A metal–organic framework functionalized with piperazine exhibiting enhanced CH4 storage†
Mingxing
Zhang
*ab,
Cong
Chen
b,
Qian
Wang
b,
Wensheng
Fu
a,
Kunlin
Huang
*a and
Wei
Zhou
*c
aDepartment of Chemistry, Chongqing Normal University, Chongqing, 401331, China. E-mail: zmx0102@hotmail.com; kunlin@cqnu.edu.cn
bState Key Laboratory of Coordination Chemistry, Collaborative Innovation Center of Advanced Microstructures, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
cNIST Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-6102, USA. E-mail: wzhou@nist.gov
First published on 21st November 2016
Abstract
NJU-Bai 19, the first cycloaliphatic ring (piperazine) functionalized MOF-505 analogue, exhibits a notably high methane storage capacity of 246.4 cm3 (STP) cm−3 (at room temperature and 65 bar); this is 93.6% of the new volumetric target of the US Department of Energy if the packing density loss is ignored. Compared with NOTT-101, when piperazine groups are inserted, the methane uptake capacity of NJU-Bai 19 at 65 bar and RT can be significantly enhanced with lower Qst. A relatively low increase was also observed in the uptake at 5 bar. Thus it has a much higher methane storage working capacity (deliverable amount of methane between 65 and 5 bar) of 185 cm3 (STP) cm−3 compared with NOTT-101 (174 cm3 (STP) cm−3). When the temperature was decreased from 298 to 273 K at working pressures between 65 bar and 5 bar, the volumetric CH4 working capacity of NJU-Bai 19 is almost unchanged (from 185 to 189 cm3 (STP) cm−3), while some of the famous MOFs with high Qst showed a decrease with a decrease in temperature. The combination of the balanced porosity and framework density and the lipophilic surface is considered to result in the increased methane storage and working capacities.
Introduction
Natural gas, as a naturally abundant fuel, consisting of nearly 95% CH4, has long been considered as a possible alternative to conventional gasoline and diesel because of its economic and environmental advantages.1 However, for future wide utilization of CH4, safe and efficient storage and transportation is still a great challenge due to its low volumetric energy density (only 0.1% of gasoline) at room temperature.2 Adsorbed natural gas (ANG) in porous solid materials has a significant advantage which is that the gas is stored as an adsorbed phase in a porous solid achieves a density competitive with that of compressed natural gas (CNG) at a lower pressure and room temperature, leading to safe and efficient storage with less energy consumption.3Recently, metal–organic frameworks (MOFs) have appeared as a new class of promising porous materials for CH4 storage,4 which were initially investigated by Kitagawa and Yaghi.5 In the context of methane storage, there has been a growing interest in the investigation of MOFs with higher volumetric methane storage capacity and working capacity. For MOFs, the working capacity determines the driving range of vehicles powered by natural gas; thus it is more important than total CH4 storage capacity. In the past decade, many MOFs with good performance for CH4 storage have been reported. For example, HKUST-1 (ref. 6) and MAF-38 (ref. 7) with high isosteric heats of CH4 adsorption (Qst) exhibit high a volumetric methane uptake of 267 and 263 cm3 (STP) cm−3 at 298 K and 65 bar, respectively. Al-soc-MOF-1 (ref. 8) and MOF-905 (ref. 10) with low Qst show a high gravimetric uptake of 0.5 g g−1 (at 85 bar and 288 K) and a high volume working capacity of 203 cm3 (STP) cm−3 (between 80 and 5 bar), respectively. UTSA-76 (ref. 9) and Co(dbp)11 show a record high volume working capacity of 197 cm3 (STP) cm−3 (between 65 and 5 bar). In fact, BASF have realized model vehicles equipped with natural gas fuel systems using MOF materials.
The popular method for increasing total methane uptakes is to enhance the interaction between CH4 and frameworks, mainly presented as higher Qst. The representative example is Ni-MOF-74,6 whose volumetric methane uptake can reach 251 cm3 (STP) cm−3 (at 298 K and 65 bar) and the Qst is 20.6 kJ mol−1. However, this strategy shows no effect on enhancing the working capacity (only 129 cm3 (STP) cm−3 for Ni-MOF-74). Moreover, high Qst may add cost and complexity to manage the exothermic heat of adsorption and endothermic heat of desorption, both of which may result in large reductions of the working capacity.11,12 In this context, there is an urgent demand to develop MOFs not only with high working capacities but also with low Qst.4b
MOFs possess one significant and unique feature which is a finely tunable structure. By inserting carefully selected functional groups, many MOFs could be systematically investigated as platforms, targeting special properties.13 We are interested in tuning MOFs with novel functional groups, which play a key role in improving the performances of MOFs. Since our group's pioneering work on introducing C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C14 and amide15 functional groups into MOFs for gas storage study, MOFs based on these two functional groups have made significant progress in gas storage.16 This motivates us to introduce new functional groups into MOFs for improving CH4 storage capacities.
C14 and amide15 functional groups into MOFs for gas storage study, MOFs based on these two functional groups have made significant progress in gas storage.16 This motivates us to introduce new functional groups into MOFs for improving CH4 storage capacities.
As far as we know, research on current functional groups for CH4 storage mainly focuses on the aromatic rings and their derivatives,8,17 so there are few MOFs constructed with a cycloaliphatic backbone. Moreover, according to previous studies,9,17a,18 methyl, ethyl and propyl functional groups as well as N atoms in MOFs have a positive effect on methane storage,9,18 so we consider introducing aliphatic fragments and N atoms in the form of piperazine to take the place of the benzene ring in NOTT-101 and get the first cycloaliphatic ring functionalized MOF [Cu2(L)(DMF)2]·3DMF·7H2O (H4L, 5,5′-(piperazine-1,4-diyl)diiso-phthalic acid; NJU-Bai 19, NJU-Bai for Nanjing University Bai's group). As expected, compared with NOTT-101,9NJU-Bai 19 exhibits a higher CH4 uptake of 246.4 cm3 g−1 (at 298 K and 65 bar) and a working capacity of 185 cm3 (STP) cm−3.
Experimental
Materials
Commercially available reagents were used as received without further purification. Elemental analyses (C, H and N) were carried out on a Perkin-Elmer 240 analyzer. The IR spectra were obtained on a VECTOR TM 22 spectrometer with KBr pellets in the 4000–400 cm−1 region. 1H NMR spectra were recorded on a Bruker DRX-500 spectrometer with tetramethylsilane as an internal reference. Thermogravimetric analyses (TGA) were performed under a N2 atmosphere (100 mL min−1) with a heating rate of 5 °C min−1 using a 2960 SDT thermogravimetric analyzer. Powder X-ray diffraction (PXRD) data were collected over the 2θ range 5–45° on a Bruker AXS D8 Advance diffractometer using Cu Kα radiation (λ = 1.5418 Å) with a routine power of 1600 W (40 kV, 40 mA) at a scan speed of 0.1 deg s−1 at room temperature. A Micromeritics ASAP 2020 was used to measure gas adsorption isotherms. To remove all the guest solvents in the framework, the fresh sample of NJU-Bai 19 was guest-exchanged with dry acetone at least 9 times, filtered and degassed at room temperature for 1 day and then at 373 K for another 24 h until the outgas rate was 5 mmHg min−1 before the measurements were made. High-pressure CH4 sorption isotherms were measured using a Sieverts-type apparatus.19Synthesis of H4L
As shown in Scheme 1, the linker H4L was synthesized by a two-step reaction procedure. Into a dried 100 mL round bottom flask was added piperazine (10.0 mmol), dimethyl 5-iodoisophthalate (30 mol), CuI (1 mmol), metformin hydrochloride (2 mmol), and Cs2CO3 (30 mmol, 3 equiv.). Later on, dry EtOH (25 mL) was added. After the mixture was stirred and refluxed for 24 hours under a N2 atmosphere, the precipitate was collected by filtration, washed with water and EtOH and dried under vacuum at 353 K to give 1 as white solid. Yield: 74% (3.5 g). The crude 1 was suspended in 150 mL of THF/methanol mixed solvent, to which 100 mL 1 M LiOH aqueous solution was added. After this mixture was stirred at room temperature for 24 hours, the solvents were removed under vacuum, and dilute hydrochloric acid was added to the remaining aqueous solution until it became acidic (pH = 2). The precipitate was collected by filtration, washed with water and dried under vacuum at 353 K to give H4L as a white solid. IR (KBr, cm−1): 3345 (br, s), 3088 (br, s), 2874 (br, s), 1733 (s), 1673 (m), 1462 (s), 1377 (m), 1341 (m), 1286 (m), 1190 (m), 1090 (m). 1H NMR (DMSO-d6, δ ppm): 13.451 (broad peak, COOH), 7.95 (s, 2H, ArH), 7.74 (s, 4H, ArH), 3.43 (s, 8H, CH2H), anal. calcd (found) for H4L, C20H18N2O8: C, 57.97 (57.88); H, 4.38 (4.50); N, 6.76 (6.61)%. MS (ESI) m/z (M − H+)−: 414.11.Synthesis of NJU-Bai 19
H4L (10.0 mg) and Cu(NO3)2·3H2O (20 mg, 0.1 mmol) were dissolved in 2 mL solvent of DMF in a vessel, to which 20 drops HBF4 were added. The vessel was sealed and heated to 75 °C for 1 day and then cooled to room temperature at a rate of 5 °C per hour. Blue block crystals of NJU-Bai 19 were filtered and washed with DMF. Yield 11.2 mg. Selected IR (cm−1): 3418 (br, s), 1655 (m), 1585 (vs), 1449 (m), 1378 (m), 1243 (w), 1001 (m), 779 (m), 730 (m). Anal. calcd (found) for NJU-Bai 19, C35H63–Cu2N7O20: C, 40.85 (40.77); H, 6.17 (6.35); N, 9.51 (9.26)%; calcd (found) for activated NJU-Bai 19, C20H14Cu2N2O8: C, 44.70 (37.10); H, 2.63 (4.35); N, 5.21 (4.36)%.Single-crystal X-ray crystallography
Single-crystal X-ray diffraction data were measured on a Bruker Apex II CCD diffractometer using graphite monochromated Mo/Kα radiation (λ = 0.71073 Å). Data reduction was made with the Bruker SAINT program. The structures were solved by direct methods and refined with the full-matrix least squares technique using the SHELXTL package. Non-hydrogen atoms were refined with anisotropic displacement parameters during the final cycles. Organic hydrogen atoms were placed in calculated positions with isotropic displacement parameters set to 1.2 × Ueq of the attached atom. The unit cell includes a large region of disordered solvent molecules, which could not be modelled as discrete atomic sites. We employed PLATON/SQUEEZE to calculate the diffraction contribution of the solvent molecules and, therefore, to produce a set of solvent-free diffraction intensities; the structures were then refined again using the data generated. Crystal data are summarized in Table S1.†Results and discussion
Solvothermal reaction of Cu(NO3)2·3H2O with H4L in a solvent mixture of DMF, H2O and fluoroboric acid gave pale-blue block-shaped crystals of NJU-Bai 19 in high yield. Single-crystal X-ray diffraction analysis reveals that like other isoreticular NbO-type MOFs, NJU-Bai 19 is also constructed from binuclear Cu(II) paddlewheel nodes, further bridged by L4− to form a 3D (4,4)-connected net. Typically, there exist two types of cages which are alternately stacked in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the formation of a 3D framework. The spherical cage is about 9.7 Å in diameter and the large shuttle-shaped cage is about 9.3 × 22.2 Å. Viewing along the c axes, there exist triangular channels whose diameters are approximately 5 Å, while along the a or b axis, isosceles triangular windows can be observed, in which the size is controlled by the length of the spaces. Obviously, the chair conformations of piperazine project into the pore, narrowing the isosceles triangular windows. The chair conformations also make the ligand L4− slightly shorter than its counterpart in NOTT-101, which further contracts this window. Calculated from the PLATON program, NJU-Bai 19 has an accessible pore volume of 71.5% (7985 Å3 out of the 11
1 ratio with the formation of a 3D framework. The spherical cage is about 9.7 Å in diameter and the large shuttle-shaped cage is about 9.3 × 22.2 Å. Viewing along the c axes, there exist triangular channels whose diameters are approximately 5 Å, while along the a or b axis, isosceles triangular windows can be observed, in which the size is controlled by the length of the spaces. Obviously, the chair conformations of piperazine project into the pore, narrowing the isosceles triangular windows. The chair conformations also make the ligand L4− slightly shorter than its counterpart in NOTT-101, which further contracts this window. Calculated from the PLATON program, NJU-Bai 19 has an accessible pore volume of 71.5% (7985 Å3 out of the 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 Å3 per unit cell volume) (Fig. 1).
170 Å3 per unit cell volume) (Fig. 1).
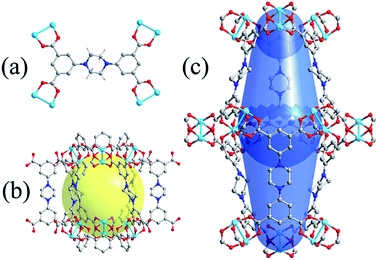 | ||
| Fig. 1 (a) Each ligand connected to four Cu(II) paddlewheel nodes; (b) the spherical-like cage of about 9.7 Å in diameter; (c) the large shuttle-shaped cage of about 9.3 × 22.2 Å. | ||
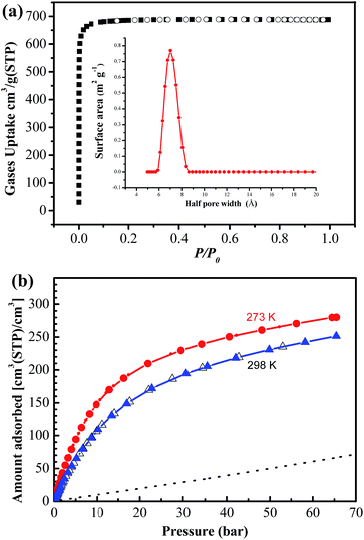
To confirm the permanent porosity of NJU-Bai 19, the acetone solvent-exchanged sample was degassed under high vacuum at 373 K for 24 hours to obtain the evacuated framework. As shown in Fig. 2a, the activated NJU-Bai 19 can take up 687 cm3 g−1 N2 at 77 K. The N2 isotherm measurements were repeated on several batches of samples using different apparatus and essentially identical results were obtained. The BET surface area and Langmuir surface area of NJU-Bai 19 are calculated to be around 2803 and 3007 m2 g−1, which are comparable to those of NOTT-101 (2805 and 3045 m2 g−1, respectively). On the basis of the maximum amount of N2 adsorbed, the calculated total pore volume is 1.063 cm3 g−1. A pore size distribution analysis by the non-local density functional theory (NLDFT) utilizing N2 gas at 77 K shows that NJU-Bai 19 exhibits a narrow distribution of micro-pores around 14 Å (Fig. 2a). Compared with NOTT-101, those values are a little lower due to the introduction of piperazine.
The unique pore functionalization and comparable porosity of NJU-Bai 19 to that of NOTT-101 prompted us to examine its methane storage capacities. Temperature-dependent high-pressure adsorption measurements were performed at the Center for Neutron Research, National Institute of Standards and Technology (NIST), using a computer-controlled Sieverts apparatus. The total volumetric methane sorption isotherms of NJU-Bai 19 at 273 K and 298 K are shown in Fig. 2. At 298 K and 35 bar, NJU-Bai 19 has a high total volumetric methane storage capacity of 200.4 cm3 (STP) cm−3, far exceeding the DOE's previous target of 180 cm3 (STP) cm−3 assuming that there is no packing loss. There are only very few MOFs whose methane storage capacities can reach 200 cm3 (STP) cm−3. With the pressure increasing to 65 bar, the volumetric methane uptake of NJU-Bai 19 can be further increased to 246.4 cm3 (STP) cm−3, which corresponds to the amount of methane stored in a CNG tank at 249 bar. Thus, NJU-Bai 19 could effectively reduce the pressure required for useful loading (246 v/v) by a factor of 3.8, down to 65 bar. Comparatively, compared to NOTT-101, although those two MOFs got almost the same pore volumes, NJU-Bai 19 shows a notably higher volumetric methane storage capacity (246.4 cm3 (STP) cm−3vs. 228.4 cm3 (STP) cm−3). In fact, the value of NJU-Bai 19 is comparable to and even higher than that of famous MOFs for CH4 storage such as NU-125,20 NU-135,21 PCN-14,6 UTSA-20 (ref. 6) and ZJNU-50 (ref. 22) (Table 1). Furthermore, at 65 bar and RT, a tank filled with NJU-Bai 19 would deliver almost four times more methane than an empty tank.
| MOFs | Total uptakea at 65 bar (35 bar) | Working capacityb at 65 bar | Ref. | ||
|---|---|---|---|---|---|
| cm3 (STP) cm−3 | g g−1 | cm3 (STP) cm−3 | Q st | ||
| a At 298 K. b The working capacity is defined as the difference in CH4 uptake between 65 and 5 bar. c The data (0.704 g cm−3) were calculated based on the crystal densities collected at 298 K; the framework density based on the He adsorption is about 0.680 g cm−3. | |||||
| NJU-Bai 19 | 246 (205) | 0.249 | 185 | 14.8 | This work |
| NOTT-101c | 228 (194) | 0.246 | 174 | 15.5 | 9 |
| HKUST-1 | 267 (227) | 0.216 | 190 | 17.0 | 6 |
| UTSA-76 | 257 (211) | 0.263 | 197 | 15.4 | 9 |
| Co(dbp) | 203 (161) | NA | 197 | 13.0 | 11 |
| PCN-14 | 230 (195) | 0.197 | 157 | 17.6 | 6 |
| MAF-38 | 263 (226) | 0.246 | 187 | 21.6 | 7 |
| Ni-MOF 74 | 260 (230) | 0.197 | 145 | 21.4 | 6 |
| UTSA-20 | 230 (184) | 0.181 | 170 | 18.2 | 17e |
| UTSA-88 | 248 (204) | 0.206 | 185 | 15.1 | 17f |
| NU-125 | 232 (182) | 0.287 | 183 | 15.5 | 20 |
| NU-135 | 230 (187) | 0.219 | 170 | 16.6 | 21 |
| ZJNU-50 | 229 (178) | 0.274 | 184 | 15.0 | 22 |
NJU-Bai 19 shows lower gravimetric capacity than some famous microporous MOFs with a higher pore volume, such as Al-soc-MOF-1,8 NU-111,23 MOF-205 (ref. 16e) and UTSA-76.9 Although NJU-Bai 19 has a lower volumetric methane storage capacity than HKUST-1 and Ni-MOF 74, it has a higher gravimetric methane storage capacity of 0.249 g g−1 than HKUST-1 (0.216 g g−1) and Ni-MOF-74 (0.197 g g−1) due to a larger pore volume and a lower framework density. This is really remarkable as NJU-Bai 19 adsorbing natural gas to such an extent may make it become commercially attractive.
Apart from its high methane storage capacities, NJU-Bai 18 also exhibits high methane working capacity, which is defined here as the difference in uptake between 5 bar and 65 bar. As discussed above, when piperazine groups are inserted, the methane uptake capacity of NJU-Bai 19 at 65 bar and RT can be significantly enhanced. However, a relatively low increase was observed in the uptake at 5 bar. As a result, the methane storage working capacity of NJU-Bai 19 can be improved from 174 cm3 (STP) cm3 in NOTT-101 to 185 cm3 (STP) cm3. Most importantly, this capacity is only slightly lower than that of some of the best MOFs, HKUST-1, UTSA-76 and Co(bdp), comparable to or higher than that of most of the other promising MOFs: NU-125, UTSA-20, NU-111,23 NU-135, PCN-14 and ZJNU-50 (Table 1). According to recent research,8,17b when temperature was decreased from 298 to 273 K at working pressures between 65 bar and 5 bar, some of the famous MOFs with high Qst showed a decrease in the volumetric CH4 working capacity with a decrease in temperature (Table S2†), the volumetric CH4 working capacity of NJU-Bai 19 is almost unchanged (from 185 to 189 cm3 (STP) cm−3). This phenomenon may be caused by the relatively low CH4 heat of adsorption of NJU-Bai 19.
To better understand the obvious improvement of the methane storage capacity and working capacity from NOTT-101 to NJU-Bai 19, we calculated the Qst from the temperature-dependent isotherms using the virial method. Considering the similar concentrations of open Cu sites24 (2.67 mmol cm−3 in NJU-Bai 19vs. 2.60 mmol cm−3 in NOTT-101), the similar binding energy of CH4 with ligands in NJU-Bai 19 and NOTT-101 (10.31 kJ mol−1vs. 10.06 kJ mol−1, Fig. S14†) and the smaller cage windows of NJU-Bai 19, we supposed it should have a higher initial Qst than NOTT-101. However, the Qst value of NJU-Bai 19 for CH4 adsorption is a little smaller than that of NOTT-101 (14.8 kJ mol−1vs. 15.5 kJ mol−1) and falls into the range of 12 kJ mol−1 to 15 kJ mol−1 which belongs to MOFs without strong CH4 binding sites.4c,6 The Qst is also lower than that of other analogues of NOTT-101 which are decorated with –CF3, F, –NO2 and –CH3. This indicates that the initial Qst value is not purely controlled by the open Cu sites, and there should be some contribution from the organic linkers in MOFs. With CH4 loading, the CH4–CH4 interactions become important, thus leading to an increase in Qst. However, we found that the Qst in NJU-Bai 19 is still smaller than that in NOTT-101 and UTSA-76 (Fig. S12†) even after high-concentration methane loadings. We also checked other probe molecules and found that the initial CO2Qst of NJU-Bai 19 is also lower than that of NOTT-101 (23.6 kJ mol−1vs. 26.6 kJ mol−1). Considering the lower CH4Qst during the process of adsorption and unchanged volumetric CH4 working capacity with a decrease in temperature, the introduction of a cycloaliphatic moiety into the organic linker may weaken the interactions between the gas and framework. This indicates that the piperazine in the organic linker can facilitate heat management during the adsorption and desorption process. This point needs further understanding although much work has been done on it as it is unusual and very interesting.
As established before,25 the methane uptake of MOFs at high pressure is mainly dominated by the second adsorption surface (mainly organic ligands). Piperazine provides a high-efficiency strategy to simultaneously introduce two kinds of CH4-favoured sites (N atoms and –CH2– groups) into MOFs. Considering the similar binding energy of CH4 with N atoms in NJU-Bai 19 and benzene ring in NOTT-101, the –CH2– groups which form the lipophilic surface may play a more important role at high CH4 loading. Moreover, to obtain high volumetric methane storage capacities, ideal MOFs should have balanced porosities and framework densities. NJU-Bai 19 has a more balanced porosity and framework density than NOTT-101 (Fig. S13†), which may be another important reason for the improved methane storage and working capacities. The combined characters indicate that NJU-Bai 19 is a potential stepping stone to MOFs which can achieve the objective of a high CH4 uptake and working capacities with smaller Qst. The piperazine functional group might open the door to constructing MOFs with a high performance using a cycloaliphatic backbone. This also motivates us to further design new MOFs with other aliphatic groups to improve their methane storage performance.
Conclusions
In summary, we have developed the first cycloaliphatic ring functionalized MOF (NJU-Bai 19) with piperazine groups. Compared with widely used aromatic ring functional groups, piperazine exhibits the following advantages. (1) Piperazine provides a high-efficiency strategy to introduce –CH2– groups into MOFs for improving CH4 storage; (2) piperazine brings a more balanced porosity and framework density than other aromatic rings; (3) piperazine may bring smaller CH4Qst, which may facilitate the heat management during the CH4 storage process; (4) compared with aromatic rings, it is easier and cheaper to introduce piperazine into MOFs. Above all, NJU-Bai 19 shows a significantly enhanced volumetric methane storage capacity of 246.4 cm3 (STP) cm−3 (at 298 K and 65 bar) and a working capacity of 185 cm3 (STP) cm−3. The combination of the balanced porosity and framework density and the lipophilic surface is considered to result in the increased methane storage and working capacities. Continuous work to develop MOFs with other new functional groups targeting higher methane storage performance is still ongoing.Acknowledgements
We thank the support of this work by the National Natural Science Foundation of China (21371091 and 21271192), Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ120632), International Science and Technology Cooperation Program of Chongqing (cstc2014-gjhz20002), and Chongqing Normal University Scientific Research Foundation Project (16XLB016). In addition, we thank Mr Yunzhi Li and Mr Fei Meng of Nanjing University for the theoretical calculations. We also gratefully acknowledge Prof. Junfeng Bai (Nanjing University) for his help and guidance.Notes and references
- (a) S. Yeh, Energy Policy, 2007, 35, 5865–5875 CrossRef; (b) R. F. Service, Science, 2014, 346, 538–541 CrossRef CAS PubMed.
- G. Whyatt, Issues affecting adoption of natural gas fuel in light- and heavy-duty vehicles, US Department of Energy, Pacific Northwest National Laboratory, Washington, 2010 Search PubMed.
- (a) A. Schoedel, Z. Ji and O. M. Yaghi, Nat. Energy, 2016, 1, 16034 CrossRef; (b) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- (a) T. A. Makal, J.-R. Li, W. Lu and H.-C. Zhou, Chem. Soc. Rev., 2012, 41, 7761–7779 RSC; (b) Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC; (c) J. A. Mason, M. Veenstra and J. R. Long, Chem. Sci., 2014, 5, 32–51 RSC.
- (a) M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Angew. Chem., Int. Ed., 1997, 36, 1725–1727 CrossRef CAS; (b) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- Y. Peng, V. Krungleviciute, I. Eryazici, J. T. Hupp, O. K. Farha and T. Yildirim, J. Am. Chem. Soc., 2013, 135, 11887–11894 CrossRef CAS PubMed.
- J.-M. Lin, C.-T. He, Y. Liu, P.-Q. Liao, D.-D. Zhou, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2016, 55, 4674–4678 CrossRef CAS PubMed.
- D. Alezi, Y. Belmabkhout, M. Suyetin, P. M. Bhatt, Ł. J. Weseliński, V. Solovyeva, K. Adil, I. Spanopoulos, P. N. Trikalitis, A.-H. Emwas and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 13308–13318 CrossRef CAS PubMed.
- B. Li, H.-M. Wen, H. Wang, H. Wu, M. Tyagi, T. Yildirim, W. Zhou and B. Chen, J. Am. Chem. Soc., 2014, 136, 6207–6210 CrossRef CAS PubMed.
- J. Jiang, H. Furukawa, Y.-B. Zhang and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 10244–10251 CrossRef CAS PubMed.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi and C. M. Brown, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- (a) J. B. Mota, A. Rodrigues, E. Saatdjian and D. Tondeur, Carbon, 1997, 35, 1259–1270 CrossRef CAS; (b) K. S. Walton and M. D. LeVan, Adsorption, 2006, 12, 227–235 CrossRef CAS.
- (a) X. L. Cui, K. J. Chen, H. B. Xing, Q. W. Yang, R. Krishna, Z. B. Bao, H. Wu, W. Zhou, X. L. Dong, Y. Han, B. Li, Q. L. Ren, M. J. Zaworotko and B. L. Chen, Science, 2016, 353, 141–144 CrossRef CAS PubMed; (b) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed; (c) L. Du, Z. Lu, K. Zheng, J. Wang, X. Zheng, Y. Pan, X. You and J. Bai, J. Am. Chem. Soc., 2012, 135, 562–565 CrossRef PubMed; (d) S. Ma, D. Sun, J. M. Simmons, C. D. Collier, D. Yuan and H.-C. Zhou, J. Am. Chem. Soc., 2008, 130, 1012–1016 CrossRef CAS PubMed; (e) A. H. Assen, Y. Belmabkhout, K. Adil, P. M. Bhatt, D. X. Xue, H. Jiang and M. Eddaoudi, Angew. Chem., Int. Ed., 2015, 54, 14353–14358 CrossRef CAS PubMed.
- Y. Hu, S. Xiang, W. Zhang, Z. Zhang, L. Wang, J. Bai and B. Chen, Chem. Commun., 2009, 7551–7553 RSC.
- (a) R. Sun, Y.-Z. Li, J. Bai and Y. Pan, Cryst. Growth Des., 2007, 7, 890–894 CrossRef CAS; (b) B. Zheng, J. Bai, J. Duan, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 133, 748–751 CrossRef CAS PubMed.
- (a) R. Yun, Z. Lu, Y. Pan, X. You and J. Bai, Angew. Chem., Int. Ed., 2013, 52, 11282–11285 CrossRef CAS PubMed; (b) M. Zhang, B. Li, Y. Li, Q. Wang, W. Zhang, B. Chen, S. Li, Y. Pan, X. You and J. Bai, Chem. Commun., 2016, 52, 7241–7244 RSC; (c) O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS PubMed; (d) O. K. Farha, A. Özgür Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944–948 CrossRef CAS PubMed; (e) H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe and J. Kim, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- (a) I. Spanopoulos, C. Tsangarakis, E. Klontzas, E. Tylianakis, G. Froudakis, K. Adil, Y. Belmabkhout, M. Eddaoudi and P. N. Trikalitis, J. Am. Chem. Soc., 2016, 138, 1568–1574 CrossRef CAS PubMed; (b) J. Cai, X. Rao, Y. He, J. Yu, C. Wu, W. Zhou, T. Yildirim, B. Chen and G. Qian, Chem. Commun., 2014, 50, 1552–1554 RSC; (c) Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC; (d) H.-M. Wen, B. Li, D. Yuan, H. Wang, T. Yildirim, W. Zhou and B. Chen, J. Mater. Chem., 2014, 2, 11516–11522 RSC; (e) Z. Guo, H. Wu, G. Srinivas, Y. Zhou, S. Xiang, Z. Chen, Y. Yang, W. Zhou, M. O'Keeffe and B. Chen, Angew. Chem., Int. Ed., 2011, 50, 3178–3181 CrossRef CAS PubMed; (f) G. Chang, B. Li, H. Wang, Z. Bao, T. Yildirim, Z. Yao, S. Xiang, W. Zhou and B. Chen, Chem. Commun., 2015, 51, 14789–14792 RSC.
- C. E. Wilmer, M. Leaf, C. Y. Lee, O. K. Farha, B. G. Hauser, J. T. Hupp and R. Q. Snurr, Nat. Chem., 2012, 4, 83–89 CrossRef CAS PubMed.
- W. Zhou, H. Wu, M. R. Hartman and T. Yildirim, J. Phys. Chem. C, 2007, 111, 16131–16137 CAS.
- C. E. Wilmer, O. K. Farha, T. Yildirim, I. Eryazici, V. Krungleviciute, A. A. Sarjeant, R. Q. Snurr and J. T. Hupp, Energy Environ. Sci., 2013, 6, 1158–1163 CAS.
- R. D. Kennedy, V. Krungleviciute, D. J. Clingerman, J. E. Mondloch, Y. Peng, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, J. T. Hupp, T. Yildirim, O. K. Farha and C. A. Mirkin, Chem. Mater., 2013, 25, 3539–3543 CrossRef CAS.
- C. Song, Y. Ling, Y. Feng, W. Zhou, T. Yildirim and Y. He, Chem. Commun., 2015, 51, 8508–8511 RSC.
- Y. Peng, G. Srinivas, C. E. Wilmer, I. Eryazici, R. Q. Snurr, J. T. Hupp, T. Yildirim and O. K. Farha, Chem. Commun., 2013, 49, 2992–2994 RSC.
- H. Wu, J. M. Simmons, Y. Liu, C. M. Brown, X.-S. Wang, S. Ma, V. K. Peterson, P. D. Southon, C. J. Kepert, H.-C. Zhou, T. Yildirim and W. Zhou, Chem.–Eur. J., 2010, 16, 5205–5214 CrossRef CAS PubMed.
- H. Wu, W. Zhou and T. Yildirim, J. Am. Chem. Soc., 2009, 131, 4995–5000 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, first-principle calculations, TGA, PXRD and heat of adsorption of CH4. CCDC 1491433. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ta06037d |
| This journal is © The Royal Society of Chemistry 2017 |