Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of functionalized unsymmetrical 1,3-butadiene-3-yne derivatives from β-halo styrene derivatives and their application in the synthesis of trisubstituted pyridines†
Vijayalakshmi Bandi,
Veerababurao Kavala,
Che-Hao Hsu,
Ashok Konala,
Bharath Kumar Villuri,
Trimurtulu Kotipalli,
Chun-Wei Kuo and
Ching-Fa Yao *
*
Department of Chemistry, National Taiwan Normal University, 88, Section 4, Tingchow Road, Taipei, Taiwan 116, Republic of China. E-mail: cheyaocf@ntnu.edu.tw
First published on 3rd October 2017
Abstract
An approach for the synthesis of functionalized unsymmetrical 1,3-butadiene-3-yne derivatives is reported starting from β-halo styrene and phenyl acetylene derivatives in the presence of PdCl2 and CuI catalysts. Functional groups such as aldehyde, cyano and ester groups are well tolerated and afford the desired functionalized dienynes. Further, these 1,3-buta-diene-3-yne derivatives are utilized for the synthesis of novel trisubstituted pyridine derivatives.
Introduction
Enynes are the predominant structures in modern organic chemistry since their derivatives are important intermediates in the synthesis of highly substituted aromatic rings and are found in various pharmaceutical drugs.1 1,3- and 1,4-disubstituted enynes are also interesting derivatives of enynes, which exist in various polymers2 and biologically active molecules.3 Owing to their applications, various methods for the synthesis of 1,3- and 1,4-disubstituted enynes are well known in the literature. A literature survey on the synthesis of 1,3-butadiene-5-yne derivatives revealed various reports with Ni,4 Pd5 and lanthanide6 and actinide7 catalysts being well documented for the trimerization of silyl alkynes and terminal alkynes. On the other hand, limited reports are available for the synthesis of 1,3-butadiene-3-yne derivatives, which are also 1,3-enynes. For instance, Wu et al. synthesized various symmetrical 1,3-diaryl-2-arylethynyl-1,3-butadienes by the Pd-catalyzed trimerization of arylalkynes.8 Later, Oro et al. reported the synthesis of 1,3-diaryl/alkyl-2-trimethylsilylethynyl-1,3-butadienes by cross-trimerization of terminal alkynes with trimethylsilylacetylene through C–H activation using rhodium(I)–pyridine-N-heterocyclic carbene catalyst.9 Both the reported methods have certain limitations such as low yields and limited scope.As a result, an efficient method for the synthesis of unsymmetrical 1,3-butadiene-3-yne derivatives is highly desirable and also to the best of our knowledge, very few reports exists in the literature for the synthesis of functionalized dienynes. Herein, we report the synthesis of functionalized unsymmetrical 1,3-butadiene-3-yne derivatives from β-halo styrene derivatives. While our work is under progress, Swamy and coworkers reported the synthesis of norbornadienes/norbornenes starting from alkynyldienols in the presence of Au catalyst.10 The starting material alkynyldienol is obtained from Sonogashira cross-coupling of 3-bromoenals to terminal alkynes followed by alkyne addition and reduction. Although they prepared dienynal derivative, further studies are not carried out on the role of the functional group during the alkyne addition step to enynal derivative.
Results and discussion
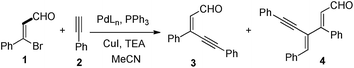
As a part of our interest in developing fascinating methodologies11 for the synthesis of variety of heterocyclic compounds, we planned to carry out Sonogashira coupling between β-bromo cinnamaldehyde and phenyl acetylene under standard reaction conditions in the presence of CuI/Pd(PPh3)2Cl2 bicatalytic system in acetonitrile solvent at room temperature for one hour. Under this reaction condition, unknown product 4 is observed in trace amounts along with the expected enynal product 3 (entry 1, Table 1). From 1H and 13C-NMR studies, X-ray crystallography studies12 the structure of 4 is confirmed. We realized that the dienynal derivative 4 is formed by the addition of one more alkyne moiety to enynal derivative 3. In order to improve the yield of dienynal 4, we screened the reaction further. There is substantial increase in the yield of dienynal 4, when we used excess of phenyl acetylene (entry 2, Table 1). Next, the yield of the desired product was slightly improved when the reaction temperature increased to 50 °C (entry 3, Table 1). Further, we added triphenyl phosphine as ligand to improve the yield of the desired product (entry 4, Table 1). However, there was no marked improvement in yield of the product was observed in this reaction. Then, we replaced Pd(PPh3)2Cl2 with simple palladium catalyst such as palladium chloride and triphenyl phosphine, to our surprise, the reaction produced good yield of the desired product after 1.5 h (entry 5, Table 1). Further, we observed dramatic improvement in the yield of the desired product when reaction time extends to 2 h, (entry 6, Table 1). Moreover, further extending the reaction showed negative impact on the reaction (entry 7, Table 1). No improvement in the yield of dienynal derivative was observed with further additional amounts of phenylacetylene (entries 8–9, Table 1). Dienynal derivative 4 is not observed, when reaction carried out in the presence of Pd(PPh3)4 (entry 10, Table 1). Next we screened the reaction with palladium acetate as catalyst. Dienynal derivative formed in 85%, when β-bromo styrene reacts with phenyl acetylene in the presence of Pd(OAc)2 (entry 11, Table 1). As the result with Pd(OAc)2 was encouraging, we screened the reaction in various conditions with Pd(OAc)2 (entries 12–14, Table 1). It was found that the reaction produced better yield in dioxane solvent in the presence of palladium acetate. Moreover, from optimization studies, it is observed that an excellent yield of the desired product 4 is obtained in 92% with 1 equiv. of β-bromo cinnamaldehyde, 2.5 equiv. of phenyl acetylene in the presence of PdCl2 (0.05 mmol), PPh3 (0.1 mmol), CuI (0.1 mmol) in acetonitrile solvent for 2 h at 50 °C.| Entrya | Pd catalyst (mmol) | Cul (mmol) | 2 (mmol) | T (°C) | Time (h) | Yield (%)b | |
|---|---|---|---|---|---|---|---|
| 3 | 4 | ||||||
| a All the reactions are carried out in 1 mmol scale using 5.6 equiv. of TEA in the acetonitrile solvent (3.0 mL).b NMR yields.c Dioxane was used as solvent. | |||||||
| 1 | PdCl2(PPh3)2 (0.1) | 0.1 | 1.2 | rt | 1 | 66 | Trace |
| 2 | PdCl2(PPh3)2 (0.05) | 0.1 | 2.5 | rt | 2 | Trace | 70 |
| 3 | PdCl2(PPh3)2 (0.05) | 0.1 | 2.5 | 50 | 1.5 | — | 74 |
| 4 | PdCl2(PPh3)2 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 1.5 | — | 76 |
| 5 | PdCl2 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 1.5 | Trace | 80 |
| 6 | PdCl2 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 2 | Trace | 92 |
| 7 | PdCl2 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 3 | Trace | 89 |
| 8 | PdCl2 (0.05)/PPh3 (0.1) | 0.1 | 3.0 | 50 | 2 | Trace | 70 |
| 9 | PdCl2 (0.05)/PPh3 (0.1) | 0.1 | 4.0 | 50 | 2 | Trace | 78 |
| 10 | Pd(PPh3)4 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 24 | 35 | — |
| 11 | Pd(OAc)2 (0.05) | 0.1 | 2.5 | 50 | 0.25 | — | 85 |
| 12 | Pd(OAc)2 (0.05)/PPh3 (0.1) | 0.1 | 2.5 | 50 | 0.25 | — | 80 |
| 13 | Pd(OAc)2 (0.1) | 0.1 | 2.5 | 50 | 2 | — | — |
| 14c | Pd(OAc)2 (0.05) | 0.1 | 2.5 | 50 | 2 | Trace | 88 |
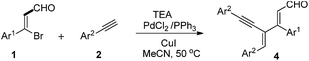
Having the optimized conditions in hand, we check the feasibility of the reaction with various substrates on both the reacting partners. The substrate scope of (Z)-3-bromo-3-arylacrylaldehyde derivatives with various aryl acetylenes in the presence of PdCl2 (0.05 mmol), PPh3 (0.1 mmol), and CuI (0.1 mmol), TEA (5.6 mmol) afforded the desired dienynal products in good to excellent yields as shown in Table 2. By fixing phenyl acetylene as one of the reacting partner, the scope of the other reacting partner (Z)-3-bromo-3-arylacrylaldehyde derivatives is examined. Electron donating substituents such as Me and OMe on the phenyl ring of (Z)-3-bromo-3-arylacrylaldehyde derivatives furnished the desired dienynal derivatives in good to excellent yields (Table 2, entries 2, 3). On the other hand, good to moderate yields of the dienyal derivatives are observed with electron withdrawing substituents like 4-Br, 3-Br and 4-Cl, 3-NO2 (Table 2, entries 4 to 7). To our delight, 1- and 2-naphthyl acrylaldehyde derivatives also afforded the desired dienyal derivatives in good yields (Table 2, entries 8 to 9). Good yields of the dienynal derivatives are obtained when disubstituted electron donating derivatives such as dimethyl, dimethoxy are subjected to optimized reaction conditions (Table 2, entries 10 to 14). Subsequently, we explored the substrate scope of 2 by fixing (Z)-3-bromo-3-phenylacrylaldehyde as one of the reacting partners. 4-Me, 4-isobutyl phenyl acetylenes furnished corresponding dienyal derivatives in moderate yields under the optimized reaction conditions (Table 2, entries 15 and 16). However, when the reaction was carried out with ortho and meta substituted phenyl acetylenes, desired dienynal derivatives were not observed (Table 2, entries 17 and 18).
| Entrya | Ar1 | Ar2 | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|---|
| 4 | |||||
| a Reaction conditions: 1 (1 mmol), 2 (2.5 equiv.), TEA (5.6 equiv.), PdCl2 (5 mol%), PPh3 (10 mol%), CuI (10 mol%) and MeCN (3.0 mL) at 50 °C.b Isolated yields.c Formation of dienyne product was not observed. | |||||
| 1 | C6H5 | C6H5 | 2 | 4a | 90 |
| 2 | 4-MeC6H4 | C6H5 | 2 | 4b | 80 |
| 3 | 4-OMeC6H4 | C6H5 | 2 | 4c | 75 |
| 4 | 4-BrC6H4 | C6H5 | 2 | 4d | 70 |
| 5 | 3-BrC6H4 | C6H5 | 2 | 4e | 68 |
| 6 | 4-ClC6H4 | C6H5 | 2 | 4f | 73 |
| 7 | 3-NO2C6H4 | C6H5 | 2 | 4g | 54 |
| 8 | 1-C10H17 | C6H5 | 2 | 4h | 70 |
| 9 | 2-C10H17 | C6H5 | 2 | 4i | 68 |
| 10 | 2,4-(CH3)2C6H3 | C6H5 | 2 | 4j | 71 |
| 11 | 2,5-(CH3)2C6H3 | C6H5 | 2 | 4k | 81 |
| 12 | 3,4-(CH3)2C6H3 | C6H5 | 2 | 4l | 62 |
| 13 | 3,4-(OCH3)2C6H3 | C6H5 | 2 | 4m | 71 |
| 14 | 4-isobutylC6H4 | C6H5 | 2 | 4n | 68 |
| 15 | C6H5 | 4-MelC6H4 | 2 | 4p | 74 |
| 16 | C6H5 | 4-isobutylC6H4 | 2.5 | 4p | 65 |
| 17c | C6H5 | 2-NO2C6H4 | 10 | 4o | — |
| 18c | C6H5 | 3-ClC6H4 | 10 | 4q | — |
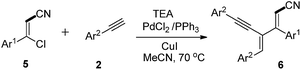
To expand scope of our methodology, we turned our attention towards other functional group such as cyano group instead of aldehyde. We prepared 3-chloro-3-phenylacrylonitrile and subjected to the optimized reaction conditions with phenyl acetylene. To our delight, the corresponding dienyne derivative 6 is isolated in 71% yield as a separable mixture of E and Z isomers (6/6′) at 70 °C (Table 3, entry 1). The structures of these dienyne E and Z isomers are further confirmed from X-ray crystallography studies.12 Electron donating groups such as 4-Me, 2-Me, 4-OMe on the phenyl ring of 3-chloro-3-arylacrylonitrile afforded mixture of dienynes with 69%, 38% and 68% respectively (Table 3, entries 2 to 4). Interestingly in the case of 2-methyl substituted acrylonitrile derivative exclusive formation of E-isomer takes place. On the other hand, in the presence of electron withdrawing group such as Cl, desired dienyne isomers obtained in 53% yield (Table 3, entry 5). To investigate the further scope of the reaction, we treated 1-naphthyl-3-chloroacrylonitrile or 2-naphthyl-3-chloroacrylonitrile with phenyl acetylene to afford corresponding dienyne derivatives in 41% and 66% yield respectively (Table 3, entries 6 and 7). In the case of 1-naphthyl group, only Z isomer is formed. Furthermore, reaction of 3-chloro-3-phenylacrylonitrile with 4-Me and 4-Cl substituted phenyl acetylenes afford the desired dienyne derivatives in 55 and 49% yields respectively (Table 3, entries 8 and 9).
| Entrya | Ar1 | Ar2 | Time (h) | 6 E/Z | Yieldb (%) | E/Z ratioc |
|---|---|---|---|---|---|---|
| a Reaction conditions: 5 (1 mmol), 2 (2.5 equiv.), TEA (5.6 equiv.), PdCl2 (5 mol%), PPh3 (10 mol%), CuI (10 mol%) and MeCN (3.0 mL) at 70 °C.b Isolated yields.c Ratio of E/Z calculated based on crude 1H NMR spectra. | ||||||
| 1 | C6H5 | C6H5 | 12 | 6a/6′a | 71 | 1:2 |
| 2 | 4-CH3C6H4 | C6H5 | 12 | 6b/6′b | 69 | 3:7 |
| 3 | 2-CH3C6H4 | C6H5 | 12 | 6c | 38 | 1:0 |
| 4 | 4-OCH3C6H4 | C6H5 | 12 | 6d/6′d | 68 | 1:2:3 |
| 5 | 4-ClC6H4 | C6H5 | 12 | 6e/6′e | 53 | 1:5 |
| 6 | 1-C10H7 | C6H5 | 12 | 6′f | 41 | 0:1 |
| 7 | 2-C10H7 | C6H5 | 12 | 6g/6′g | 66 | 5:4 |
| 8 | C6H5 | 4-CH3C6H4 | 14 | 6h/6′h | 55 | 3:5 |
| 9 | C6H5 | 4-ClC6H4 | 14 | 6i/6′i | 49 | 2:5 |
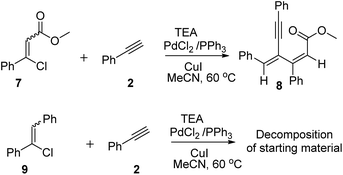
The scope of the present protocol is further elaborated with the ester functional group by examining with methyl 3-chloro-3-phenylacrylate under the optimized reaction conditions. To our delight, methyl 3-chloro-3-phenylacrylate 7 reacts with phenyl acetylene in the presence of PdCl2, PPh3, and CuI, TEA in CH3CN solvent at 60 °C afforded the desired dienyne derivative 8 in 76% yield. From 1H and 13C-NMR studies, X-ray crystallography studies12 the structure of 8 is confirmed. To investigate the role of functional group in the present protocol, we prepared chloro stilbene derivative 9 and treated with phenyl acetylene under the optimized reaction conditions. To our surprise desired dienyne derivative is not observed which confirms the vital role of functional group i.e., CHO, CN, COOEt in β-halo styrene derivative in affording the desired 1,3-butadiene-3-yne derivatives as shown in Scheme 1.
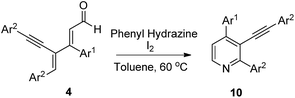
In order to enhance the synthetic utility of the current protocol, novel 2,3,5-trisubstituted pyridine derivatives are prepared from dienyal derivatives 4 and phenyl hydrazine in the presence of iodine as shown in Table 4. Trisubstituted pyridine derivatives are also obtained when the reaction carried with glycine methyl ester and NH4Cl albeit in low yield under similar conditions. Reaction of dienynal 4a with phenyl hydrazine in the presence of iodine in DCM at rt, furnished the dienyne hydrazine intermediate. However, when we replaced DCM with toluene and enhanced the temperature to 60 °C, to our delight trisubstituted pyridine is obtained in 66% yield (Table 4, entry 1). The structure of 10a is further confirmed from X-ray crystallography studies.12
| Entrya | Ar1 | Ar2 | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4 (0.5 mmol), phenyl hydrazine (0.75 mmol), I2 (0.15 mmol), toluene (4.0 mL) at 60 °C.b Isolated yields. | |||||
| 1 | C6H5 | C6H5 | 9 | 10a | 66 |
| 2 | 4-OMeC6H4 | C6H5 | 9 | 10b | 68 |
| 3 | 4-ClC6H4 | C6H5 | 10 | 10c | 62 |
| 4 | 3-NO2C6H4 | C6H5 | 11 | 10d | 58 |
| 5 | 1-C10H17 | C6H5 | 10 | 10e | 46 |
| 6 | 2-C10H17 | C6H5 | 10 | 10f | 53 |
| 7 | 4-MeC6H4 | C6H5 | 15 | 10g | 58 |
| 8 | 3,4-(MeO)2C6H3 | C6H5 | 16 | 10h | 50 |
| 9 | C6H5 | 4-MeC6H4 | 20 | 10i | 56 |
Both electron donating and withdrawing substituents afford the desired trisubstituted pyridine derivatives in moderate to good yield. For instance, with methoxy and methyl substituents desired trisubstituted pyridine derivative is isolated in moderate yields (Table 4, entries 2, 7–9). Electron withdrawing groups such as chloro and nitro substituents afford the pyridine derivative in 62% and 58% yield respectively (Table 4, entry 3 and 4). However, 46% and 53% yields of pyridine derivatives were obtained successively in the case of 1-naphthyl and 2-naphthyl derivatives (Table 4, entries 5 and 6).
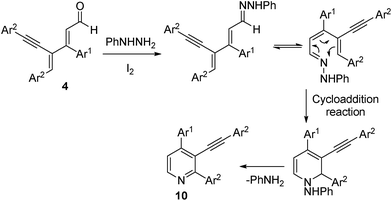
The proposed mechanism for the formation of pyridine derivatives is shown in Fig. 1. In the presence of iodine, dienyal derivative 4 reacts with phenyl hydrazine to form hydrazone intermediate. This hydrazone intermediate will undergo electrocyclic reaction followed by the elimination of aniline affords the desired pyridine derivative 10. To the best of our knowledge no reports are available in the literature in which phenyl hydrazine acts as nitrogen source for the synthesis of pyridine derivatives.
Experimental section
General information
Reagents and solvents were purchased from various commercial sources and were used directly without any further purification. Column chromatography was performed with 63–200 mesh silica gel. 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively. Chemical shifts are reported in parts per million (d) using chloroform as internal standards and coupling constants are expressed in Hertz. Melting points were recorded using an electro thermal capillary melting point apparatus and are uncorrected.General procedure for the synthesis of (E)-4-((E)-benzylidene)-3,6-diphenylhex-2-en-5-ynal derivatives (4a–4o)
Triethyl amine (567 mg, 5.6 equiv.), palladium chloride (9 mg, 5 mol%), triphenyl phosphine (26 mg, 10 mol%), copper iodide (20 mg, 10 mol%) and phenyl acetylene (255 mg, 2.5 equiv.) were added successively to a stirred solution of (Z)-3-bromo-3-phenylacrylaldehyde (210 mg, 1.0 mmol) in acetonitrile under nitrogen atmosphere. The reaction mixture was heated to 50 °C and monitored by TLC. After completion of the reaction, the resulting reaction mixture was cooled to room temperature and solvent was removed under reduced pressure. Now add ethyl acetate and filtered through celite pad to remove metal catalysts. Later organic layer was washed with brine and dried over MgSO4. The resulting crude compound was purified by flash column chromatography (eluent: petroleum ether/ethyl acetate) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on silica gel to afford desired product.
1 on silica gel to afford desired product.
General procedure for the synthesis of (E)-4-((E)-benzylidene)-3,6-diphenylhex-2-en-5-ynenitrile derivatives (6a–6j)
Triethyl amine (567 mg, 5.6 equiv.), palladium chloride (9 mg, 5 mol%), triphenyl phosphine (26 mg, 10 mol%), copper iodide (20 mg, 10 mol%) and phenyl acetylene (255 mg, 2.5 equiv.) were added successively to a stirred solution of 3-chloro-3-phenylacrylnitrile (164 mg, 1.0 mmol) in acetonitrile under nitrogen atmosphere. The reaction mixture was heated to 70 °C and monitored by TLC. After completion of the reaction, the resulting reaction mixture was cooled to room temperature and solvent was removed under reduced pressure. Now add ethyl acetate and filtered through celite pad to remove metal catalysts. Later organic layer was washed with brine. The organic layer was separated; dried over anhydrous MgSO4 and filtered the dried organic layer, then concentrated it to obtain the crude product. The resulting crude compound was purified by flash column chromatography (eluent: petroleum ether/ethyl acetate) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on silica gel to afford desired product.
1 on silica gel to afford desired product.
Procedure for the synthesis of methyl (E)-4-((E)-benzylidene)-3,6-diphenylhex-2-en-5-ynoate (8)
Triethyl amine (567 mg, 5.6 equiv.), palladium chloride (9 mg, 5 mol%), triphenyl phosphine (26 mg, 10 mol%), copper iodide (20 mg, 10 mol%) and phenyl acetylene (255 mg, 2.5 equiv.) were added successively to a stirred solution of methyl 3-chloro-3-phenylacrylate (197 mg, 1.0 mmol) in acetonitrile under nitrogen atmosphere. The reaction mixture was heated to 60 °C and monitored by TLC. After completion of the reaction, the resulting reaction mixture was cooled to room temperature and solvent was removed under reduced pressure. Now add ethyl acetate and filtered through celite pad to remove metal catalysts. Later organic layer was washed with brine. The organic layer was separated; dried over anhydrous MgSO4 and filtered the dried organic layer, then concentrated it to obtain the crude product. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate) 20
petroleum ether/ethyl acetate) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on silica gel to afford desired product.
1 on silica gel to afford desired product.
General procedure for the synthesis of 2,4-diphenyl-3-(phenylethynyl)pyridine derivatives (10a–10f)
Phenyl hydrazine (162 mg, 1.5 mmol.), and iodine (76 mg, 0.3 mmol) were added successively to a stirred solution of (E)-4-((E)-benzylidene)-3,6-diphenylhex-2-en-5-ynal (334 mg, 1.0 mmol) in toluene. The reaction mixture was heated to 60 °C and monitored by TLC. After completion of the reaction, the resulting reaction mixture was cooled to room temperature and treated with hypo solution to quench iodine. The organic layer was extracted with ethyl acetate, washed with brine. The organic layer was separated; dried over anhydrous MgSO4 and filtered the dried organic layer, then concentrated it to obtain the crude product. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate) 10
petroleum ether/ethyl acetate) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on silica gel to afford desired product.
1 on silica gel to afford desired product.
Spectral data of compounds
Conclusions
In conclusion, an approach for the synthesis of unsymmetrical functionalized (CHO, CN, COOEt) 1,3-butadiene-3-yne derivatives is reported starting from β-halo styrene derivatives and phenyl acetylenes in the presence of PdCl2 and CuI catalysts. Good yields and wide substrate scope makes this protocol noteworthy. Also, these 1,3-buta-diene-3-ynals are utilized for the synthesis of novel 2,3,5-trisubstituted pyridine derivatives. Further efforts to synthesize complex heterocyclic derivatives from 1,3-buta-diene-3-ynals are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the Ministry of Science and Technology of the Republic of China (MOST 103-2113-M-003-008-MY3), National Taiwan Normal University (103-07-C) and Instrumentation Centre at National Taiwan Normal University is gratefully acknowledged. The authors are grateful to Ms. Hsiu-Ni Huan, Ms. Chiu-Hui He and Ting-Shen Kuo for providing HRMS, NMR spectral and Crystallographic data respectively.Notes and references
- (a) B. M. Trost and J. T. Masters, Chem. Soc. Rev., 2016, 45, 2212 RSC; (b) M. Gholami and R. R. Tykwinski, Chem. Rev., 2006, 106, 4997 CrossRef CAS PubMed; (c) D. J. Faulkner, Nat. Prod. Rep., 2001, 18, 1 RSC.
- (a) Z. Liu, I. Schmidt, P. Thamyongkit, R. S. Loewe, D. Syomin, J. R. Diers, Q. Zhao, V. Misra, J. S. Lindsey and D. F. Bocian, Chem. Mater., 2005, 17, 3728 CrossRef CAS; (b) M. Gholami and R. R. Tykwinski, Chem. Rev., 2006, 106, 4997 CrossRef CAS PubMed.
- (a) M. Dörfler, N. Tschammer, K. Hamperl, H. Hübner and P. Gmeiner, J. Med. Chem., 2008, 51, 6829 CrossRef PubMed; (b) T. H. Jones, R. M. M. Adams, T. F. Spande, H. M. Garrafo, T. Kaneko and T. R. Schultz, J. Nat. Prod., 2012, 75, 1930 CrossRef CAS PubMed.
- (a) N. Matsuyama, H. Tsurugi, T. Satoh and M. Miura, Adv. Synth. Catal., 2008, 350, 2274 CrossRef CAS; (b) K. Ogata, H. Murayama, J. Sugasawa, N. Suzuki and S.-i. Fukuzawa, J. Am. Chem. Soc., 2009, 131, 3176 CrossRef CAS PubMed; (c) K. Ogata, J. Sugasawa, Y. Atsuumi and S.-i. Fukuzawa, Org. Lett., 2010, 12, 148 CrossRef CAS PubMed; (d) K. Ogata, Y. Atsuumi and S.-i. Fukuzawa, Org. Lett., 2011, 13, 122 CrossRef CAS PubMed.
- (a) B. M. Trost, M. T. Sorum, C. Chan, A. E. Harms and G. Rühter, J. Am. Chem. Soc., 1997, 119, 698 CrossRef CAS; (b) J. Krause, G. Cestaric, K.-J. Haack, K. Seevogel, W. Storm and K.-R. Pörschke, J. Am. Chem. Soc., 1999, 121, 9807 CrossRef CAS.
- A. Haskel, T. Straub, A. K. Dash and M. S. Eisen, J. Am. Chem. Soc., 1999, 121, 3014 CrossRef CAS.
- (a) T. Straub, A. Haske and M. S. Eisen, J. Am. Chem. Soc., 1995, 117, 6364 CrossRef CAS; (b) A. K. Dash, I. Gourevich, J. Q. Wang, J. Wang, M. Kapon and M. S. Eisen, Organometallics, 2001, 20, 5084 CrossRef CAS.
- Y.-T. Wu, W.-C. Lin, C.-J. Liu and C.-Y. Wu, Adv. Synth. Catal., 2008, 350, 1841 CrossRef CAS.
- (a) L. Rubio-Pérez, R. Azpíroz, A. D. Giuseppe, V. Polo, R. Castarlenas, J. J. Pérez-Torrente and L. A. Oro, Chem.–Eur. J., 2013, 19, 15304 CrossRef PubMed; (b) R. Azpíroz, L. Rubio-Pérez, R. Castarlenas, J. J. Pérez-Torrente and L. A. Oro, ChemCatChem, 2014, 6, 2587 CrossRef.
- A. Uruvakili, G. Gangadhararao and K. C. Kumara Swamy, Org. Biomol. Chem., 2015, 13, 10060 CAS.
- (a) C.-Y. Huang, V. Kavala, C.-W. Kuo, A. Konala, T.-H. Yang and C.-F. Yao, J. Org. Chem., 2017, 82, 1961 CrossRef CAS PubMed; (b) S. S. Ichake, A. Konala, V. Kavala, C.-W. Kuo and C.-F. Yao, Org. Lett., 2017, 19, 54 CrossRef CAS PubMed; (c) T.-H. Yang, C.-W. Kuo, V. Kavala, A. Konala, C.-Y. Huang and C.-F. Yao, Chem. Commun., 2017, 53, 1676 RSC; (d) A. Konala, C.-W. Kuo, L. Lin, T.-T. Chiang, C.-Y. Huang, T.-H. Yang, V. Kavala and C.-F. Yao, Chem. Commun., 2016, 52, 7870 RSC; (e) V. Kavala, C.-C. Wang, Y.-H. Wang, C.-W. Kuo, D. Janreddy, W.-C. Huang, T.-S. Kuo, C. H. He, M.-L. Chen and C.-F. Yao, Adv. Synth. Catal., 2014, 356, 2609 CrossRef CAS.
- See ESI† for further details. 4a (CCDC no. 1557911), 6a (CCDC no. 1556913), 6′a (CCDC no. 1556912), 8 (CCDC no. 1556914), 10a (CCDC no. 1557912): (a) W. Yang, Z. Ruan, Y. Wang, K. V. Kirk, Z. Ma, B. J. Arey, C. B. Cooper, R. Seethala, J. H. M. Feyen and J. K. Dickson Jr, J. Med. Chem., 2009, 52, 1204 CrossRef CAS PubMed; (b) A. Basnet, P. Thapa, R. Karki, Y. Na, Y. Jahng, B.-S. Jeong, T. C. Jeong, C.-S. Lee and E.-S. Lee, Bioorg. Med. Chem., 2007, 15, 4351 CrossRef CAS PubMed; (c) H. Huang, J. Cai, L. Tang, Z. Wang, F. Li and G.-J. Deng, J. Org. Chem., 2016, 81, 1499 CrossRef CAS PubMed; (d) S. M. Gomha and K. K. Dawood, J. Heterocycl. Chem., 2017, 54, 1943 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1557911, 1556913, 1556912, 1556914 and 1557912. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra07128k |
| This journal is © The Royal Society of Chemistry 2017 |