAB exhibits a more positive ORR onset potential (0.89 V, see Fig. 3e) and a slightly smaller n value (Fig. 3f) as compared to Ag/N-rGO. These results suggest that the ORR activity of Ag/FNG cannot depend only on the Ag particles, although Ag is critical to gain improved kinetics. Therefore, the Ag/Fe2O3 heterojunction is mainly responsible for the synergistic ORR enhancement in Ag/FNG.
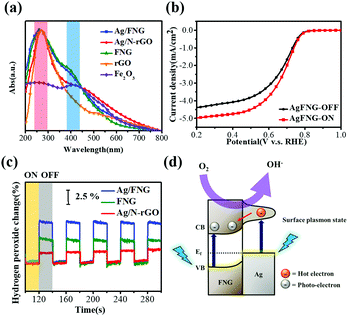
We further explore the photoelectrochemical ORR of Ag/FNG under the direct laser irradiation penetrating massive water medium at room temperature (see the ESI†), where drastic local heat and temperature increases at the electrodes are negligible to influence the ORR performance. The UV-vis spectra of Ag/FNG and the control samples were acquired first to identify the absorption energy among them (Fig. 4a). The band at 269 nm (labelled by the red zone) is due to the π → π* transition of reduced graphene oxide,18 since all the samples that contain rGO possess this band but Fe2O3. The shoulder band at 405 nm (labelled by the blue zone) can be attributed to the band gap absorption of Fe2O3.19 The surface plasmonic absorption of the Ag nanoparticles is also observed in Ag/N-rGO within the blue zone.20 Thus, the light source of a 405 nm laser used in this study can both stimulate the band gap excitation of Fe2O3 and the surface plasmon of Ag. Different from the reported light sources with continuous wavelengths,9,21 our studies of the LORR behaviors can be focused on the Ag/Fe2O3 heterojunction only by minimizing the potential interference that comes from other parts of the composites, such as graphene material excitation. The laser-coupled LSV curves of Ag/FNG (Fig. 4b) show similar onset potentials with a higher current density than the dark condition by 12.6%. The identical onset potentials suggest no change in the active site configuration, yet the quantity of catalytically reduced oxygen is increased under laser irradiation. Higher plateau currents in Ag/FNG reveal an increase in the oxygen molecule conversion equilibrium over time.
By comparing the difference of peroxide yields under laser-on/off conditions (Fig. 4c), the highly repeatable signals further indicate no potential decay of the photoactive sites due to laser irradiation. The laser-assisted peroxide inhibition of Ag/FNG, FNG, and Ag/N-rGO is shown to be 7.5%, 4.1%, and 2%, respectively. Moreover, the change in H2O2 yield of the heterojunction-free sample (Fig. S6, ESI†) is measured to be 4.0% which is comparable to that of FNG. The highest inhibition capability of Ag/FNG, which is even greater than the summation of bandgap excitation (FNG) and plasmonic resonance (Ag/N-rGO), as well as the noble metal alloys,9 reveals the strong photoelectrochemical interaction between Ag and Fe2O3 for peroxide depression. At the Ag/Fe2O3 heterojunction, the laser-excited hot electrons at the Ag nanoparticles can quickly transfer to the Fe2O3 conduction band with the lowered Schottky barrier, as illustrated in Fig. 4d.19,22 Together with the photoelectrons due to laser excitation on Fe2O3, the localization of these charges at the interfaces makes recombination less likely, to which the nearly four-electron reduction and further H2O2 inhibition can be attributed.9 It is also likely that the laser-activated Ag and Fe enhances the adsorption/desorption of oxygen simultaneously at the interface, which improves the mass transport and ORR kinetics for the increased plateau currents. To the best of our knowledge, Ag/FNG is the first LORR example achieving efficient kinetics and activities realized by the specific formation of Ag/Fe2O3 heterojunctions on graphene through the synthesis as demonstrated in this work.
In summary, we developed a two-step preparation to realize the formation of Ag/Fe2O3 heterojunctions on N-doped graphene, which has been experimentally confirmed as the origin of plateau currents and synergistic ORR enhancement achieving n values of 3.9 over a wide potential range with a minimized potential loss. N-doped graphene by DMF synthesis enhances the current density and lowers the onset potentials. Ag/FNG is capable of inhibiting peroxide yields under visible-light LORR, as a new example for the future design of a low-cost, photo-active composite to replace noble metal electrocatalysts. The detailed mechanisms regarding the photoelectrochemical ORR are currently under active investigation.
We acknowledge the financial support from the Ministry of Science and Technology, Taiwan under grant 106-2113-M-110-010. We also thank the NSYSU-NUK Joint Research Project, NSYSUNUK 107-P005.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) X. Ge, A. Sumboja, D. Wuu, T. An, B. Li, F. W. T. Goh, T. S. A. Hor, Y. Zong and Z. Liu, ACS Catal., 2015, 5, 4643–4667 CrossRef; (b) C. H. Kuo, I. M. Mosa, S. Thanneeru, V. Sharma, L. Zhang, S. Biswas, M. Aindow, S. Pamir Alpay, J. F. Rusling, S. L. Suib and J. He, Chem. Commun., 2015, 51, 5951–5954 RSC.
- T. N. Lambert, J. A. Vigil, S. E. White, C. J. Delker, D. J. Davis, M. Kelly, M. T. Brumbach, M. A. Rodriguez and B. S. Swartzentruber, J. Phys. Chem. C, 2017, 121, 2789–2797 Search PubMed.
- Z. S. Wu, S. Yang, Y. Sun, K. Parvez, X. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 9082–9085 CrossRef PubMed.
- (a) B. Zhao, Y. Zheng, F. Ye, X. Deng, X. Xu, M. Liu and Z. Shao, ACS Appl. Mater. Interfaces, 2015, 7, 14446–14455 CrossRef PubMed; (b) S. Ren, S. Ma, Y. Yang, Q. Mao and C. Hao, Electrochim. Acta, 2015, 178, 179–189 CrossRef; (c) M. Sun, Y. Dong, G. Zhang, J. Qu and J. Li, J. Mater. Chem. A, 2014, 2, 13635–13640 RSC.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jonsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- S.-A. Park, H. Lim and Y.-T. Kim, ACS Catal., 2015, 5, 3995–4002 CrossRef.
- (a) F. H. B. Lima, C. D. Sanches and E. A. Ticianelli, J. Electrochem. Soc., 2005, 152, A1466 CrossRef; (b) K. Lee, M. S. Ahmed and S. Jeon, J. Power Sources, 2015, 288, 261–269 CrossRef.
- B. Zhang, S. Wang, W. Fan, W. Ma, Z. Liang, J. Shi, S. Liao and C. Li, Angew. Chem., Int. Ed., 2016, 55, 14748–14751 CrossRef PubMed.
- S. C. Lin, C. S. Hsu, S. Y. Chiu, T. Y. Liao and H. M. Chen, J. Am. Chem. Soc., 2017, 139, 2224–2233 CrossRef PubMed.
- (a) Y. Xiong, M. Ren, D. Li, B. Lin, L. Zou, Y. Wang, H. Zheng, Z. Zou, Y. Zhou, Y. Ding, Z. Wang, L. Dai and H. Yang, J. Catal., 2017, 354, 160–168 CrossRef; (b) L. Guo, K. Liang, K. Marcus, Z. Li, L. Zhou, P. D. Mani, H. Chen, C. Shen, Y. Dong, L. Zhai, K. R. Coffey, N. Orlovskaya, Y.-H. Sohn and Y. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 34970–34977 CrossRef PubMed; (c) Z. Zheng, W. Xie, M. Li, Y. H. Ng, D.-W. Wang, Y. Dai, B. Huang and R. Amal, Nano Energy, 2017, 41, 233–242 CrossRef.
- (a) C. H. Chen, E. C. Njagi, S. Y. Chen, D. T. Horvath, L. Xu, A. Morey, C. Mackin, R. Joesten and S. L. Suib, Inorg. Chem., 2015, 54, 10163–10171 CrossRef PubMed; (b) M. Özacar, A. S. Poyraz, H. C. Genuino, C.-H. Kuo, Y. Meng and S. L. Suib, Appl. Catal., A, 2013, 462–463, 64–74 CrossRef.
- R. Krishna, D. M. Fernandes, C. Dias, J. Ventura, E. Venkata Ramana, C. Freire and E. Titus, Int. J. Hydrogen Energy, 2015, 40, 4996–5005 CrossRef.
- (a) D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef PubMed; (b) W. Y. Kao, W. Q. Chen, Y. H. Chiu, Y. H. Ho and C. H. Chen, Sci. Rep., 2016, 6, 37174 CrossRef PubMed.
- W. J. Jiang, L. Gu, L. Li, Y. Zhang, X. Zhang, L. J. Zhang, J. Q. Wang, J. S. Hu, Z. Wei and L. J. Wan, J. Am. Chem. Soc., 2016, 138, 3570–3578 CrossRef PubMed.
- (a) D. A. Slanac, A. Lie, J. A. Paulson, K. J. Stevenson and K. P. Johnston, J. Phys. Chem. C, 2012, 116, 11032–11039 CrossRef; (b) P. Singh and D. A. Buttry, J. Phys. Chem. C, 2012, 116, 10656–10663 CrossRef.
- X. Liu and W. Hu, RSC Adv., 2016, 6, 29848–29854 RSC.
- (a) B. Men, Y. Sun, Y. Tang, L. Zhang, Y. Chen, P. Wan and J. Pan, Ind. Eng. Chem. Res., 2015, 54, 7415–7422 CrossRef; (b) E. J. Lim, S. M. Choi, M. H. Seo, Y. Kim, S. Lee and W. B. Kim, Electrochem. Commun., 2013, 28, 100–103 CrossRef.
- Y. Zhang, H.-L. Ma, Q. Zhang, J. Peng, J. Li, M. Zhai and Z.-Z. Yu, J. Mater. Chem., 2012, 22, 13064 RSC.
- X. Li, Z. Wang, Z. Zhang, L. Chen, J. Cheng, W. Ni, B. Wang and E. Xie, Sci. Rep., 2015, 5, 9130 CrossRef PubMed.
- N. F. Adegboyega, V. K. Sharma, K. Siskova, R. Zboril, M. Sohn, B. J. Schultz and S. Banerjee, Environ. Sci. Technol., 2013, 47, 757–764 CrossRef PubMed.
- Q. He, F. Zhou, S. Zhan, N. Huang and Y. Tian, Appl. Surf. Sci., 2018, 430, 325–334 CrossRef.
- D. Tsukamoto, A. Shiro, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, ACS Catal., 2012, 2, 599–603 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and figures. See DOI: 10.1039/c8cc03136c |
| This journal is © The Royal Society of Chemistry 2018 |