The improved ion clustering and conductivity of a di-quaternized poly(arylene ether ketone sulfone)-based alkaline fuel cell membrane†
Geetanjali
Shukla
ab and
Vinod K.
Shahi
 *ab
*ab
aElectro-Membrane Processes Division, CSIR-Central Salt and Marine Chemicals Research Institute, Council of Scientific & Industrial Research, Gijubhai Badheka Marg, Bhavnagar 364 002, Gujarat, India. E-mail: vkshahi@csmcri.org; vinodshahi1@yahoo.com; Fax: +91-0278-2566970; Tel: +91-278-2569445
bAcademy of Scientific and Innovative Research, CSIR-Central Salt and Marine Chemicals Research Institute, Council of Scientific & Industrial Research, Gijubhai Badheka Marg, Bhavnagar 364 002, Gujarat, India
First published on 11th April 2017
Abstract
Herein, we designed a hydrophobic–hydrophilic phase-separated poly(arylene ether ketone sulfone) (PAEKS) random copolymer-based di-quaternized stable and highly conductive alkaline membrane (AM). These membranes were prepared by bromination and mono- and di-quaternization using bi-functional 1,4-diazabicyclo[2,2,2]octane (DABCO) as the quaternization agent, which showed improved ion clusters. The reported method avoids the use of hazardous choloromethyl methyl ether (CMME), and the hydrophilic–hydrophobic blocks were responsible for the formation of OH− conductive channels. Furthermore, steric hindrance (due to methyl bonded second functional group) and the micro-phase separated structure were responsible for the improved alkaline resistance and formation of ion-clusters. The structure of PAEKS was confirmed by 1H NMR and FTIR spectroscopy, whereas the molecular weight was determined by gel permeation chromatography (GPC). Transmission electron microscopy (TEM) images showed well phase-separated ionic clusters. The well optimized di-quaternized alkaline membrane with a 68% degree of bromination (di-DQP-OH (DB: 68%)) exhibited a significantly improved ion-exchange capacity (2.45 meq g−1), hydroxide ion conductivity (4.68 × 10−2 S cm−1), and activation energy (6.61 kJ mol−1) along with good thermal, mechanical, and dimensional stabilities. These properties of the AMs confirm their potential applications in alkaline fuel cells.
1. Introduction
Proton exchange fuel cells (PMFCs) with a state-of-art acidic polymer electrolyte membrane (PEM) (such as Nafion®, a perfluorinated ionomer developed by DuPont) have been considered as an advanced electrochemical energy conversion technology.1–3 However, several disadvantages such as slow electrode kinetics, precious metal electrocatalysts, and low methanol barrier limit the further applications of PMFCs.4–6 To address these problems, the acidic PEMs have been replaced with alkaline membranes (AMs), where the methanol flux is reduced due to OH− mobility in the opposite direction.7,8 A variety of polymers such as poly(ether ketone),9,10 polyethersulfones,11–13 polystyrenes,14 poly(phenylene oxide)s,15 poly(ether-imide),16 radiation-grafted fluorinated polymers,17 and organic–inorganic hybrid composites18 have been explored for developing AMs after chloromethylation. In particular, chloromethylation is achieved using chloromethyl methyl ether (CMME), which is carcinogenic and potentially harmful to human health.19–21 Herein, we proposed bromination using N-bromosuccinimide (NBS) to avoid the use of CMME. Furthermore, chloromethylation can be achieved at a single position of the benzene ring without any side reaction or cross-linking.22 To avoid these problems, a green three-step method, polycondensation, bromination, and quaternization, was reported.22,23 Chen et al. anchored quaternary ammonium groups with the bis(3,5-dimethyl-4-hydroxyphenyl)-3,5-dimethylphenylmethane monomer unit and achieved 70% functionalization.22 However, the membrane showed instability and a high degree of swelling due to the high molality of the cationic functional groups. It was suggested that for improved membrane conductivity, distinct ion clustering or hydrophilic–hydrophobic phase separation can be created by synthesizing random copolymers with hydrophilic and hydrophobic units.22,24,25 Herein, we also proposed a random copolymer of poly(arylene ether ketone sulfone) (PAEKS) containing well-separated hydrophilic and hydrophobic domains.Generally, the low conductivity of AMs may be circumvented by grafting alkaline functional groups in high molality with the main polymer.8,11,24,26–28 In this case, bi-functional 1,4-diazabicyclo[2,2,2]octane (DABCO) was proposed to achieve the high molality of quaternary ammonium groups.13,27 The di-quaternized polymer with a bunch of OH− ionic clusters was responsible for the improved conductivity without any structural deterioration. This strategy was used to prepare the di-quaternized poly(ether ether ketone)-based AM containing two cations with a side chain using DABCO.27 However, to achieve good phase separation and conductivity, well-separated hydrophobic–hydrophilic segments were essentially required.29
We designed a well hydrophobic–hydrophilic phase-separated poly(arylene ether ketone sulfone)-based random copolymer; this copolymer contained tetra-phenyl methane groups as active sites for grafting the di-quaternizing agent (DABCO) to fabricate the stable and highly conductive AMs with improved ion clusters. The chemical and thermal stabilities of the di-quaternized membranes were also explored along with their properties. The proposed strategy may be used as an advantage for developing multiple quaternized and distinct phase-separated conducting random copolymers.
2. Experimental
2.1. Materials
4,4′-Difluorobenzophenone (DFBP) (99%) and 4,4′-sulfonyldiphenol (SDP) (98%) were obtained from Sigma-Aldrich Chemicals. 2,2-Bis(4-hydroxy-3,5-dimethylphenyl)propane (BHDMPP) (98%) and 1,4-diazabicyclo[2,2,2]octane (DABCO) (98%) were received from TCI Chemicals. Toluene, dry dimethylacetamide (DMAc), N-methyl-2-pyrrolidinone (NMP), dimethyl sulfoxide (DMSO), potassium carbonate (K2CO3), tetrahydrofuran (THF), N-bromosuccinimide (NBS), 1,1,2,2-tetrachloroethane (TCE), and benzoyl peroxide (BPO) of analytical grade were obtained from commercial sources and used as received without purification. Deionized (DI) water was used for all the experiments.2.2. The synthesis and bromination of poly(arylene ether ketone sulfone) (PAEKS)
PAEKS was synthesized via a nucleophilic substitution and polycondensation reaction (Scheme 1). In a typical procedure, DFBP (2.0 g), SDP (1.0 g), BHDMPP (1.0 g), K2CO3 (2.7 g), DMAc (15.0 mL), and toluene (8.0 mL) were charged into a three-necked round-bottomed flask equipped with a magnetic stirrer and Dean–Stark trap condenser with a N2 gas inlet and outlet. The polymerization reaction was carried out at 140–150 °C (oil bath) in a N2 environment under constant stirring for 3 h. The reaction temperature was increased to 175–180 °C and was maintained for 24 h to remove the water produced due to toluene distillation. The obtained light yellow viscous liquid mixture was cooled to 30 °C and precipitated in hot water. The resultant fibrous precipitate was successively washed several times with DI water and methanol and dried in a vacuum oven at 60 °C for two days.Bromination of PAEKS was carried out via radical substitution using NBS in TCE solvent and BPO as an initiator (Scheme 1).30 In a typical procedure, PAEKS (1.0 g) and TCE (20 mL) were charged in a 100 mL three-necked round-bottomed flask equipped with a magnetic stirrer and a condenser with a N2 inlet and outlet. After complete dissolution, NBS (0.6307 g) and BPO (0.0429 g) were added and reaction mixture was heated at 85 °C under a N2 atmosphere for 5 h. Afterwards, the mixture was cooled to 30 °C, precipitated with excess of methanol, repeatedly washed with hot methanol to remove the impurities (if any), filtered and dried in vacuum oven at 60 °C for 24 h to obtain a light yellow solid brominated PAEKS (Br-PAEKS).
2.3. Quaternization of Br-PAEKS and membrane preparation
DABCO and methyl iodide were used as the quaternizing agent for preparing the mono- and di-quaternized PAEKS (mono-DQP-Br and di-DQP-Br, respectively). In a typical procedure for the preparation of mono-DQP-Br, Br-PAEKS (1.0 g) and DABCO (1.5 g) were added to the desired volume of NMP; the reaction mixture was stirred for 6 h at 30 °C and precipitated with methanol. The obtained precipitate was washed several times with methanol and dried under vacuum at 60 °C. The di-DQP-Br was prepared by dissolving mono-DQP-Br in NMP in the presence of methyl iodide under constant stirring at 30 °C for 24 h, precipitated with an excess of methanol, and dried in a vacuum oven at 60 °C.For the membrane preparation, mono-DQP-Br or di-DQP-Br (7 wt%) was dissolved in NMP to form a viscous solution that was transformed into a thin film of desired thickness on a cleaned glass plate with the help of a doctor blade and dried in vacuum at 60 °C for 24 h. Dried membrane sample was thoroughly washed with water and equilibrated in NaOH solution (1.0 M) for 24 h to converted into OH− form. The obtained membranes were named as mono-DQP-OH or di-DQP-OH, washed several times with DI water, and finally with NaOH solution before characterization. The average thickness of the membrane thin film was measured to be about 150 μm.
2.4. Characterization
Detailed instrumental analysis for the 1H NMR spectra (500 MHz), FT-IR spectra, wide-angle X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), optical images, thermogravimetric analysis (TGA), gas permeation chromatography (GPC), and mechanical strength are included in the Section S1 of the ESI.†The water uptake (WU) of the membranes may be defined as the ratio between the weight of the dry (Wdry) and wet (Wwet) membrane samples and was estimated by
 | (1) |
The ion exchange capacity (IEC) defines the moles of ionic moieties present per unit mass of the dry membrane, measured using an acid–base titration method. Dry membrane samples (mono-DQP-OH or di-DQP-OH) of a known weight (Wdry) were equilibrated in NaCl (0.1 M) for 24 h, and the liberated NaOH was titrated with acid (HCl) using phenolphthalein as an indicator via the following equation:
 | (2) |
The hydration number (λ) for different membranes was calculated via the following equation using the molecular weight of water (MH2O).
 | (3) |
The swelling ratio (SR) was obtained using the length of the wet and dry membrane samples (Lwet and Ldry, respectively).
 | (4) |
The in-plane hydroxide conductivity of the mono-DQP-OH or di-DQP-OH membranes was measured in DI water using a four-electrode AC impedance potentiostat/galvanostat frequency response analyzer (Eco Chemie, B.V. Utrecht, The Netherlands Auto Lab, model PGSTAT 302N) over the 1–106 Hz frequency range. The membrane was sandwiched between two in-house made stainless steel circular electrodes (4.0 cm2). Direct current (dc) and sinusoidal alternating current (ac) were supplied to the respective electrodes to record the frequency at a 1 μA s−1 scanning rate. The membrane resistance was determined from the Nyquist plots using the fit and simulation method. The membrane resistance (Rm) was measured in equilibration with deionised water and the membrane conductivity (κm) was estimated using the following equation:
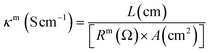 | (5) |
The activation energy (Ea) was estimated from the slope of Arrhenius plot (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) κmvs. 1/T) using the following equation:
κmvs. 1/T) using the following equation:
 | (6) |
The procedure used for the measurement of methanol permeability has been included in Section S2 of the ESI.†
2.5. Direct methanol fuel cell performance
The single-cell DMFC performance for the best assessed di-DQP-OH AEM membrane was studied with the help of a MTS-150 manual fuel cell test station (Electro Chem Inc.), equipped with a controlled fuel flow and pressure, and temperature regulation attached with an electronic load control ECL-150 (Electro Chem Inc.). The anode was made by coating a slurry of the catalyst (50 wt% Pt + 50 wt% Ru on carbon), 5 wt% Nafion ionomer solution, 2-propanol, and DI water (catalyst ink), and 5.0 mg cm−2 catalyst ink, which was uniformly loaded on the desired membrane area with the help of paint brush. Similarly, the catalyst ink of the cathode was also prepared without Ru.31 The measurements were performed in the air mode of operation at 10 psi pressure with a 2 M methanol feed at the anode side with a pressure of 7 psi at 70 °C.3. Results and discussion
3.1. The synthesis and structural characterization of the random copolymer and functionalized derivatives
The poly(arylene ether ketone sulfone) (PAEKS) random copolymer was synthesized via a nucleophilic substitution and polycondensation reaction according to Scheme 1. The protons attached to the phenylene rings (PAEKS) exhibited 1H NMR peaks around 6.5–8.5 ppm (c, d, e, f, and g), whereas the presence of the aliphatic region was confirmed by the peaks around 1.5–2.2 ppm (a and b) (Fig. 1a). Characteristic FTIR bands around 1649, 1582, 1237 and 1102 cm−1 confirmed the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, S
C, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C groups, respectively, in PAEKS (Fig. 2a). The molecular weights Mn and Mw and poly-dispersity index (PDI) for PAEKS were found to be 70.2 kDa, 138.6 kDa, and 1.97, respectively. Controlled bromination of PAEKS (the degree of bromination (DB) was estimated by the integral ratio of the bromobenzyl proton peak to the bromobenzyl proton peak + unreacted benzyl proton peak and was found to be 56–68%) was achieved by varying the reaction temperature, time and NBS content (Fig. S1, ESI†).32,33 In the 1H NMR spectra, the peaks around 4.2–4.5 ppm (b2) confirmed successful bromination (Fig. 1b), and the brominated PAEKS was designated as Br-PAEKS. The FTIR spectrum (C–Br: 621 cm−1) also confirmed successful bromination (Fig. 2b). A small alteration in the molecular weights (Mn: 68.1 kDa and Mw: 149.8 kDa) and PDI (2.20) (GPC study) also suggested the formation of Br-PAEKS.
O and C–O–C groups, respectively, in PAEKS (Fig. 2a). The molecular weights Mn and Mw and poly-dispersity index (PDI) for PAEKS were found to be 70.2 kDa, 138.6 kDa, and 1.97, respectively. Controlled bromination of PAEKS (the degree of bromination (DB) was estimated by the integral ratio of the bromobenzyl proton peak to the bromobenzyl proton peak + unreacted benzyl proton peak and was found to be 56–68%) was achieved by varying the reaction temperature, time and NBS content (Fig. S1, ESI†).32,33 In the 1H NMR spectra, the peaks around 4.2–4.5 ppm (b2) confirmed successful bromination (Fig. 1b), and the brominated PAEKS was designated as Br-PAEKS. The FTIR spectrum (C–Br: 621 cm−1) also confirmed successful bromination (Fig. 2b). A small alteration in the molecular weights (Mn: 68.1 kDa and Mw: 149.8 kDa) and PDI (2.20) (GPC study) also suggested the formation of Br-PAEKS.
Br-PAEKS (with varied DB) was functionalized by grafting DABCO, and the resultant mono-quaternized PAEKS (mono-DQP-OH) exhibited signals around 4.3 ppm (b2) and 2.4–3.0 ppm (h and i) in the 1H NMR spectrum (Fig. 3a). However, di-quaternized PAEKS (di-DQP-OH) was prepared by further quaternization of mono-DQP-OH and showed similar signals in addition to a new signal at around 3.3 ppm (j) (Fig. 3b). This observation was also supported by the FTIR spectrum and an absorption band around 1371 cm−1 confirmed the successful formation of a C–N bond, and the new peaks around 3348 and 3320 cm−1 (Fig. 2c and d) were due to the –OH stretching vibration.13
3.2. The morphology of the mono- and di-functionalized DQP-OH alkaline membranes
For an effective architecture of hydroxide ion conductive channels in the alkaline membrane, hydrophilic–hydrophobic micro-phase separation is the most desired requirement.30 Representative SEM images (surface and cross-section) of the mono-DQP-OH and di-DQP-OH (DB: 68%) membranes revealed smooth, dense and homogeneous surface structures, and a change in the surface morphology after di-functionalization for the mono-DQP-OH membrane was observed (Fig. 4).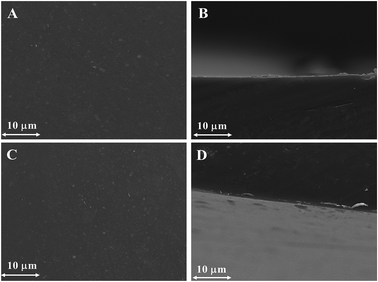 | ||
| Fig. 4 The SEM images (surface and cross-section) of: (A & B) mono-DQP-OH-68 and (C & D) di-DQP-OH-68 AMs. | ||
The dark and bright areas in the TEM images of the mono-DQP-OH and di-DQP-OH (DB: 68%) membranes (Fig. 5A and D) show the ionic hydrophilic and hydrophobic domains, respectively.24,27 Uniformly attached ionic clusters in the mono-DQP-OH membrane phase are relatively smaller than those observed in the di-DQP-OH membrane. The ionic clusters (∼2 nm) of the latter were interconnected ionic channels and their morphology was similar to that of Nafion.34,35 The larger size and number of ionic clusters in the di-DQP-OH alkaline membrane may be attributed to its high IEC (2.45 meq g−1) value, which is responsible for the improved micro-phase separation.27
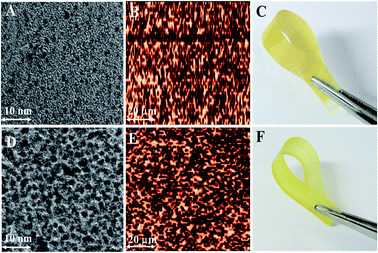 | ||
| Fig. 5 The (A & D)-TEM, (B & E)-AFM phase and (C & F)-optical images of the mono-DQP-OH-68 & di-DQP-OH-68 AMs, respectively. | ||
The AFM phase images of the mono-DQP-OH and di-DQP-OH (DB: 68%) membranes in the tapping mode at room temperature also showed the dark and bright regions, representing the hydrophilic and hydrophobic domains, respectively (Fig. 5B and E).36–38 The optical images showed the semi-transparent tough and flexible nature of these membranes (Fig. 5C and F). The reported di-functionalization strategy demonstrates enhanced ionic clustering and thus micro-phase separation, which is suitable for an efficient alkaline membrane. In the XRD spectra, mono-DQP-OH (DB: 68) showed two peaks around 20.35° and 8.91°, whereas di-DQP-OH (DB: 68) exhibited a broadened peak around 8.16° (Fig. S2, ESI†). The mild shift and broadening of the peak confirmed the hydrophilic nature of di-DQP-OH.
3.3. The thermal, mechanical and chemical stabilities
The TGA curves obtained for the mono-DQP-OH and di-DQP-OH (DB: 68%) alkaline membranes showed a three-step weight loss (Fig. S3, ESI†). The first weight loss (30–150 °C) was assigned to the loss of absorbed and bound water, whereas the second weight loss around 300–350 °C was attributed to the degradation of the quaternary ammonium groups. The third weight loss (>350 °C) was attributed to the decomposition of the main polymer chain. In each step, the weight loss for the di-DQP-OH (DB: 68%) AM was higher compared with that of the mono-DQP-OH (DB: 68%) AM, which was attributed to the large clusters of its hydrophilic groups.The mechanical stability of the AMs was studied using the stress–strain curve, and the tensile strength of mono-DQP-OH (DB: 68%) AM (26.56 MPa) was found to be significantly high compared with that of di-DQP-OH (DB: 68%) AM (17.32 MPa). The tensile strength was significantly reduced due to the high concentration of quaternary ammonium groups in spite of the relatively high molecular weights (Mn: 68.1 and Mw: 149.8 kDa).
Chemical stability is a serious challenge for fuel cell AMs under strongly basic, oxidative, and hydrolytic (elevated temperature) conditions. Under harassed oxidative (treatment with Fenton's reagent at 80 °C for 1 h) and hydrolytic (treatment at 140 °C for 24 h under pressurized steam) conditions, both membranes (mono-DQP-OH and di-DQP-OH (DB: 68%)) were unbroken and showed about 1.8%–3.8% κm loss (Table 1). After treatment with 2.0 M NaOH at 65 °C for 120 h, these membranes retained their toughness and appearance, and di-DQP-OH AMs (loss in κm: 16.6%) exhibited better alkaline stability compared with mono-DQP-OH membrane (loss in κm: 18.6%). The alkaline stability of the mono-DQP-OH and di-DQP-OH AEMs with low DB was comparatively high, and the AEMs with high DB degraded in a relatively fast manner. However, to achieve high conductivity, a higher degree of bromination (amination) was necessary. Thus, a complete optimization of the degree of bromination was carried out, and mono-DQP-OH-68 showed about 18.6% weight loss after alkaline treatment against a 16.6% weight loss observed for di-DQP-OH-68. Furthermore, the long term (336 h) alkaline stability data for mono-DQP-OH-68, di-DQP-OH-62, and di-DQP-OH-68 membranes revealed the stable nature of di-DQP-OH-68 under alkaline conditions (Fig. 6).
| Degree of bromination (%) | Stability of mono-DQP-OH | Stability of di-DQP-OH | ||||
|---|---|---|---|---|---|---|
| Oxidative κm loss (%) | Hydrolytic κm loss (%) | Alkaline κm loss (%) | Oxidative κm loss (%) | Hydrolytic κm loss (%) | Alkaline κm loss (%) | |
| a Alkaline stability carried out in 2 M NaOH for 5 days at 65 °C. | ||||||
| 56 | 2.5 ± 0.15 | 1.8 ± 0.10 | 4.1 ± 0.10 | 3.1 ± 0.10 | 2.4 ± 0.06 | 3.3 ± 0.17 |
| 62 | 3.2 ± 0.20 | 2.6 ± 0.20 | 8.1 ± 0.20 | 3.9 ± 0.10 | 2.8 ± 0.15 | 7.8 ± 0.15 |
| 68 | 3.8 ± 0.11 | 3.1 ± 0.10 | 18.6 ± 0.10 | 4.6 ± 0.20 | 3.6 ± 0.15 | 16.6 ± 0.15 |
 | ||
| Fig. 6 The conductivity profile obtained for the mono-DQP-OH-68, di-DQP-OH-62 and di-DQP-OH-68 AMs after long-term alkaline treatment (2.0 M NaOH at 65 °C). | ||
Moreover, the FT-IR spectra of the treated and untreated di-DQP-OH and mono-DQP-OH membranes showed all the characteristic bands; however, the slightly reduced intensity of the treated membrane was observed due to partial E1 and E2 nucleophilic substitution reactions (Fig. S4 and S5(A & B), ESI†).26 Furthermore, these observations confirmed the alkaline stability of the membranes even under high temperature conditions. The reported strategy for preparing the di-DQP-OH AMs avoids alkaline degradation of the quaternary ammonium groups due to the steric hindrance between the methyl and second functional group, and the hydrophilic–hydrophobic micro-phase separated structure is responsible for the formation of ion-clusters.
3.4. Water uptake (WU), swelling ratio (SR), and ion-exchange capacity (IEC) under hydrated conditions
The presence of water in the membrane phase solvates the fixed-ionic groups and is essential for ion-pair separation and membrane conductivity.23 However, an excessive water uptake will cause membrane swelling and thus membrane instability.39 The WU values obtained for the mono-DQP-OH and di-DQP-OH AMs increased with the DB value or density of the functional groups (Table 2). As reference, the mono-DQP-OH and di-DQP-OH (DB: 68%) AMs showed 39.48% and 49.47% WU against the 15.36% and 18.52% SR values, respectively. Water absorption is beneficial for the formation of interconnected OH− conducting pathway (ionic clusters). The WU and SR values (at 30 °C and 65 °C) obtained for the mono-DQP-OH and di-DQP-OH membranes are included in Table S1 (ESI†). The mono-DQP-OH (DB = 68) membrane (IEC: 2.16 meq g−1) at 30 °C showed an SR value of about 15.36%, which increased to 19.62% at 65 °C due to the increased WU (48.18%). Similar behaviour was also observed for the WU and SR values of di-DQP-OH (DB = 68) (IEC: 2.45 meq g−1). The high WU and SR values of these membranes at high temperatures (65 °C) may be attributed to the increased mobility of water in the membranes. Moreover, the IEC observed for the mono-DQP-OH (DB: 68%) AM (2.16 meq g−1) was significantly increased to 2.45 meq g−1 for the di-DQP-OH (DB: 68%) AM. The hydration number (λ) (moles of water per mole of quaternary ammonium group) responsible for the formation of ionic clusters increased with the functional group concentration (Table 2).| DB (%) | Mono-DQP-OH | Di-DQP-OH | ||||||
|---|---|---|---|---|---|---|---|---|
| WUa (%) | IECb (meq g−1) | κ m × 10−2 (S cm−1) | λ | WU (%) | IEC (meq g−1) | κ m × 10−2 (S cm−1) | λ | |
| a Measuring error: 0.01%. b Measuring error: 0.01 meq g−1. c Measuring error: 0.01 × 10−2 S cm−1, measured at 30 °C. | ||||||||
| 56 | 28.86 ± 0.03 | 1.96 ± 0.04 | 3.32 ± 0.01 | 8.18 | 36.26 ± 0.08 | 2.18 ± 0.02 | 3.76 ± 0.02 | 9.24 |
| 62 | 34.15 ± 0.06 | 2.04 ± 0.02 | 3.46 ± 0.02 | 9.30 | 44.18 ± 0.04 | 2.36 ± 0.01 | 4.18 ± 0.03 | 10.41 |
| 68 | 39.48 ± 0.05 | 2.16 ± 0.05 | 3.71 ± 0.02 | 10.01 | 49.47 ± 0.03 | 2.45 ± 0.01 | 4.68 ± 0.01 | 11.20 |
The WU data for the mono-DQP-OH and di-DQP-OH AMs shows the segregation of the hydrophilic domains from the hydrophobic domains (polymer chain), which also effects membrane morphology and dimensional instability. In the case of the di-DQP-OH AM, the large ionic clusters (relatively high λ value) also promote the IEC for di-DQP-OH (DB: 68%). Thus, it is necessary to study the trade-off behaviour of the mono-DQP-OH and di-DQP-OH AMs with varied DB in terms of the desirable membrane properties and mechanical/dimensional stability.
3.5. Hydroxide conductivity (κm)
Membrane conductivity is an inclusive effect of the WU, SR, and hydroxide ion concentration in the matrix. The mono-DQP-OH (DB: 68%) and di-DQP-OH (DB: 68%) AMs exhibited a conductivity of 3.71 × 10−2 and 4.68 × 10−2 S cm−1 at 30 °C, respectively. The relatively high conductivity observed for the di-DQP-OH AMs may be attributed to the high WU, IEC and thus, the high concentration of OH− in the membrane phase. In the case of the di-DQP-OH AM, the architecture of efficient hydroxide ion conductive channels with large ionic clusters was responsible for the improved ionic conductivity. Furthermore, the comparable methanol permeability found for di-DQP-OH (68%) (1.28 × 10−7 cm2 s−1) and mono-DQP-OH (68%) (1.47 × 10−7 cm2 s−1) AMs also suggested the effectiveness of the well phase-separated morphology being impervious to fuel-crossover even after grafting of functional groups with high molalities.The membrane conductivity of both AMs (mono-DQP-OH (DB: 68%) and di-DQP-OH (DB: 68%) followed an approximate Arrhenius-type temperature dependence, which may be due to the promoted thermal activation of the water molecules (Fig. 7).40 The apparent activation energy (Ea) for mono-DQP-OH (DB: 68%) (7.28 kJ mol−1) and di-DQP-OH (DB: 68%) (6.61 kJ mol−1) were relatively low compared with quaternary ammonium functionalized aromatic polymers (Fig. S6, ESI†).10,41 Hydroxide ion solvation and migration across the AM is important and provides information that is helpful for the design of membranes. It is reported that a relatively uniform separation of rigid hydrophobic polymer chains and hydrophilic ionic clusters produce continuous channels for hydroxide ion transport. The reported AMs with significant phase separation provide ion conducting channels for hydroxide ion transport via both vehicular (standard diffusion) and Grotthuss (proton hopping) mechanisms.42,43 Furthermore, the lowered activation energy barrier for hydroxide diffusion in di-DQP-OH-68 indicates a significant enhancement of ion transport in the AM at elevated temperatures.
 | ||
| Fig. 7 The membrane conductivity of the mono-DQP-OH & di-DQP-OH (DB: 68%) AMs at different temperatures. | ||
The ionic conductivity value found for the synthesized di-DQP-OH (DB: 68%) AM was found to be superior compared with other AMs based on either poly(ether ketone) or poly(arylene sulfone) reported in the literature (Table 3).23,33,38,44–47
3.6. Fuel cell performance
The fuel cell performance of the di-DQP-OH (DB: 68%) AM with 2.45 meq g−1 IEC and 4.68 × 10−2 S cm−1 conductivity in OH− was also assessed in a single cell. The current–voltage polarization curve for the di-DQP-OH (DB: 68%) membrane was obtained at 50 °C with 100% relative humidity and ambient pressure (Fig. 8). The high open circuit voltage (OCV) (0.83 V) and peak power density (44.5 mW cm−2) (at 153 mA cm−2 current density) may be assigned to the low permeation of reactant gaseous through the membrane, which also favoured the reaction kinetics in the alkaline medium. It appears that the membrane–electrode interfacial properties and gas-diffusion layer played significant roles. In this case, serious attention was rendered to design and characterize the highly functionalized (di-quaternary ammonium groups) membrane matrix with hydrophilic–hydrophobic phase separation. The data suggested that the reported strategy and particularly di-DQP-OH (DB: 68%) AM showed strong potential for its use in alkaline fuel cells.4. Conclusions
The architecture of the hydrophilic–hydrophobic phase separated efficient ion conducting channels in AMs is highly desirable to improve the ionic conductivity. We synthesised a well phase separated poly(arylene ether ketone sulfone) random copolymer containing tetra-phenyl methane groups via a nucleophilic substitution and polycondensation reaction. Multiple (mono- and di-) quaternized groups were grafted on the main polymer backbone via a bromination di-quaternization route using DABCO as a bi-functional quaternization agent. It was observed that the di-DQP-OH AM (DB: 68%; IEC: 2.45 meq g−1) showed a larger size and number of ionic clusters, which were responsible for the improved micro-phase separation. In the case of the di-DQP-OH AM, the efficient hydroxide ion conductive channels and large ionic clusters were responsible for the improved membrane performance. Both AMs, (mono-DQP-OH (DB: 68%) and di-DQP-OH (DB: 68%), showed 7.28–6.61 kJ mol−1 ion transport activation energy, which was comparatively low. Furthermore, di-DQP-OH (DB: 68%) exhibited an 0.83 V OCV and 44.5 mW cm−2 peak power density at a 153 mA cm−2 current density under fuel cell testing conditions. The reported AMs with excellent thermal, mechanical and chemical stabilities appear to be promising candidates for use in alkaline fuel cells. The di-functionalization strategy demonstrates the improved conductivity and ionic clustering, which are useful to fabricate multi-functional polyamines.Acknowledgements
Registration number: CSIR-CSMCRI-052/2017. This study was supported by the Department of Science and Technology (Govt. of India) (Project No. DST/INT/UK/P-55/2014). The central instrumental facilities of the CSIR-CSMCRI (Analytical Science Division) are also acknowledged.References
- X. H. Li, Y. F. Yu, Q. F. Liu and Y. Z. Meng, ACS Appl. Mater. Interfaces, 2012, 4, 3627–3635 CAS.
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K. I. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS PubMed.
- B. P. Tripathi and V. K. Shahi, Prog. Polym. Sci., 2011, 36, 945–979 CrossRef CAS.
- J. Pan, Y. Li, J. Han, G. Li, L. Tan, C. Chen, J. Lu and L. Zhuang, Energy Environ. Sci., 2013, 6, 2912–2915 CAS.
- S. Gu, R. Cai, T. Luo, Z. Chen, M. Sun, Y. Liu, G. He and Y. Yan, Angew. Chem., Int. Ed., 2009, 48, 6499–6502 CrossRef CAS PubMed.
- A. Jasti and V. K. Shahi, J. Mater. Chem. A, 2013, 1, 6134–6137 CAS.
- J. R. Varcoe and R. C. T. Slade, Fuel Cells, 2005, 5, 187 CrossRef CAS.
- T. J. Clark, N. J. Robertson, H. A. Kostalik, E. B. Lobkovsky, P. F. Mutolo, H. D. Abruna and G. W. Coates, J. Am. Chem. Soc., 2009, 131, 12888–12889 CrossRef CAS PubMed.
- Y. Xiong, Q. L. Liu and Q. H. Zeng, J. Power Sources, 2009, 193, 541–546 CrossRef CAS.
- A. Jasti, S. Prakash and V. K. Shahi, J. Membr. Sci., 2013, 428, 470–479 CrossRef CAS.
- M. R. Hibbs, M. A. Hickner, T. M. Alam, S. K. McIntyre, C. H. Fujimoto and C. J. Cornelius, Chem. Mater., 2008, 20, 2566–2573 CrossRef CAS.
- L. Li and Y. Wang, J. Membr. Sci., 2005, 262, 1–4 CrossRef CAS.
- G. Das, B. J. Park and H. H. Yoon, J. Mater. Chem. A, 2016, 4, 15554–15564 CAS.
- K. Kneifel and K. Hattenbach, Desalination, 1980, 34, 77–95 CrossRef CAS.
- L. Wu, T. Xu, D. Wu and X. Zheng, J. Membr. Sci., 2008, 310, 577–585 CrossRef CAS.
- G. Wang, Y. Weng, D. Chu, D. Xie and R. Chen, J. Membr. Sci., 2009, 326, 4–8 CrossRef CAS.
- J. R. Varcoe and R. C. T. Slade, Electrochem. Commun., 2006, 8, 839–843 CrossRef CAS.
- Y. Wu, C. Wu, T. Xu, X. Lin and Y. Fu, J. Membr. Sci., 2009, 329, 236–245 CrossRef CAS.
- T. Xu, Z. Liu, Y. Li and W. Yang, J. Membr. Sci., 2008, 320, 232–239 CrossRef CAS.
- S. Singh, A. Jasti, M. Kumar and V. K. Shahi, Polym. Chem., 2010, 1, 1302–1312 RSC.
- M. Kumar, S. Singh and V. K. Shahi, J. Phys. Chem. B, 2010, 114, 198–206 CrossRef CAS PubMed.
- D. Chen and M. A. Hickner, Macromolecules, 2013, 46, 9270–9278 CrossRef CAS.
- J. Si, S. Lu, X. Xu, S. Peng, R. Xiu and Y. Xiang, ChemSusChem, 2014, 7, 3389–3395 CrossRef CAS PubMed.
- M. Tanaka, K. Fukasawa, E. Nishino, S. Yamaguchi, K. Yamada, H. Tanaka, B. Bae, K. Miyatake and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 10646–10654 CrossRef CAS PubMed.
- R. Gao, D. Wang, J. R. Heflin and T. E. Long, J. Mater. Chem., 2012, 22, 13473–13476 RSC.
- G. Merle, M. Wessling and K. Nijmeijer, J. Membr. Sci., 2011, 377, 1–35 CrossRef CAS.
- X. Wu, W. Chen, X. Yan, G. He, J. Wang, Y. Zhang and X. Zhu, J. Mater. Chem. A, 2014, 2, 12222–12231 CAS.
- M. Tanaka, M. Koike, K. Miyatake and M. Watanabe, Macromolecules, 2010, 43, 2657–2659 CrossRef CAS.
- C. H. Park, S. Y. Lee, D. S. Hwang, D. W. Shin, D. H. Cho, K. H. Lee, T. W. Kim, T. W. Kim, M. Lee, D. S. Kim, C. M. Doherty, A. W. Thornton, A. J. Hill, M. D. Guiver and Y. M. Lee, Nature, 2016, 532, 480–483 CrossRef PubMed.
- P. Y. Xu, K. Zhou, G. L. Han, Q. G. Zhang, A. M. Zhu and Q. L. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 6776–6785 CAS.
- H. Y. Li and Y. L. Liu, J. Mater. Chem. A, 2014, 2, 3783–3793 CAS.
- J. Yan and M. A. Hickner, Macromolecules, 2010, 43, 2349–2356 CrossRef CAS.
- D. Chen and M. A. Hickner, ACS Appl. Mater. Interfaces, 2012, 4, 5775–5781 CAS.
- E. M. W. Tsang, Z. Zhang, A. C. C. Yang, Z. Shi, T. J. Peckham, R. Narimani, B. J. Frisken and S. Holdcroft, Macromolecules, 2009, 42, 9467–9480 CrossRef CAS.
- C. Wang, D. W. Shin, S. Y. Lee, N. R. Kang, G. P. Robertson, Y. M. Lee and M. D. Guiver, J. Mater. Chem., 2012, 22, 25093–25101 RSC.
- B. D. Ratner and V. V. Tsukruk, Scanning probe microscopy of polymers, ACS Symposium Series 694, American Chemical Society, Washington, DC, 1998 Search PubMed.
- G. Nisato, B. D. Ermi, J. F. Douglas and A. Karim, Macromolecules, 1999, 32, 2356–2364 CrossRef CAS.
- J. Wang, Z. Zhao, F. Gong, S. Li and S. Zhang, Macromolecules, 2009, 42, 8711–8717 CrossRef CAS.
- Y. S. Kim, L. M. Dong, M. A. Hickner, T. E. Glass, V. Webb and J. E. McGrath, Macromolecules, 2003, 36, 6281–6285 CrossRef CAS.
- A. A. Kornyshev, A. M. Kuznetsov, E. Spohr and J. Ulstrup, J. Phys. Chem. B, 2003, 107, 3351–3356 CrossRef CAS.
- X. Yan, G. He, S. Gu, X. Wu, L. Du and H. Zhang, J. Membr. Sci., 2011, 375, 204–211 CrossRef CAS.
- C. Chen, Y. L. S. Tse, G. E. Lindberg, C. Knight and G. A. Voth, J. Am. Chem. Soc., 2016, 138, 991–1000 CrossRef CAS PubMed.
- M. E. Tuckerman, D. Marx and M. Parrinello, Nature, 2002, 417, 925–929 CrossRef CAS PubMed.
- E. A. Weiber and P. Jannasch, J. Membr. Sci., 2015, 481, 164–171 CrossRef CAS.
- X. Li, Y. Yu and Y. Meng, ACS Appl. Mater. Interfaces, 2013, 5, 1414–1422 CAS.
- X. Li, Q. Liu, Y. Yu and Y. Meng, J. Mater. Chem. A, 2013, 1, 4324–4335 CAS.
- B. Wang, W. Sun, F. Bu, X. Li, H. Na and C. Zhao, Int. J. Hydrogen Energy, 2016, 41, 3102–3112 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The supporting information provides instrumental details for the 1H NMR and FTIR spectra, TGA, WXRD, SEM, and TEM (Section S1); methanol permeability (Section S2); the 1H NMR spectra for the different degrees of bromination (Fig. S1); XRD patterns (Fig. S2); TGA spectra (Fig. S3); Arrhenius plot (Fig. S6). See DOI: 10.1039/c7se00097a |
| This journal is © The Royal Society of Chemistry 2017 |





