Performance evaluation of a yeast biorefinery as a sustainable model for co-production of biomass, bioemulsifier, lipid, biodiesel and animal-feed components using inexpensive raw materials†
RaviRanjan
Kumar
a,
Gunaseelan
Dhanarajan
a,
Moumita
Bhaumik
a,
Jayita
Chopra
ab and
Ramkrishna
Sen
 *a
*a
aDepartment of Biotechnology, Indian Institute of Technology, Kharagpur, WestBengal-721302, India. E-mail: rksen@yahoo.com; Tel: +91-3222-283752
bPK Sinha Center for Bioenergy, Indian Institute of Technology, Kharagpur-721302, WestBengal, India
First published on 24th April 2017
Abstract
Oleaginous yeasts have gained increasing attention as feedstock for biodiesel and other value-added products due to their high growth rates coupled with lipid accumulation abilities. However, the development of a technologically and economically sustainable biodiesel manufacturing process from yeasts necessitates the use of low-cost substrates and co-production of value-added by-products in a biorefinery model. Thus, the present study endeavors to concomitantly produce lipid and bioemulsifier from the isolated oleaginous yeast Pichia guilliermondii using inexpensive raw materials. Various low-cost raw materials, such as molasses, crude glycerol, distillery wastewater (DWW) and corn steep liquor (CSL), are tested to develop a judicious combination of substrates for the optimal production of yeast biomass and lipid for biodiesel application. Among the various combinations tested, crude glycerol when supplemented with CSL and mineral salts results in a maximum biomass concentration of 24.47 ± 0.78 g L−1 with 52.09 ± 2.03% lipid on a dry weight basis. Gas chromatographic analysis of the transesterified yeast lipid reveals that the compositions of fatty acid methyl esters vary with the substrates used for lipid production. However, the biodiesel properties are found to comply with the international standards, ASTM D6751 and EN14214. Studies on the emulsification activity reveal the extracellular production of bioemulsifer by the oleaginous yeast. Further biochemical analysis of the lipid-extracted biomass shows that it contains up to 24.6% ± 0.83% protein and 44.2% ± 1.41% carbohydrate, which indicate its potential use as animal feed. A preliminary cost estimate of lipid production shows the economic advantages of cheaper raw materials over synthetic media. Thus, as a proof of a novel biorefinery concept, an efficient and sustainable yeast biorefinery is successfully developed for the concomitant production of biodiesel, bioemulsifier and animal feed-components with simultaneous valorization of waste as low-cost substrates.
1. Introduction
Since the beginning of the industrial revolution, the global energy demand has been growing at a dramatic rate and has led to the depletion of the fossil fuel reserves and a consequent increase in the energy prices. The conventional energy scenario has been further compounded by global warming driven climate change due to emissions of green house gases by major industries. Based on the resolutions adopted in the United Nations Framework Conventions on Climate Change, including the recent convention in Paris, Governments are mandating the use of renewable sources for fuel, food, feed and chemicals. This calls for the development and production of multiple products in a refinery model. The term ‘biorefinery’ refers to the conversion and value-addition of biomass into fuels and chemicals through the incorporation of clean and green processes to make the process cost competitive.1,2To date, lignocellulose material (LCMs) and microalgae have been studied extensively as biomass feedstock for biorefineries.3,4 Recently, microalgal biorefineries have been at the center of scientific attraction due to the novel concept of producing biofuels along with multiple value-added products while remediating wastewater and sequestering carbon-dioxide.5–7 However, the issues involved in the availability of land and water, biomass productivity, dependence on climatic variations and longer incubation or gestation period pose pertinent questions on the feasibility and sustainability of LCM or microalgae based biorefineries.8,9
These limitations need to be overcome, since there is a demand to revolutionize the future energy scenario. Recently, oleaginous yeasts have been considered as alternative biofuel feedstock to lignocellulose and microalgal biomass because of their ability to grow in conventional fermenters at high rates by utilizing low-cost industrial by-products and waste as growth media.10 In the typical biorefinery concept, yeast feedstock is developed to derive low-value-high-volume products, such as biodiesel, crude glycerol, and adhesives as well as high-value-low-volume products such as polyunsaturated fatty acids and carotenoids with high antioxidant activities as components of functional foods and nutraceuticals, and also bioemulsifiers for potential healthcare, food and cosmetic applications.11–13 Moreover, yeast biomass may serve as good quality probiotics as health and wellness products.14 Since the biochemical composition of yeast biomass includes significant amounts of carbohydrate and proteins, the residual defatted biomass can be effectively utilized as animal feed.15 Therefore, oleaginous yeasts are emerging as the most favored feedstock in a biorefinery model for the production of biofuel and value added products and have achieved the status of potential industrial significance as ideal alternatives to other known renewable resources.
The literature is replete with instances where yeast has been reported as a versatile system for the biotechnological production of platform chemicals including biofuel in a biorefinery model. The studies reported on yeast-based biorefinery leveraging waste as raw materials have mostly failed to show significantly higher lipid contents along with the production of multiple value-added products.16,17 This information, thus, necessitates and calls for the development of a true yeast biorefinery that is more productive and sustainable, which is the challenge for us. Our preliminary studies involving seven oleaginous yeast isolates indicated the suitability of one isolate, which was characterized as Pichia guilliermondii,18 as a potentially superior feedstock for a biorefinery, mainly due to its ability to grow at higher rates in waste-containing media and produce relatively greater amounts of lipid and some value-added products including a bioemulsifier. This amply justifies the use of Pichia guilliermondii in the present study to develop and demonstrate a model yeast biorefinery for the concomitant production of lipid, biodiesel, crude glycerol, bio emulsifier and animal feed.
2. Material and methods
2.1 Microorganism and culture conditions
Oleaginous yeast, P. guillierrmondii, was isolated from an oil mill in Kharagpur, India for use in this study. The stock culture was maintained on a nutrient agar slant at 4 °C. Inoculum (10% v/v) was prepared in MGYP media for all experiments.18 The culture conditions were maintained at 28 °C in an incubator shaker (180 rpm).2.2 Use of inexpensive substrates for lipid production
In this study, molasses, crude glycerol and distillery waste water (DWW) were used as carbon sources, and corn steep liquor (CSL) was used as the nitrogen source for lipid production from oleaginous yeast. Molasses, crude glycerol, DWW and CSL were collected from a local market in Kharagpur, Eastern Biodiesel Technologies Pvt. Ltd, Kolkata, IFB Agro Limited, Noorpur, and Shukhjit Starch and Chemicals Ltd, Malda, West Bengal respectively. CSL and DWW were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to separate the solids present before use as a production medium.
000 rpm for 10 min to separate the solids present before use as a production medium.
Shake flask experiments were performed using the abovementioned inexpensive substrates in different combinations for 96 h. The substrates were judiciously mixed with CSL in different concentrations (10, 20 and 30 g L−1). Synthetic media containing mineral salt and refined glycerol (5.0% w/v) were used as control media.18 After optimizing the concentration of CSL in the media, mineral salts (KH2PO4 – 1, K2HPO4 – 0.122, MgSO4 – 0.7, NaCl – 0.5, CaCl2·2H2O – 0.246 g L−1) were added for further improvement of the lipid content. To ensure reproducibility, all experiments were performed in triplicate. The error bars represent the standard deviation of all the experiments.
2.3 Biomass and lipid production in 14 L stirred tank reactor (STR)
The best media combination containing crude glycerol (5% w/v), CSL (20 g L−1) and mineral salt was chosen as the production media for the reactor study. A New Brunswick BioFlo 415, Eppendorf Inc, fermenter (working volume: 10 L) was used. The culture was grown for 120 h at 28 °C and 180 rpm with an air flow rate of 1.5 (vvm). 10% (v/v) inoculum of exponentially growing cells was used as the seed culture.2.4 Analytical methods
A. Microscopy. Cells were subjected to nile red staining (1 μg mL−1 nile red in acetone) for visualization under a fluorescence microscope (Olympus IX51, Japan). Based on the size and intensity of the yellow–gold fluorescence emitted by the lipid droplets, the lipid content was qualitatively determined.
B. Gravimetric analysis. Dry biomass was disrupted using an ultrasonicator (Q SONICA, Q125 USA) for 10 min in pulse mode (45 s on; 15 s off) at 50% amplitude, and lipids were extracted according to the Bligh and Dyer method19 in a lipid extractor (J.P SELECTA Spain, Extractor fat Det-Gras N 6SAM, no. 4002842). The lipid containing phase was collected in pre-weighed glass vials, whereas the solvent phase was recovered. Neutral lipids were recovered in the hexane phase for further transesterification reaction. Excess solvent was evaporated in a vacuum evaporator, and the amount of neutral lipid obtained was measured and stored at 4 °C for further use.
2.5 Emulsification activity of the cell-free broth
The cell-free broth, obtained after harvesting, was subjected to microfiltration, followed by ultrafiltration (Lab scale Millipore TFF) with a molecular weight cut off (MWCO) of 0.45 μm and 10 kDa, respectively. Emulsification activity was estimated by mixing an equal amount of retentate and diesel. The mixture was vortexed for 10 min at a very high speed and left undisturbed for 24 h. Emulsification activity was calculated from the following equation.202.6 Carbohydrate and protein estimation in de-oiled biomass
Biomass was suspended in deionized water and incubated for 1 h at 90 °C with 0.4 mL of HCl (1 N). Carbohydrate was precipitated by adding two volumes of absolute cold ethanol and stored at −20 °C overnight. The sample was analyzed to estimate the carbohydrate content via the phenol sulphuric acid method.For protein estimation, 0.5 N NaOH (1 mL) was added to the biomass and extracted for 10 min at 50 °C with occasional stirring. The supernatant was collected after cooling and centrifugation, and alkali extraction was repeated 2 times. Furthermore, the protein content was estimated using the Lowry method.
2.7 Transesterification and FAME analysis
The yeast lipids obtained from the shake flask study, as mentioned in Section 2.2, were enzymatically transesterified by lipase (Steapsin, SRL, India) in a packed bed reactor (PBR) (KC Engineers (P) Ltd., India). The column was packed with alginate beads (3% sodium alginate) of diameter 1 mm. The packing height of the column was maintained at 15 cm with operating conditions of 37 °C for 2 h at a flow rate of 1 mL min−1. This was followed by extraction of the fatty acid methyl esters (FAMEs) in n-hexane. Quantification of the FAME was performed using the method reported by Dinesh Kumar et al.5 The FAMEs were analyzed by gas chromatography (Thermo Fisher Scientific-Chemito Ceres 800 Plus) equipped with a flame ionization detector and BPX 70 capillary column (30 m × 0.25 mm). The operating conditions were as follows: injector temperature, 260 °C; detector temperature, 280 °C; injection volume, 1 μL; split ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and oven temperature starting at 70 °C for 1 min, and increasing at 5 °C min−1 to 180 °C for 10 min and 6 °C min−1 to 220 °C for 11 min.
25 and oven temperature starting at 70 °C for 1 min, and increasing at 5 °C min−1 to 180 °C for 10 min and 6 °C min−1 to 220 °C for 11 min.
2.8 Analysis of biodiesel properties
The fuel properties of the methyl esters, namely cetane number, cloud point, iodine number, higher heating value, specific gravity and viscosity, were calculated using the equations of Hoekman et al.21 and compared with the US biodiesel standard ASTM D6751 and EU biodiesel standard EN 14214 for their suitability as biodiesel.2.9 Preliminary economic assessment of raw material cost for lipid production
The cost of raw materials was calculated for the production of 1 kg lipid. For each media combination, the number of batches (10 L) required to produce 1 kg lipid was calculated. Furthermore, the cost of the raw material for a single batch was estimated for each media combination. Thus, the amount of media required for the total number of batches to produce lipid (1 kg) was calculated.3. Result and discussion
3.1 Effect of different low cost substrates on biomass and lipid production
From preliminary experiments, the optimum concentrations of carbon sources, such as molasses and crude glycerol, for the improved production of lipid were found to be 6.0% (w/v) and 5.0% (w/v), respectively (results not shown), whereas DWW was used as such (100% v/v). Among them, crude glycerol facilitated the maximum biomass growth. Furthermore, the media was supplemented with different concentrations of CSL (10, 20 and 30 g L−1) to increase the yeast growth and lipid accumulation. It was found that the increase in CSL concentration up to 20 g L−1 improved the biomass concentration and lipid content (Fig. 1A–C). However, the biomass concentration was observed to decrease upon increasing the CSL concentration to 30 g L−1. This might be due to the presence of toxic components present in the CSL at inhibitory concentrations. Crude glycerol and CSL containing media resulted in a maximum biomass concentration of 23.3 ± 0.89 g L−1 with 22 ± 1.04% (w/w) lipid content. Molasses-CSL media showed 21.5 ± 0.81 g L−1 yeast concentrations with 27% ± 0.98% lipid content, whereas 15.8 g L−1 biomass with 34% lipid content was achieved using DWW–CSL media. In order to further improve the biomass and lipid production, critical mineral salts reported for oleaginous yeast cultivation were supplemented into the media.It was observed that the presence of mineral salts particularly improved the lipid accumulation, whereas the maximum biomass concentration remained almost constant. Molasses, crude glycerol and DWW supplemented with CSL (20 g L−1) and mineral salts resulted in 42.82% ± 1.7%, 52.09% ± 2.03% and 39.21% ± 1.41% lipid content, respectively, whereas culture grown in only MSM resulted in 49.48% ± 2.3% lipid content (Fig. 1D). The biomass concentration (g L−1) and lipid content (%) from various cultures on different substrates are shown in Table 1. Our result is in agreement with that of Karatay et al.22 who reported lipid content (46.8%) by culturing C. tropicalis in molasses (8%) and (NH4)2SO4 (1.0 g L−1). Kitcha et al.23 demonstrated biomass yields of 9.17 and 10.45 g L−1 and lipid contents of 41.50% and 53.28% by culturing Kodamaea ohmeri and Trichosporonoides spathulata, respectively, while utilizing crude glycerol and ammonium sulphate as fermentation media. Our result corroborates the abovementioned study that used the same media combination, whereas Sankh et al.10 reported a biomass yield of 15.5 g L−1 and lipid content of 23% by culturing P. kudriavzevii in crude glycerol and CSL. Moreover, Schneider et al.24 reported a lipid yield of 0.75 g L−1 and biomass yield of 5.22 g L−1 by culturing Rhodotorula glutinis in brewery wastewater.
| S. no | Strain | Carbon | Nitrogen | Biomass (g L−1) | Lipid (%) | Reference |
|---|---|---|---|---|---|---|
| a DWW – distillery waste water, CSL – corn steep liquor. | ||||||
| 1 | Y. lipolytica | Industrial fat | NH2SO4 & yeast extract | 8.7 | 44.0 | Papanikolaou et al.28 |
| 2 | L. starkeyi | Glucose and xylulose | Yeast extract NH2SO4 | 17.7 | 44.6 | Zhao et al.29 |
| 14.7 | 44.9 | |||||
| 3 | T. fermentans | Rice straw hydrolysate | Yeast extract & peptone | 28.6 | 40.1 | Huang et al.30 |
| 4 | R. glutinis | Monosodium glutamate waste water | NA | 25.0 | 20.0 | Xue et al.31 |
| 5 | P. kudriavzevii | Glycerol | CSL | 15.5 | 23.0 | Sankh et al.10 |
| 6 | R. glutinis IIP-30 | Molasses | NA | 18.37 | 8.0 | Johnson et al.32 |
| 7 | R. toruloides | Glucose | NH2SO4 & yeast powder | 18.2 | 76.0 | Li et al.33 |
| 8. | C.albidus | Volatile fatty acids | NH4Cl | 1.15 | 27.0 | Fei et al.34 |
| 9 | C. protothecoides | Volatile fatty acids | Urea | 0.65 | 48.7 | Fei et al.35 |
| 10 | P. guilliermondii | Molasses | CSL | 22.42 | 42.82 | This study |
| Crude glycerol | CSL | 24.47 | 52.09 | |||
| DWW | CSL | 15.80 | 39.21 | |||
| Refined glycerol | NH2SO4 | 10.21 | 49.48 | |||
The influence of adding mineral salts in media, such as KH2PO4 and MgSO4, on lipid biosynthesis has been reported in the literature. KH2PO4 acts as a buffering agent and helps to maintain cell integrity during growth and plays a significant role in phospholipid formation.25 Magnesium ions are an important co-factor for acetyl CoA carboxylase and thus, enhance lipid synthesis by promoting its activity.26 This confirms that the supplementation of minerals salts in the media is a pivotal strategy to increase the lipid content in oleaginous yeasts. Furthermore, by implementing the fed batch mode and controlling the operation parameters, such aeration, agitation, pH and temperature, lipid content and biomass yield may be further increased. Moreover, the genome of P. guilliermondii has been sequenced recently and is publicly available (http://www.broad.mit.edu). Hence, this yeast is amenable to further genetic modification and metabolic engineering to improve lipid production.
The fluorescence microscopic images of the cells grown in different media combinations are observed as intact lipid droplets fluorescing inside the cell after nile red staining (Fig. 2).
The time course profile of biomass growth in different media combinations is shown in Fig. S1 in the ESI.† The analysis of the growth pattern shows that the log phase was initiated at 48 h of fermentation, and biomass increased exponentially until 72 h in all cases. After this point, at about 96 h, due to the nitrogen limited condition in the media, lipid accumulation starts and the growth rate reached the stationary phase with a biomass yield of 22.4 ± 0.91, 24.47 ± 0.78 and 15.75 ± 0.72 g L−1 in MCS, GCS and DCS media, respectively, whereas the culture grown in MSM resulted in a biomass yield of 10.21 ± 0.36 g L−1. Final CDW in each media combination is shown in Fig. 1. It is well known that microorganisms grown in media with excess carbon and a limited nitrogen content are suitable for lipid accumulation.27 No further growth is possible due to the assimilation of the carbon source and nitrogen limitation by the microorganisms. The carbon flux is then channelled into lipid synthesis for oil accumulation.
| Fatty acid | MCS | GCS | DCS | MSM |
|---|---|---|---|---|
| a MCS – molasses + CSL (20 g L−1) + salts, GCS – crude glycerol + CSL (20 g L−1) + salts, DCS – DWW + CSL (20 g L−1) + salts, and MSM – minerals salt medium. | ||||
| Caprylic (C8:0) | 13.2 | ND | 1.9 | ND |
| Lauric (C12:0) | 16.5 | 1.1 | ND | 5.5 |
| Tridecanoic (C13:0) | ND | 11 | 6.8 | ND |
| Palmitic (C16:0) | ND | 15 | 22.5 | 31.8 |
| Heptadecanoic (C17:0) | 4.1 | 1 | ND | ND |
| Oleic (18:1) | 38.8 | 14.7 | 8.3 | 23.8 |
| γ-Linolenic (18:3) | ND | 22.9 | 42.9 | ND |
| Arachidic (C20:0) | ND | 6.4 | 8.3 | ND |
| Stearic (C18:0) | ND | 2.2 | 2.5 | 25.1 |
| Myristic (14:0) | 4.3 | 1.2 | 0.9 | 2.2 |
| Myristoleic (14:1) | 8.6 | ND | ND | 4.8 |
| Pentadecanoic (C15:0) | 1.8 | ND | ND | 1.8 |
| Tricosanoic (C23:0) | ND | ND | ND | ND |
| Undecanoic (C11:0) | 6.6 | ND | ND | ND |
| Palmitoleic (16:1) | 6 | 3.3 | 5.8 | 4.9 |
| Behenic acid (C22:0) | ND | 1.5 | ND | ND |
| Lignoceric acid (24:0) | ND | 19.7 | ND | ND |
In our investigation, oleic acid (C18:1) was the major fatty acid present in the FAME obtained from the different media combinations, whereas linolenic acid (C18:3) was found in the FAME obtained from the GCS and DCS media combination. The ratio of saturated![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) unsaturated fatty acid for MCS, GCS and DCS was (59.1
unsaturated fatty acid for MCS, GCS and DCS was (59.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.9), (46.5
40.9), (46.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53.4) and (42.9
53.4) and (42.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57.0), respectively. The fatty acid profile obtained from our investigation is similar to that obtained from conventional plant oils. However, the fatty acid profile from the lipid produced by the Pichia strain was found to be dominated by oleic acid when grown in glycerol and CSL based media.10 Johnson et al.32 reported 36.4% and 23.5% oleic and palmitic acid (C16:0), respectively, by culturing Rhodotorula glutinis IIP-30 in molasses based media, which is similar to our result for the FAME obtained from the MCS media combination. Linoleic acid (50%) was reported by Schneider et al.22 while culturing Rhodotorula glutinis in distillery effluents, whereas we achieved 42.9% linoleic acid in the FAME obtained from the DCS media combination. In terms of biodiesel properties, as the chain length increases, fuel properties, such as cetane number, heat of combustion and melting point, also increase. Saturated fatty acid contributes to a higher cetane number, shorter ignition delay times and improved oxidative stability, whereas unsaturated fatty acid is beneficial in terms of cold flow and pour point properties. Since the FAME obtained from GCS and DCS media has a higher percentage of unsaturated fatty acid, it can serve as a good substrate for biodiesel production from oleaginous yeast.
57.0), respectively. The fatty acid profile obtained from our investigation is similar to that obtained from conventional plant oils. However, the fatty acid profile from the lipid produced by the Pichia strain was found to be dominated by oleic acid when grown in glycerol and CSL based media.10 Johnson et al.32 reported 36.4% and 23.5% oleic and palmitic acid (C16:0), respectively, by culturing Rhodotorula glutinis IIP-30 in molasses based media, which is similar to our result for the FAME obtained from the MCS media combination. Linoleic acid (50%) was reported by Schneider et al.22 while culturing Rhodotorula glutinis in distillery effluents, whereas we achieved 42.9% linoleic acid in the FAME obtained from the DCS media combination. In terms of biodiesel properties, as the chain length increases, fuel properties, such as cetane number, heat of combustion and melting point, also increase. Saturated fatty acid contributes to a higher cetane number, shorter ignition delay times and improved oxidative stability, whereas unsaturated fatty acid is beneficial in terms of cold flow and pour point properties. Since the FAME obtained from GCS and DCS media has a higher percentage of unsaturated fatty acid, it can serve as a good substrate for biodiesel production from oleaginous yeast.
| Sample | Viscosity (mm s−2) | Specific gravity (kg L−1) | Cetane number | Cloud point (°C) | Iodine number | HHV |
|---|---|---|---|---|---|---|
| a MCS – molasses + CSL (20 g L−1) + salts, GCS – crude glycerol + CSL (20 g L−1) + salts, DCS – DWW + CSL (20 g L−1) + salts, and MSM – minerals salt medium. | ||||||
| MCS | 4.89 | 0.87 | 59.56 | 13.36 | 49.63 | 39.40 |
| GCS | 4.49 | 0.87 | 55.39 | 5.01 | 96.10 | 40.50 |
| DCS | 4.75 | 0.87 | 58.08 | 10.40 | 66.10 | 39.79 |
| MSM | 5.11 | 0.87 | 61.95 | 18.15 | 22.93 | 38.77 |
| ASTM D6751 | 1.9–6.0 | 0.85 | 47 | −3 to 12 | NA | NA |
| EN14214 | 3.5–5.0 | 0.86–0.9 | 51 | NA | 120 max | NA |
Cetane number depicts the ignition property of the fuel, which is the delay in the ignition of the engine. The minimum values for cetane number as per ASTM D6751 and EN14214 are 47 and 51, respectively, which correspond to our result of 59.56, 55.39 and 58.08 in different media combinations, as mentioned in Table 3. The energy content of the oil is represented in terms of higher heating value (HHV), where a higher value is desirable for this property, which was found in the same range in all the media combinations. Cloud point refers to the low temperature property of biodiesel, thus a higher cloud point is undesirable, which is due to the presence of methyl esters longer than C12. Iodine number is a measure of unsaturated fatty acid and is specified by the mass of iodine utilized by 100 g oil. In our result, the maximum iodine number was found in the FAME from the GCS media, followed by DCS and MCS with the same combination. Since a higher degree of saturation will cause the formation of deposits in the fuel line on heating due to polymerization of glycerides, it is regarded as one of the important fuel properties. Furthermore, the specific gravity of biodiesel from different media combinations conforms to the ASTM D6751 and EN 14214 standards.
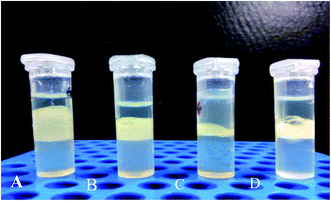
3.2 Emulsification activity
The emulsification activity of cell free supernatant from the different media combinations is illustrated in Fig. 3. The GCS media shows the maximum emulsification activity of 56.25% ± 1.74% followed by the MCS (37.5% ± 1.42%), MSM (37.5% ± 1.08%) and DCS media (18.75% ± 0.76%). The surface tension of the GCS media was 53.1 mN m−1. The inorganic salts present in the media may affect the emulsification activity. Cirigliano and Carman36 reported the emulsification activity of 50.1% by culturing Candida lipolytica, which corroborates to our result.3.3 Carbohydrate and protein content in de-oiled biomass
In addition to lipid, oleaginous yeasts contain a significant amount of carbohydrate and protein. An important perspective to reduce the production cost for biodiesel production is to make use of the defatted biomass as animal feed. The carbohydrate and protein contents in the yeast cultured using different media combinations are illustrated in Table 4. The maximum carbohydrate content was found in the DCS media, whereas the protein content was found to be higher in the yeast cells grown in MCS media, followed by DCS and GCS media. This application of de-oiled biomass as animal feed could kindle tremendous interest in the biorefinery arena.| Biochemical composition | MCS | GCS | DCS | MSM |
|---|---|---|---|---|
| a MCS – molasses + CSL (20 g L−1) + salts, GCS – crude glycerol + CSL (20 g L−1) + salts, DCS – DWW + CSL (20 g L−1) + salts, and MSM – minerals salt medium. | ||||
| Carbohydrate (%) | 41.7 ± 1.75 | 35.8 ± 1.32 | 44.2 ± 1.41 | 37.4 ± 1.79 |
| Protein (%) | 24.6 ± 0.83 | 12.41 ± 0.58 | 16.3 ± 0.63 | 19.1 ± 0.70 |
3.4 Process integration for the development of yeast biorefinery
In this study, we demonstrate an integrated yeast biorefinery involving the simultaneous production of biodiesel and bioemulsifier along with an animal feed component that was obtained from the residual biomass. From the shake flask experiments, the performance of the best media combination (GCS) was validated in a 14 L STR. It should be noted that crude glycerol as a carbon source and corn steep liquor as a nitrogen source were strategically fed into the reactor as low cost production media for biomass growth and lipid accumulation. The time course profiles for substrate concentration, biomass, lipid production and emulsification activity are shown in Fig. 4. The lipid content in crude glycerol supplemented with CSL and mineral salt was found to be 52.04% ± 1.92%, and the biomass yield was 25.96 ± 1.24 g L−1. The emulsification activity of the retentate was 53.49% ± 1.87%, whereas the carbohydrate and protein content was 32.09% ± 1.25% and 12.15% ± 0.52%, respectively. | ||
| Fig. 4 Time course growth profile, crude glycerol consumption, emulsification activity and lipid content of P. guillierrmondii growth in 14 L STR. | ||
Mathews et al.37 reported a ‘feed + fuel’ biorefinery approach by utilizing Saccharomyces cerevisiae for the production of bioethanol, in addition to yeast biomass as a source of single-cell protein (SCP) in the animal feed supplement. This model demonstrates a three-fold increase in ethanol production from 27 Gl to over 92 Gl. In our study, we develop a proof-of-concept for a fuel, feed and healthcare based biorefinery approach, which is as follows: (i) lipid extracted from yeast biomass can be transesterified for biofuel application, (ii) defatted biomass containing protein and carbohydrate, and unreacted lipid after transesterification can be potentially utilized as animal feed components and (iii) bioemulsifier obtained from the retentate can be used as a value-added product for healthcare application. The crude glycerol obtained during lipid transesterification as a by-product can be used as a carbon source in the production media to reduce the media cost. The permeate obtained after ultrafiltration can be recycled as a medium supplement for fermentation, thus ensuring zero discharge from the overall process. Moreover, the solvent used for extraction can be recovered and reused for the same.
| C3H8O3 + aO2 + bNH4 → 1.966CH1.65O0.5N0.135 + dCO2 + eH2O + 0.001488C32H58O11 | (1) |
Using the determined values of the stoichiometric coefficients by C, H, N & O balance, eqn (1) becomes
| C3H8O3 + 1.418O2 + 0.265NH4 → 1.966CH1.65O0.5N0.135 + 0.986CO2 + 2.865H2O + 0.001488C32H58O11 | (2) |
From eqn (2), it is estimated that 36 g carbon in crude glycerol is utilized during the fermentation, in which 23.59 g C is utilized for biomass growth, 11.83 g C in the form of CO2 for respiration, and 0.57 g C for bioemulsifier production (Fig. 5). From the total biomass, 11.8 g C is channeled into lipid synthesis, while the remaining carbon is fixed into the non-lipid part of the biomass. It is observed that neutral lipid constitutes around 8.26 g C, in which 7.04 g C is channeled into biodiesel, 0.63 g C into glycerol and 1.24 g C in unreacted lipid. It should be noted that around 23% C from crude glycerol was utilized for the production of biodiesel, glycerol and bio emulsifier. Upon adopting suitable reactor strategies, the percentage of carbon fixation into the products can be further improved.
3.5 Preliminary economic assessment of the raw material cost for lipid production using different low-cost substrates
At present, biodiesel containing fatty acid methyl ester (FAME) is undergoing a transformation from a demonstration fuel to a commercialized product. The high cost of the feed stock is the major obstacle in the commercialization of microbial biodiesel production. For lipid production, glucose is mainly used as the carbon source, which costs $500 per ton at the international market price. Therefore to overcome its economic feasibility, an assessment of the raw material cost was conducted for the production of microbial oil using a low cost industry by product. In the current study, an attempt was made to estimate the cost of 1 kg microbial lipid from different fermentation media. The cost of different media combinations to produce 1 kg lipid is illustrated in Table 5. Based on the current market prices sourced from the relevant industries, molasses costs around $8.01 kg−1, whereas the cost of CSL and crude glycerol is estimated to be $7.0 and $6.67 kg−1. It is important to note that DWW was procured free of cost from IFB Agro Limited, Noorpur, India. Based on these prices, the costs of the DCS and GCS media were sixteen and twelve times less than that of MSM. However Fei et al.34,35 reported a raw material cost $0.95 and $0.60 kg−1 using volatile fatty acid for yeast and microalgae in a race way pond, respectively. It should be emphasized here that the preliminary cost analysis of the fermentation media was computed, taking into account the different low cost raw materials and other media ingredients. Furthermore, life cycle analysis (LCA) can be implemented to study the economic feasibility of this approach as a sustainable biorefinery model.| Fermentation media | Yield of lipid (g) | No. of batches to produce lipid (1 kg) | Medium cost per batch | Media cost for lipid (INR/kg) | Media cost for lipid (USD/kg) |
|---|---|---|---|---|---|
| a MCS – molasses + CSL (20 g L−1) + salts, GCS – crude glycerol + CSL (20 g L−1) + salts, DCS – DWW + CSL (20 g L−1) + salts, and MSM – minerals salt medium. | |||||
| MCS | 9.60 | 10.41 | 15.42 | 160.59 | 2.40 |
| GCS | 12.74 | 7.84 | 5.35 | 41.97 | 0.62 |
| DCS | 6.17 | 16.20 | 2.01 | 32.54 | 0.48 |
| MSM | 5.05 | 19.80 | 26.79 | 530.97 | 0.79 |
4. Conclusion
The present study demonstrates the development of a sustainable and integrated yeast biorefinery model that is capable of utilizing waste for the concurrent production of commercially important products, namely, lipid, biodiesel, bioemulsifier, crude glycerol and animal feed component. The oleaginous yeast P. guilliermondii could efficiently grow in different media containing low-cost substrates, such as molasses, crude glycerol, and distillery waste water, as carbon sources, along with corn steep liquor as a cheap nitrogen source. Moreover, the overall carbon balance for the integrated process and preliminary cost-benefit analysis of the different media combinations indicate the potential feasibility of the yeast biorefinery. To the best of our knowledge, this study offers a solid proof of concept for building a yeast biorefinery to derive multiple products, including a biofuel, by judiciously valorizing wastes and thus, is expected to contribute positively and significantly to the contemporary scientific literature on biorefineries.Acknowledgements
The authors thankfully acknowledge the Department of Biotechnology, Government of India for the financial support under a sponsored project (Grant No. BT/PR6909/PBD/26/391/2013, 21/03/2014). The authors gratefully acknowledge IIT Kharagpur for all the research facilities. The authors are also thankful to Dinesh Kumar Ramalingam and Dr Chinmay Hazra for their kind help and suggestions. JC thankfully acknowledges the Ministry of New and Renewable Energy, Govt. of India, for her fellowship.References
- S. Octave and D. Thomas, Biochimie, 2009, 91, 659–664 CrossRef CAS PubMed.
- F. Cherubini and S. Ulgiati, Appl. Energy, 2010, 87, 47–57 CrossRef CAS.
- X. P. Gao, Y. Yu and H. W. Wu, ACS Sustainable Chem. Eng., 2013, 1, 1371–1380 CrossRef CAS.
- D. R. Vardon, B. R. Moser, W. Zheng, K. Witkin, R. L. Evangelista, T. J. Strathmann, K. Rajagopalan and B. K. Sharma, ACS Sustainable Chem. Eng., 2013, 1, 1286–1294 CrossRef CAS.
- R. Dineshkumar, S. K. Dash and R. Sen, RSC Adv., 2015, 5, 73381–73394 RSC.
- G. Subramanian, G. Yadav and R. Sen, RSC Adv., 2016, 6, 72897–72904 RSC.
- G. Yadav, A. Karemore, S. K. Dash and R. Sen, Bioresour. Technol., 2015, 191, 399–406 CrossRef CAS PubMed.
- A. Karemore and R. Sen, RSC Adv., 2015, 5, 70929–70938 RSC.
- A. Karemore and R. Sen, RSC Adv., 2016, 6, 29486–29496 RSC.
- S. Sankh, M. Thiru, S. Saran and V. Rangaswamy, Fuel, 2013, 106, 690–696 CrossRef CAS.
- I. R. Sitepu, L. A. Garay, R. Sestric, D. Levin, D. E. Block, J. B. German and K. L. Boundy-Mills, Biotechnol. Adv., 2014, 32, 1336–1360 CrossRef CAS PubMed.
- C. Calvo, F. L. Toledo, C. Pozo, M. V. Martinez-Toledo and J. Gonzalez-Lopez, J. Food, Agric. Environ., 2004, 2, 238–243 CAS.
- Y. Miura, K. Kondo, T. Saito, H. Shimada, P. D. Fraser and N. Misawa, Appl. Environ. Microbiol., 1998, 64, 1226–1229 CAS.
- T. Silva, M. Reto, M. Sol, A. Peito, C. M. Peres, C. Peres and F. X. Malcata, LWT-Food Sci. Technol., 2011, 44, 1349–1354 CrossRef CAS.
- I. M. P. L. V. O. Ferreira, O. Pinho, E. Vieira and J. G. Tavarela, Trends Food Sci. Technol., 2010, 21, 77–84 CrossRef CAS.
- S. H. Razani, S. M. Mousavi, H. M. Yeganeh and I. Marc, J. Microbiol. Biotechnol., 2007, 17, 1591–1597 Search PubMed.
- T. Schneider, S. Graeff-Hönninger, W. T. French, R. Hernandez, W. Claupein, W. E. Holmes and N. Merkt, J. Combust., 2012, 15341, 1–9 CrossRef.
- J. Chopra, R. Dineshkumar, M. Bhaumik, G. Dhanarajan, R. Kumar and R. Sen, RSC Adv., 2016, 6, 70364–70373 RSC.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- D. G. Cooper and B. G. Goldenberg, Appl. Environ. Microbiol., 1987, 53, 224–229 CAS.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros and M. Natarajan, Renewable Sustainable Energy Rev., 2012, 16, 143–169 CrossRef CAS.
- S. E. Karatay and G. Donmez, Bioresour. Technol., 2010, 101, 7988–7990 CrossRef CAS PubMed.
- S. Kitcha and B. Cheirsilp, Energy Procedia, 2011, 9, 274–282 CrossRef.
- T. Schneider, S. Graeff-Honninger, W. T. French, R. Hernandez, N. Merkt, W. Claupein, M. Hetrick and P. Pham, Energy, 2013, 61, 34–43 CrossRef CAS.
- D. Dasgupta, S. K. Suman, D. Pandey, D. Ghosh, R. Khan, D. Agrawal, R. K. Jain, V. T. Vadde and D. K. Adhikari, SpringerPlus, 2013, 2, 159–169 CrossRef PubMed.
- H. J. Janssen, M. H. A. Ibrahim, D. Broker and A. Steinbuchel, AMB Express, 2013, 3, 38–46 CrossRef PubMed.
- C. Ratledge, Biochimie, 2004, 86, 807–815 CrossRef CAS PubMed.
- S. Papanikolaou, I. Chevalot, M. Komaitis, G. Aggelis and I. Marc, Antonie van Leeuwenhoek, 2001, 80, 215–224 CrossRef CAS PubMed.
- X. Zhao, X. L. Kong, Y. Y. Hua, B. Feng and Z. B. Zhao, Eur. J. Lipid Sci. Technol., 2008, 110, 405–412 CrossRef CAS.
- C. Huang, M. H. Zong, H. Wu and Q. P. Liu, Bioresour. Technol., 2009, 100, 4535–4538 CrossRef CAS PubMed.
- F. Y. Xue, J. X. Miao, X. Zhang, H. Luo and T. W. Tan, Bioresour. Technol., 2008, 99, 5923–5927 CrossRef CAS PubMed.
- V. W. Johnson, M. Singh, V. S. Saini, D. K. Adhikari, V. Sista and N. K. Yadav, J. Ind. Microbiol., 1995, 14, 1–4 CrossRef CAS.
- Y. H. Li, Z. B. Zhao and F. W. Bai, Enzyme Microb. Technol., 2007, 41, 312–317 CrossRef CAS.
- Q. Fei, H. N. Chang, L. A. Shang, J. D. R. Choi, N. Kim and J. Kang, Bioresour. Technol., 2011, 102, 2695–2701 CrossRef CAS PubMed.
- Q. Fei, R. Fu, L. Shang, C. J. Brigham and H. N. Chang, Bioprocess Biosyst. Eng., 2015, 38, 691–700 CrossRef CAS PubMed.
- M. C. Cirigliano and G. M. Carman, Appl. Environ. Microbiol., 1984, 48, 747–750 CAS.
- J. A. Mathews, H. Tan, M. J. B. Moore and G. Bell, Energy Policy, 2011, 39, 4932–4938 CrossRef.
- F. R. Accorsini, M. J. R. Mutton, E. G. M. Lemos and M. Benincasa, Braz. J. Microbiol., 2012, 43, 116–125 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00010c |
| This journal is © The Royal Society of Chemistry 2017 |