Benzothiazole- and benzoxazole-linked porous polymers for carbon dioxide storage and separation†
Mohammad Gulam
Rabbani
*a,
Timur
Islamoglu
b and
Hani M.
El-Kaderi
*b
aDepartment of Chemistry, University of Wisconsin-Platteville, 1 University Plaza, Platteville, Wisconsin 53818-3099, USA. E-mail: rabbanim@uwplatt.edu; Fax: +1 608 342 1559; Tel: +1 608 342 7344
bDepartment of Chemistry, Virginia Commonwealth University, Richmond, VA 23284-2006, USA. E-mail: helkaderi@vcu.edu; Fax: +1 804 828 8599; Tel: +1 804828 7505
First published on 14th November 2016
Abstract
Incorporation of CO2-philic heteroatoms (i.e. N, S, and O) into porous organic polymers has been instrumental in achieving selective CO2 capture. Here, we report the synthesis of porous benzothiazole and benzoxazole linked polymers which have sulfur and oxygen atoms, respectively, in addition to the nitrogen functionality. Their structural properties have been analyzed and compared to their analogous benzimidazole linked polymers which have only nitrogen heteroatoms. The polymers exhibit high surface areas (SABET = 698–1011 m2 g−1), high physicochemical stability, and considerable CO2 storage capacity. Low pressure gas uptake experiments were used to calculate the binding affinity of small gas molecules and revealed that the polymers have high heats of adsorption (Qst) for CO2 (28.7–33.6 kJ mol−1). Comparison of CO2 uptakes and Qst values of benzothiazole-, benzoxazole- and benzimidazole-linked polymers demonstrated that smaller pores facilitate CO2 adsorption with higher Qst values and the total CO2 uptake capacity mainly depends on the surface areas provided that the pore sizes are significantly small in lower micropore regions. The reported polymers also show moderate to high adsorption selectivity for CO2/N2 (40–78) and CO2/CH4 (5.7–7.8) as determined from the Ideal Adsorbed Solution Theory (IAST) calculation using pure gas isotherms at 298 K.
Introduction
The design and synthesis of porous organic architectures has received considerable attention in recent years because of their potential in catalysis,1–3 sensing,4–7 optoelectronics,8–10 and gas storage and separation.11–17 Porous adsorbents have been suggested as alternative CO2 capturing media to aqueous amine solutions, which suffer from several shortcomings such as toxicity, volatility, decomposition and most importantly considerable energy penalty during regeneration processes. Accordingly, several crystalline and amorphous organic polymers having nitrogen heterogeneity in their porous architectures were synthesized and their superior performance in gas storage and separation applications was demonstrated.11–13 Diverse synthetic routes have been developed to construct porous organic polymers featuring specific pore functionality and adjustable pore metrics to enable a wide range of applications.18–22 For example, incorporation of nitrogen atoms has been one of the most effective approaches for enhancing the adsorption properties of porous materials toward carbon dioxide (CO2) over other gases.23–29 Along this line, we recently reported a simple synthetic route to prepare highly porous benzimidazole-linked polymers (BILPs) by condensation reactions between aryl aldehyde and diamine containing building units.23,30–33 This template-free approach was very effective in attaining highly porous polymers with remarkable CO2 storage capacity and high selectivity levels over N2 and CH4 typically found in flue gas and methane rich gas mixtures. According to Density Functional Theory (DFT) calculations, we found that CO2 interacts most strongly with the Lewis basic nitrogen sites (N-imine) while the protonated nitrogen sites are not effective for CO2 capture.34 This is because of the delocalization of the lone pairs of sp3 hybridized nitrogen in aromatic systems which makes the nitrogen much less basic compared to the sp2 hybridized one in the imidazole. The outcome of DFT studies on CO2-BILP interactions encouraged us to consider replacing the less basic imidazole sites (N–H) with other heteroatoms such as oxygen and sulfur to investigate its effect on CO2 capture performances (Fig. 1). Such structural and electronic changes are expected to increase the polarity of the pore surface and thereby increase the number of potential binding sites for CO2.Benzoxazole-based polymers have been widely investigated as processable materials for membrane fabrication and used in gas mixture separation.35,36 Although there are a few successful examples, studies on the synthesis of porous polymers containing benzothiazole and benzoxazole units still remain scarce.37–39 Recently, Yavuz and co-workers39 reported benzoxazole linked polymers synthesized via annealing of the intermediate polymeric material at 400 °C. Although they have reported surface areas as high as 600 m2 g−1, the surface area drops dramatically when the linkers are extended indicating the limitation of the synthetic route. Echegoyen and co-workers37 reported bifunctionalized POPs containing benzothiazole and imine moieties. Because of the imine linked framework, the polymers are subject to hydrolysis which limits their usage under industrial conditions. Very recently and while this manuscript was under revision, McGrier et al. published benzobisoxazole-linked two-dimensional COFs.40 However, the CO2 uptakes of these crystalline COFs drop with increasing surface areas and pore sizes, which poses a question whether the surface functionalities or pore sizes or both determine CO2 uptakes.
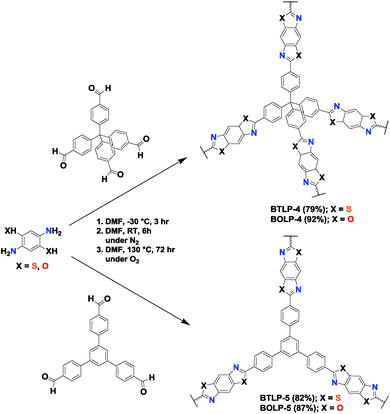
With these considerations in mind, we report here the synthesis of benzothiazole- and benzoxazole-linked polymers (BTLPs and BOLPs) via simple condensation reactions of aryl aldehydes with ortho-aminothiophenol and ortho-aminophenol, respectively. All BTLPs and BOLPs are microporous and have high porosity and physicochemical stability. The performance of the new polymers was investigated in CO2 storage and its separation from N2, and CH4. Their CO2 uptakes were compared to understand the effect of heteroatoms and the pore size distribution on CO2 adsorption.
Results and discussion
The synthesis of BTLPs and BOLPs was accomplished by the condensation reactions illustrated in Scheme 1. The proposed mechanism for the formation of the benzothiazole or benzoxazole linkages consists of two steps; the formation of an in situ aniline Schiff base that undergoes subsequent cyclo-dehydrogenation in the presence of molecular oxygen to afford the desired polymers. Four different polymers were constructed using 2D and 3D building units. A schematic representation of the synthesis of BTLP-4, BTLP-5, BOLP-4 and BOLP-5 is shown in Scheme 2. In a typical experiment for the preparation of BTLP-4, a homogeneous solution of 2,5-diamino-1,4-benzenedithiol dihydrochloride in dimethylformamide (DMF) was cooled to −30 °C, and then treated dropwise with tetrakis(4-formylphenyl)methane (TFPM) dissolved in DMF to afford a yellow suspension which is most likely imine-linked networks. The resultant suspension was bubbled with air for 10 minutes and then heated for three days at 130 °C to afford BTLP-4 as a yellow powder. Benzoxazole-linked polymers were synthesized under the same conditions using coupling reactions of 2,5-diaminohydroquinone dihydrochloride with 2D and 3D aryl aldehydes. Since the addition of aryl aldehydes to aminothiophenol or aminophenol resulted in immediate solid formation, slow addition of aldehyde and the use of low temperatures during the initial polymerization stages were maintained for optimizing the porosity of polymers. The first step is acid-catalyzed (HCl in this case) even at low temperature (−30 °C) whereas exposure to molecular oxygen and heating over an extended period of time are required for thiazole or oxazole ring formation. Both BTLPs and BOLPs were characterized by spectral and analytical methods, while porosity was investigated by argon sorption measurements. As expected, BTLPs and BOLPs are insoluble in common organic solvents and they remain intact upon washing with 2 M solutions of HCl or NaOH which reflects their high chemical stability. The thermal stability of the polymers was confirmed by TGA and both types of polymers remain stable up to ∼400 °C under N2 (Fig. S1, ESI†). The polymers are amorphous as evidenced by powder X-ray diffraction analysis (Fig. S2, ESI†). Because of their amorphous nature, it is hard to predict the actual structure of these polymers and we expect the networks to have randomly interpenetrated nets. It is noteworthy to mention that these five membered rings possess significantly high chemical stability. This irreversible nature of bond formation of these rings limits reorganizing the networks and makes it very difficult to obtain uniform topology. The physical morphology of the polymer particles was studied via SEM images (Fig. S3, ESI†). In the case of BTLPs, aggregation of spherical particles of around 300 nm in diameter was observed. On the other hand, BOLP-4 shows poorly defined aggregation of spheres of around 100 nm in diameter. The formation of the thiazole and oxazole based five membered rings was confirmed by ATR-IR and 13C cross-polarization with magic angle spinning (CP-MAS) NMR spectroscopic methods. The IR spectra (Fig. S4, ESI†) contain a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band at around 1620 cm−1. A new band appears at around 820 cm−1 for all BTLPs and BOLPs and this is assigned to C–S–C and C–O–C stretching bands. In the case of BOLPs, this band is slightly upshifted. The spectra also showed strongly attenuated C
N stretching band at around 1620 cm−1. A new band appears at around 820 cm−1 for all BTLPs and BOLPs and this is assigned to C–S–C and C–O–C stretching bands. In the case of BOLPs, this band is slightly upshifted. The spectra also showed strongly attenuated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches that are present in the spectra of aldehyde monomers. The 13C CP-MAS NMR spectra contain a signal around 162 ppm that corresponds to NC(Ph)X in the benzothiazole or benzoxazole units. A sharp difference was observed for benzene C peaks at 115 and 101 ppm for benzothiazole and benzoxazole units, respectively. Additional signals in the aromatic range arise from the phenyl rings of the building units (Fig. S5, ESI†).
O stretches that are present in the spectra of aldehyde monomers. The 13C CP-MAS NMR spectra contain a signal around 162 ppm that corresponds to NC(Ph)X in the benzothiazole or benzoxazole units. A sharp difference was observed for benzene C peaks at 115 and 101 ppm for benzothiazole and benzoxazole units, respectively. Additional signals in the aromatic range arise from the phenyl rings of the building units (Fig. S5, ESI†).
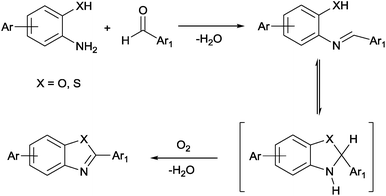 | ||
| Scheme 1 Proposed mechanism for the formation of benzothiazole and benzoxazole rings via condensation reactions. | ||
The porosity of the polymers was examined by argon sorption–desorption measurements at 87 K. The polymers were degassed at 120 °C and 1.0 × 10−5 bar for 12 h to remove any remaining guest molecules before porosity measurements. The Ar isotherms depicted in Fig. 2 show a rapid Ar uptake at very low pressure indicating a predominant microporous nature followed by a gradual increase in Ar uptake which is most likely due to the presence of large micropores and mesopores. The increase in uptake at a relative pressure above P/Po = 0.9 might be due to Ar condensation in interparticular voids while the hysteresis is consistent with the flexible nature of the polymers.41 It is worth noting that a drastic Ar condensation in interparticular voids is observed in BOLPs which have a smaller particle size compared to BTLPs. Applying the Brunauer–Emmett–Teller (BET) model within the pressure range of P/Po = 0.05–0.10 resulted in SABET = 698–1011 m2 g−1 (Table S1†). These values are comparable to the reported surface areas of benzimidazole linked polymers (599–1497 m2 g−1),23,42–44 polymers of intrinsic microporosity (PIMs)45 and azo-linked polymers (ALPs).46,47 Since both types of polymers are amorphous, it is hard to predict the extent of crosslinking and its impact on the pore size distribution (PSD). Density functional theory (DFT) calculation using experimental adsorption isotherms is an excellent means to predict the PSD of such amorphous polymers. The pore size distribution (PSD) of the synthesized BTLPs and BOLPs was estimated from the adsorption branch of Ar isotherms by nonlocal density functional theory (NLDFT). PSD studies indicated the existence of three major regions that peak at ca. 8, 10 and 16 Å in addition to some broad distributions in the mesoporous range for BTLPs which is most likely due to interparticular voids which act like large sized pores. Similar PSDs were observed for BOLPs with two major regions that peak at ca. 8 and 10 Å. Pore volumes were calculated from single point measurements at P/Po = 0.80 and found to be 0.41–0.53 cm3 g−1 as tabulated in Table 1.
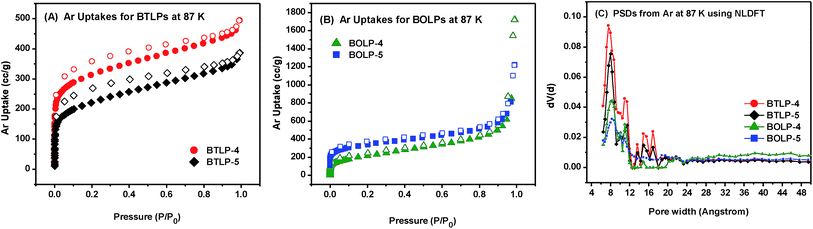 | ||
| Fig. 2 Argon uptake isotherms at 87 K. (A) BTLP-4 and BTLP-5. (B) BOLP-4 and BOLP-5, adsorption (filled) and desorption (empty). (C) Pore size distribution (PSD) of BTLPs and BOLPs. | ||
| Polymer | Porosity | CO2 at 1 bard | Selectivitye | |||||
|---|---|---|---|---|---|---|---|---|
| SABETa | PSDb (Å) | PVc | 273 K | 298 K | Q st | CO2/N2 | CO2/CH4 | |
| a SA (BET) specific surface area (m2 g−1) was calculated from the Ar 87 K isotherm. b Pore size distribution (PSD) was calculated using the zeolites/silica (spher./cylindr. pores, NLDFT ads.) model. c Pore volume at P/Po = 0.80. d Gas uptake in mg g−1 and the isosteric enthalpies of adsorption (Qst) in kJ mol−1. e Selectivity (mol mol−1) was calculated from initial slope calculations at 273 K and 298 K. f From previously published BILPs.23 g Surface areas were estimated from N2 isotherms at 77 K; CO2 uptake was estimated at 273 and 295 K at 1.2 bar, and selectivity was calculated at 273 and 295 K from the initial slope.40 | ||||||||
| BTLP-4 | 1011 | 7.55 | 0.53 | 190 | 119 | 28.7 | 41 (32) | 6.9 (5.7) |
| BTLP-5 | 705 | 7.93 | 0.41 | 139 | 87 | 29.1 | 45 (42) | 6.9 (5.7) |
| BOLP-4 | 698 | 8.68 | 0.54 | 136 | 87 | 33.6 | 55 (55) | 9.4 (6.9) |
| BOLP-5 | 759 | 8.30 | 0.52 | 129 | 79 | 32.9 | 54 (65) | 8.8 (6.6) |
| BILP-4f | 1135 | 6.8 | 0.65 | 235 | 158 | 28.7 | 79 (32) | 10 (7) |
| BILP-5f | 599 | 6.8 | 0.36 | 128 | 87 | 28.8 | 95 (36) | 10 (6) |
| BBO-COF-1g | 891 | 13.4 | 0.42 | 151 | 92 | 30.2 | 36 (35) | — |
| BBO-COF-2g | 1106 | 18.4 | 0.55 | 112 | 70 | 27.8 | 31 (31) | — |
In order to achieve low cost adsorbent regeneration without applying heat, only physisorption interactions between the gas molecules and the adsorbent are needed. For small gas storage and selective uptake in porous organic solids, engineering the chemical and physical nature of the pores is required along with appreciable porosity to attain appreciable storage and separation levels under ambient conditions. Such requirements have been recognized by both theoretical and experimental methods which indicated that only moderate porosity, very narrow pores, and chemical functionalities within the pores i.e. N, S, O, F, etc. are needed for selective CO2 binding and separation from N2 and CH4.48 On the other hand, alkyl amine functionalized POPs29,49–51 and MOFs52 lead to chemisorption interactions between CO2 and the adsorbent which require heat to regenerate the material. Therefore, to investigate the impact of microporosity and the heteroatom-rich pore walls of BTLPs and BOLPs on the uptake and selective binding of CO2, we collected CO2, N2 and CH4 isotherms and calculated their respective isosteric heats of adsorption (Qst). The adsorptive capacity at ambient pressure and their respective binding affinities were established according to the virial method and the results are summarized in Table 1.
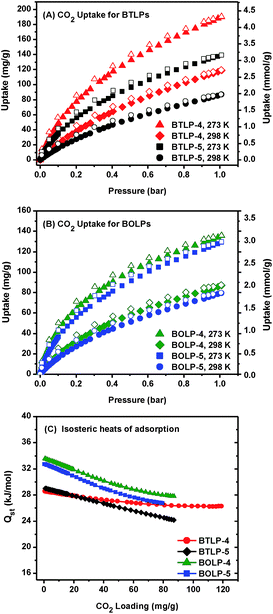
The CO2 isotherms are fully reversible and exhibit a steep rise at low pressures (Fig. 3A and B). The highest CO2 uptake was observed for BTLP-4 which was 190 mg g−1 at 273 K and 1 bar. BOLP-4 has a slightly lower uptake of 136 mg g−1 at 273 K and 1 bar. For comparison, these uptakes are much higher than the reported values of azo-COPs (85–112 mg g−1 at 273 K and 54–67 mg g−1 at 298 K)25 and are comparable to those of other porous organic polymers in the literature.53–56 The Qst for CO2 was calculated from the isotherms collected at 273 K and 298 K by using the virial method (Fig. S8 and S9, ESI†). At zero coverage, the Qst values were found to be ∼29 kJ mol−1 for BTLPs and 33 kJ mol−1 for BOLPs (Fig. 3C). These values are slightly lower than those of the best performing benzimidazole linked polymer BILP-10 (38), but are higher than those of most of the POPs in the literature.57–61 As summarized in Table 1, the Qst values of BTLPs are similar to those of BILP analogues. On the other hand, the Qst value of BOLPs is slightly higher than those of BTLP and BILP analogues. In order to interpret these results for the three analogous polymers, it is necessary to consider both atomic heterogeneity and the pore size distribution. Based on the pKa values of the conjugate acids of benzimidazole (5.5),62 benzothiazole (1.2),63 and benzoxazole (−0.13)63 moieties, it is expected that Qst for acidic CO2 should follow the order of Qst(BILP) > Qst(BTLP) > Qst(BOLP). Experimental Qst values are not fully consistent with this trend, although they are closely spaced. Slightly higher Qst for BOLPs can be explained by considering the PSD effect. BOLPs possess PSDs that peak mainly around 8 Å, while BTLPs and BILPs possess additional PSD in higher regions around 16 Å (Fig. 2C and ref. 23). This suggests that smaller pores in BOLPs facilitate the stabilization of adsorbed CO2 molecules in parallel to surface functionality. This is probably through the multiwall interactions to the adsorbed gas molecules. This idea is further supported by the recently reported crystalline BBO-COF-2 which is made from the same building units of our BOLP-5.40 The lower Qst (27.8 kJ mol−1) of BBO-COF-2 is due to the presence of a relatively larger pore size distribution (18.4 Å). The same work also reported structurally similar crystalline BBO-COF-1 which has a higher Qst (30.2 kJ mol−1) and is consistent with its smaller PSD (13.4 Å).40 In addition to PSD, the higher electronegativity of oxygen in BOLPs compared to that of nitrogen and sulphur in BILPs and BTLPs provides higher polarity to the frames which enhances the CO2 adsorption through higher dipole–quadruple interactions.
It is interesting to quantify how these surface functionalities and the PSD affect CO2 uptake. For better comparison, CO2 uptakes at 273 K and 1 bar for all polymers listed in Table 1 are plotted as a function of surface area (Fig. S10, ESI†). Interestingly, CO2 uptakes vary almost linearly with surface areas for those polymers having dominant PSD in lower micropore regions. On the other hand, BBO-COF-2 which has a larger PSD in the upper micropore regions (18.4 Å) significantly deviates from the linearity. BBO-COF-1 with a PSD of 13.4 Å also deviates but to a smaller extent. These findings emphasize that PSD plays the key role in the CO2 sorption of these heteroatom-functionalized polymers. It is also demonstrated that total CO2 uptake capacity mainly depends on the surface area provided that PSD falls within the lower micropore region.
We also collected CH4 uptakes at 273 K and 298 K up to 1 bar (Fig. S11 and S12, ESI†). Isotherms are completely reversible and exhibit maximum uptakes of 14.0–21.5 mg g−1 at 273 K and 1 bar. The Qst for CH4 was calculated from adsorption data collected at 273 K and 298 K from the virial method and found to be 22–26 kJ mol−1 at zero coverage.
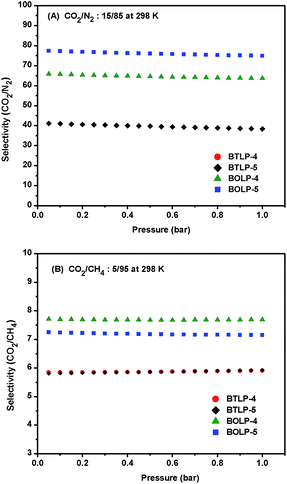
Given the remarkable physicochemical stability of BTLPs and BOLPs and their ability to bind CO2 with moderate affinities, we examined their CO2/N2 selectivity from single-component gas isotherms using the initial slope method (Fig. S13 and S14, ESI†), and these are summarized in Table 1. The highest selectivity was observed for BOLP-5 (65 at 298 K) which is consistent with the fact that O decorated and smaller pores are less favourable for nitrogen adsorption. The initial-slope calculation method was also applied to estimate the CO2/CH4 selectivity at 273 K and 298 K and was found to be in the range of ∼5.7 to 9.4. CH4 has higher adsorption potential than N2 at the abovementioned temperatures and as a result, CO2/CH4 selectivity values are much lower than those recorded for CO2/N2. We also estimated the CO2 adsorption selectivity for the flue gas composition (CO2/N2 = 15/85) and natural gas composition (CO2/CH4 = 5/95) using Ideal Adsorbed Solution Theory (IAST) at 298 K as shown in Fig. 4. The IAST results indicate that BOLP-5 has the highest selectivity (78) for CO2/N2. The selectivity values from IAST calculations are slightly higher than the values obtained from the initial slope calculations. This is probably because IAST calculation counts the selectivity for a gas mixture of a certain composition ratio using two pure single component adsorption isotherms while the initial slope calculation only counts the low-pressure data of pure component adsorption isotherms. IAST also provides the selectivity over the entire pressure range which is much more comprehensive than the initial slope method.
Experimental
Materials and methods
All chemicals were purchased from commercial suppliers (Sigma-Aldrich, Acros Organics, or Frontier Scientific) and used without further purification, unless otherwise noted. Tetrakis(4-formylphenyl)methane (TFPM) and 1,3,5-tris-(4-formylphenyl)benzene (TFPB) were synthesized according to published methods.1,2 Solution 1H and 13C NMR spectra were obtained on a Varian Mercury-300 MHz NMR spectrometer (75 MHz carbon frequency). 13C cross-polarization magic angle spinning (CP-MAS) NMR spectra for solid samples were taken at Spectral Data Services, Inc. Spectra were obtained with the samples on a Tecmag-based NMR spectrometer, operating at a H-1 frequency of 363 MHz, using a contact time of 1 ms and a delay of three seconds for the CPMAS experiment; the samples were spun at 7.0 kHz. Thermogravimetric analysis (TGA) was carried out using a TA Instruments Q-5000IR series thermal gravimetric analyzer with the samples held in 50 μL platinum pans under an atmosphere of N2 (heating rate 5 °C min−1). For Scanning Electron Microscopy Imaging (SEM), the sample was prepared by dispersing the material onto a sticky carbon surface attached to a flat aluminum sample holder. The sample was then coated with platinum at a pressure of 1 × 10−5 mbar in a nitrogen atmosphere for 90 seconds before imaging. Images were taken on a Hitachi SU-70 Scanning Electron Microscope. Powder X-ray diffraction data were collected on a Panalytical X'pert pro multipurpose diffractometer (MPD). The samples were mounted on a sample holder and measured using Cu Kα radiation with a 2θ range of 1.5–35. FT-IR spectra were obtained on a Nicolet-Nexus 670 spectrometer furnished with an attenuated total reflectance accessory. Porosity and gas sorption experiments were performed using a Quantachrome Autosorb 1-C volumetric analyser using adsorbates of UHP grade. In a typical experiment, a sample was loaded into a 9 mm large bulb cell (Quantachrome) of known weight and then hooked up to the Autosorb 1-C and degassed at 120 °C for 12 h. The degassed sample was refilled with nitrogen, weighed precisely and then transferred back to the analyser. The temperatures for adsorption measurements were controlled by using a refrigerated bath of liquid nitrogen (77 K) or liquid argon (87 K) or a temperature controlled water bath (273 K and 298 K). Hydrogen isotherms were collected at 77 K and 87 K. Carbon dioxide and methane isotherms were collected at 273 and 298 K. Pore Size Distribution (PSD) was calculated using a spherical/cylindrical pore (zeolite) NLDFT equilibrium model from the Ar isotherm.Synthesis of BTLP-4
A 250 mL Schlenk flask was charged with 160 mg (0.65 mmol) of 2,5-diamino-1,4-benzenedithiol dihydrochloride and 60 mL of anhydrous DMF. The solution was cooled to ca. −30 °C and a solution of TFPM (141 mg, 0.33 mmol) in anhydrous DMF (30 mL) was added drop-wise. The temperature was maintained around −30 °C until the formation of a light yellow solid product was completed and then allowed to rise to room temperature and maintained overnight. The flask containing the reaction mixture of light yellow solids was flushed with air for 10 minutes and capped. The reaction mixture was then heated in an oven at 130 °C for 3 days to afford a fluffy yellow polymer which was isolated by filtration over a glass frit and subsequently washed with DMF, acetone, water, 2.0 M HCl, 2.0 M NaOH, water, and acetone in succession. During acid and base treatments, the polymers were soaked for around 5 minutes and filtered and this process was repeated at least 3 times. The product was then immersed in acetone/CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for one day, during which the activation solvent was decanted and freshly replenished twice. After filtration, the product was dried at 120 °C under vacuum to give BTLP-4 (227 mg, yield 79%) as a bright yellow fluffy solid. Anal. calcd for C41H20N4S4·4H2O: C, 65.27%; H, 3.16%; N, 8.35%; S, 16.45%. Found: C, 64.04%; H, 3.67%; N, 7.29%; S, 16.68%.
1, v/v) for one day, during which the activation solvent was decanted and freshly replenished twice. After filtration, the product was dried at 120 °C under vacuum to give BTLP-4 (227 mg, yield 79%) as a bright yellow fluffy solid. Anal. calcd for C41H20N4S4·4H2O: C, 65.27%; H, 3.16%; N, 8.35%; S, 16.45%. Found: C, 64.04%; H, 3.67%; N, 7.29%; S, 16.68%.
Synthesis of BTLP-5
This polymer was synthesized following the methods mentioned above for BTLP-4 using 2,5-diamino-1,4-benzenedithiol dihydrochloride (160 mg, 0.65 mmol) and 1,3,5-(4-formylphenyl)benzene (170 mg, 0.44 mmol). After drying, the final product BTLP-5 was obtained as a yellow fluffy solid (210 mg, 82% yield). Anal. calcd for C72H36N6S6·6H2O: C, 70.56%; H, 3.65%; N, 6.91%; S, 11.83%. Found: C, 67.27%; H, 3.76%; N, 6.54%; S, 14.97%.Synthesis of BOLP-4
This polymer was synthesized following the methods mentioned above for BTLP-4 using 2,5-diaminohydroquinone dihydrochloride (150 mg, 0.70 mmol) and TFPM (152 mg, 0.35 mmol). After drying, the final product BOLP-4 was obtained as an ash colored fluffy solid (205 mg, 92% yield). Anal. calcd for C41H20N4O4·3H2O: C, 70.38%; H, 4.38%; N, 8.71%; O, 16.28%. Found: C, 71.71%; H, 3.82%; N, 8.16%; O, 16.31%.Synthesis of BOLP-5
This polymer was synthesized following the methods mentioned above for BTLP-4 using 2,5-diaminohydroquinone dihydrochloride (150 mg, 0.70 mmol) and TFPB (184 mg, 0.47 mmol). After drying, the final product BOLP-4 was obtained as an ash colored fluffy solid (220 mg, 87% yield). Anal. calcd for C72H36N6O6·5H2O: C, 73.63%; H, 4.07%; N, 7.43%; O, 14.97%. Found: C, 73.84%; H, 3.96%; N, 7.18%; O, 15.03%.Conclusions
In conclusion, we have reported the synthesis of highly porous benzothiazole and benzoxazole linked polymers which are structurally analogous to benzimidazole-linked polymers. These polymers exhibit significant CO2 adsorption capacity![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) high CO2 adsorption selectivity over N2 and CH4. Systematic CO2 adsorption studies demonstrated that smaller pores facilitate CO2 adsorption with higher Qst while the total CO2 uptake varies linearly with surface area provided that the pore size falls within the lower micropore region. The synthesis of BTLPs and BOLPs, thus, provides numerous benefits to the scientific community: (a) tuning the pore sizes in a lower micropore region among BTLPs, BOLPs and BILPs, which demonstrates that the pore size plays key roles in selective adsorption of CO2, (b) establishing a common synthetic procedure to prepare varieties of heteroatom-functionalized porous polymers which could be used in many applications beyond gas storage and gas separation, and (c) inclusion of S and O in porous architectures is very attractive in preparation of heterogeneous catalysts.
high CO2 adsorption selectivity over N2 and CH4. Systematic CO2 adsorption studies demonstrated that smaller pores facilitate CO2 adsorption with higher Qst while the total CO2 uptake varies linearly with surface area provided that the pore size falls within the lower micropore region. The synthesis of BTLPs and BOLPs, thus, provides numerous benefits to the scientific community: (a) tuning the pore sizes in a lower micropore region among BTLPs, BOLPs and BILPs, which demonstrates that the pore size plays key roles in selective adsorption of CO2, (b) establishing a common synthetic procedure to prepare varieties of heteroatom-functionalized porous polymers which could be used in many applications beyond gas storage and gas separation, and (c) inclusion of S and O in porous architectures is very attractive in preparation of heterogeneous catalysts.
Acknowledgements
This research was supported by Virginia Commonwealth University. Gas uptake studies were partially supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award Number DE-SC0002576. M. G. R. thanks the University of Wisconsin–Platteville for startup funds.Notes and references
- P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819–835 CrossRef CAS.
- Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC.
- Q. Sun, Z. Dai, X. Meng, L. Wang and F.-S. Xiao, ACS Catal., 2015, 5, 4556–4567 CrossRef CAS.
- X. Liu, Y. Xu and D. Jiang, J. Am. Chem. Soc., 2012, 134, 8738–8741 CrossRef CAS PubMed.
- N. A. Rakow, M. S. Wendland, J. E. Trend, R. J. Poirier, D. M. Paolucci, S. P. Maki, C. S. Lyons and M. J. Swierczek, Langmuir, 2010, 26, 3767–3770 CrossRef CAS PubMed.
- Y. Yuan, H. Ren, F. Sun, X. Jing, K. Cai, X. Zhao, Y. Wang, Y. Wei and G. Zhu, J. Mater. Chem., 2012, 22, 24558–24562 RSC.
- Y. Yuan, H. Ren, F. Sun, X. Jing, K. Cai, X. Zhao, Y. Wang, Y. Wei and G. Zhu, J. Phys. Chem. C, 2012, 116, 26431–26435 CAS.
- J.-X. Jiang, A. Trewin, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1777–1781 RSC.
- S. Wan, F. Gándara, A. Asano, H. Furukawa, A. Saeki, S. K. Dey, L. Liao, M. W. Ambrogio, Y. Y. Botros, X. Duan, S. Seki, J. F. Stoddart and O. M. Yaghi, Chem. Mater., 2011, 23, 4094–4097 CrossRef CAS.
- Y. Xu, L. Chen, Z. Guo, A. Nagai and D. Jiang, J. Am. Chem. Soc., 2011, 133, 17622–17625 CrossRef CAS PubMed.
- Z. Chang, D.-S. Zhang, Q. Chen and X.-H. Bu, Phys. Chem. Chem. Phys., 2013, 15, 5430–5442 RSC.
- R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS.
- R. Dawson, A. I. Cooper and D. J. Adams, Polym. Int., 2013, 62, 345–352 CrossRef CAS.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 988 CrossRef CAS PubMed.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- M. G. Rabbani, A. K. Sekizkardes, Z. Kahveci, T. E. Reich, R. Ding and H. M. El-Kaderi, Chem.–Eur. J., 2013, 19, 3324–3328 CrossRef CAS PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS PubMed.
- Z. Xiang and D. Cao, J. Mater. Chem. A, 2013, 1, 2691–2718 CAS.
- X. Zou, H. Ren and G. Zhu, Chem. Commun., 2013, 49, 3925–3936 RSC.
- K. T. Jackson, M. G. Rabbani, T. E. Reich and H. M. El-Kaderi, Polym. Chem., 2011, 2, 2775–2777 RSC.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2012, 24, 1511–1517 CrossRef CAS.
- P. Arab, M. G. Rabbani, A. K. Sekizkardes, T. Islamoglu and H. M. El-Kaderi, Chem. Mater., 2014, 26, 1385–1392 CrossRef CAS.
- H. A. Patel, S. H. Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun., 2013, 4, 1357 CrossRef PubMed.
- P. Pandey, A. P. Katsoulidis, I. Eryazici, Y. Wu, M. G. Kanatzidis and S. T. Nguyen, Chem. Mater., 2010, 22, 4974–4979 CrossRef CAS.
- A. K. Sekizkardes, S. Altarawneh, Z. Kahveci, T. Islamoglu and H. M. El-Kaderi, Macromolecules, 2014, 47, 8328–8334 CrossRef CAS.
- T. Islamoglu, T. Kim, Z. Kahveci, O. M. El-Kadri and H. M. El-Kaderi, J. Phys. Chem. C, 2016, 120, 2592–2599 CAS.
- W. G. Lu, J. P. Sculley, D. Q. Yuan, R. Krishna, Z. W. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 7480–7484 CrossRef CAS PubMed.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2011, 23, 1650–1653 CrossRef CAS.
- M. G. Rabbani, T. E. Reich, R. M. Kassab, K. T. Jackson and H. M. El-Kaderi, Chem. Commun., 2012, 48, 1141–1143 RSC.
- M. G. Rabbani, A. K. Sekizkardes, O. M. El-Kadri, B. R. Kaafarani and H. M. El-Kaderi, J. Mater. Chem., 2012, 22, 25409–25417 RSC.
- S. Altarawneh, L. Nahar, I. U. Arachchige, A. a. O. El-Ballouli, K. M. Hallal, B. R. Kaafarani, M. G. Rabbani, R. K. Arvapally and H. M. El-Kaderi, J. Mater. Chem. A, 2015, 3, 3006–3010 CAS.
- S. Altarawneh, S. Behera, P. Jena and H. M. El-Kaderi, Chem. Commun., 2014, 50, 3571–3574 RSC.
- M. Calle and Y. M. Lee, Macromolecules, 2011, 44, 1156–1165 CrossRef CAS.
- C. H. Jung, J. E. Lee, S. H. Han, H. B. Park and Y. M. Lee, J. Membr. Sci., 2010, 350, 301–309 CrossRef CAS.
- V. S. P. K. Neti, X. Wu, P. Peng, S. Deng and L. Echegoyen, RSC Adv., 2014, 4, 9669–9672 RSC.
- X. Zhu, C. Tian, T. Jin, J. Wang, S. M. Mahurin, W. Mei, Y. Xiong, J. Hu, X. Feng, H. Liu and S. Dai, Chem. Commun., 2014, 50, 15055–15058 RSC.
- H. A. Patel, D. Ko and C. T. Yavuz, Chem. Mater., 2014, 26, 6729–6733 CrossRef CAS.
- D. A. Pyles, J. W. Crowe, L. A. Baldwin and P. L. McGrier, ACS Macro Lett., 2016, 5, 1055–1058 CrossRef CAS.
- J. T. Culp, M. R. Smith, E. Bittner and B. Bockrath, J. Am. Chem. Soc., 2008, 130, 12427–12434 CrossRef CAS PubMed.
- A. K. Sekizkardes, T. Islamoglu, Z. Kahveci and H. M. El-Kaderi, J. Mater. Chem. A, 2014, 2, 12492–12500 CAS.
- S. Altarawneh, T. Islamoglu, A. K. Sekizkardes and H. M. El-Kaderi, Environ. Sci. Technol., 2015, 49, 4715–4723 CrossRef CAS PubMed.
- T. Islamoglu, S. Behera, Z. Kahveci, T.-D. Tessema, P. Jena and H. M. El-Kaderi, ACS Appl. Mater. Interfaces, 2016, 8, 14648–14655 CAS.
- N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS.
- P. Arab, E. Parrish, T. Islamoglu and H. M. El-Kaderi, J. Mater. Chem. A, 2015, 3, 20586–20594 CAS.
- H. A. Patel, S. H. Je, J. Park, Y. Jung, A. Coskun and C. T. Yavuz, Chem.–Eur. J., 2014, 20, 772–780 CrossRef CAS PubMed.
- C. E. Wilmer, O. K. Farha, Y. S. Bae, J. T. Hupp and R. Q. Snurr, Energy Environ. Sci., 2012, 5, 9849–9856 CAS.
- S. Sung and M. P. Suh, J. Mater. Chem. A, 2014, 2, 13245–13249 CAS.
- H. A. Patel and C. T. Yavuz, Faraday Discuss., 2015, 183, 401–412 RSC.
- S. J. Garibay, M. H. Weston, J. E. Mondloch, Y. J. Colon, O. K. Farha, J. T. Hupp and S. T. Nguyen, CrystEngComm, 2013, 15, 1515–1519 RSC.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocella, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- N. Popp, T. Homburg, N. Stock and J. Senker, J. Mater. Chem. A, 2015, 3, 18492–18504 CAS.
- C. Shen and Z. Wang, J. Phys. Chem. C, 2014, 118, 17585–17593 CAS.
- G. Li, B. Zhang and Z. Wang, Macromolecules, 2016, 49, 2575–2581 CrossRef CAS.
- S. Wu, Y. Liu, G. Yu, J. Guan, C. Pan, Y. Du, X. Xiong and Z. Wang, Macromolecules, 2014, 47, 2875–2882 CrossRef CAS.
- G. Y. Li, B. Zhang, J. Yan and Z. G. Wang, J. Mater. Chem. A, 2014, 2, 18881–18888 CAS.
- O. Buyukcakir, S. H. Je, D. S. Choi, S. N. Talapaneni, Y. Seo, Y. Jung, K. Polychronopoulou and A. Coskun, Chem. Commun., 2016, 52, 934–937 RSC.
- T. Ratvijitvech, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Polymer, 2014, 55, 321–325 CrossRef CAS.
- R. Muhammad, P. Rekha and P. Mohanty, Greenhouse Gases: Sci. Technol., 2016, 6, 150–157 CrossRef CAS.
- T. Islamoglu, M. G. Rabbani and H. M. El-Kaderi, J. Mater. Chem. A, 2013, 1, 10259–10266 CAS.
- H. Walba and R. W. Isensee, J. Org. Chem., 1961, 26, 2789–2791 CrossRef CAS.
- J. A. Zoltewicz and L. W. Deady, in Advances in Heterocyclic Chemistry, ed. A. R. Katritzky and A. J. Boulton, Academic Press, 1978, vol. 22, pp. 71–121 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ta06342j |
| This journal is © The Royal Society of Chemistry 2017 |