Influence of blended learning on outcomes of students attending a general chemistry course: summary of a five-year-long study
P.
Bernard
 *,
P.
Broś
and
A.
Migdał-Mikuli
*,
P.
Broś
and
A.
Migdał-Mikuli
Department of Chemical Education, Jagiellonian University in Kraków, ul. Ingardena 3, 30-060 Kraków, Poland. E-mail: pawel.bernard@uj.edu.pl
First published on 26th May 2017
Abstract
The development of the Internet, communication technologies and teaching methods creates new opportunities for the modernisation of academic classes. Many studies on the application of new educational models indicate that they are both more effective and preferred by students over classical approaches. Additionally, combining various education methods and didactic tools is a common approach, ensuring a high degree of flexibility in the courses and the ability to satisfy the expectations and needs of students with various inclinations, learning styles and intelligence types. In this study, an attempt was made to determine the effects of blended learning on the outcomes of biophysics students attending a general chemistry course. The blended learning model applied covered a combination of classically organised classes, flipped classes, and elements of distance learning supplemented with various multimedia resources. The study had a quantitative character and involved analysis of variance for comparison of student cohorts taking the course in classical and in modernised forms. The study was run for five consecutive years and covered 98 students. The obtained results indicated a statistically significant increase in the students' outcomes after the classes were modernised.
Introduction
The first college chemistry course plays an important role in the academic trajectory of college students (Tai et al., 2006). This basic course functions as a screening device for many science fields. Experience has indicated that a portion of students may not be prepared to begin a general chemistry course upon entering university (Coley, 1973; Allenbaugh and Herrera, 2014; Shedlosky-Shoemaker and Fautch, 2015). The course is especially challenging for non-chemistry majors, who have various backgrounds, not only in chemistry but also in subjects such as mathematics and physics. However, proper knowledge and skills in chemistry are crucial in many areas, including a wide range of medical and biological studies, such as biology, biophysics, and biotechnology (Sadler and Tai, 2007). Therefore, it is important to develop syllabuses and didactic methods that will help students to learn basic chemistry and increase their chances of successfully completing their general chemistry courses.Evolution of academic courses
Academic courses are changing constantly. The changes are caused not only by changes in the subject area but also by the evolution of teaching methods, availability of new technologies, and changes in society and social expectations. In the past ten years, there was a strong trend in higher education towards complementing classic face to face (F2F) classes with elements of computer-based distance education (Bloomfield et al., 2010). This approach is based on the development of information and communication technologies (ICTs) and the expectations of students. This method also creates an opportunity to use various teaching methods and didactic materials. The computer-based part is usually delivered via an online e-learning platform. The digital resources ensure a high level of interactivity and a significant level of autonomy in the order presentation and forms in which the provided materials are used. The role of online materials is not only to summarise the classes but also to enable a flipped approach (Baepler et al., 2014). A flipped classroom moves part of the learning content to a student-centred out-of-class setting; therefore, part of the course content is subsequently being covered by the students as homework (Eichler and Peeples, 2016). Additionally, the F2F classes are changing from the classic instructor-centred theatre into an active learning classroom (Freeman et al., 2014) with multiple lecturer–student interactions, often conducted by clickers (Classroom Response Systems) (Fies and Marshall, 2006; MacArthur and Jones, 2008). Taken together, these properties create a blended learning (BL) environment in which a hybrid of approaches described by Driscoll (2002) is working:• Combination of modes of web-based technology
• Combination of various pedagogical approaches
• Combination of any form of instructional technology with face-to-face instructor-guided training
• Combination of an instructional technology with actual job tasks in order to create a harmonious effect of learning and working.
This method is, in fact, an approach described by Graham (2006), where he defines blended learning as a combination of instruction from two historically separate models of teaching and learning: traditional F2F learning systems and distributed learning systems with computer-based techniques. The evolution affects not only lectures but also workshops, seminars and laboratory classes. In the case of laboratories, an increase in inquiry-based instructions, problem-based and context-based exercises is easily noticeable (Hofstein, 2004; Hofstein and Mamlok-Naaman, 2007; Russell and Weaver, 2011).
Modernisation of a general chemistry course for biophysics
Considering students' difficulties with general chemistry classes and the current trends in higher education, a complex modernisation of the course was proposed and implemented. The process started in 2005 when preliminary studies were conducted (Migdał-Mikuli et al., 2008). The pilot study let the authors identify the main sources of students' difficulties, select a pedagogical approach, and design a model implementing BL techniques. The aim of the modernisation was to develop a student-centred learning environment that motivates students, helps them to bridge the gap between upper secondary school and university, and equalises chances to successfully complete the course, regardless of prior chemical skills and knowledge.The original course covered 90 hours (7 ECTS), divided into three types of classes: lecture (30 h), seminar (15 h), and laboratory (45 h). All three types of classes were run in parallel; therefore, each week, the students attended 2 hours of lectures, 1 hour of seminars and 3 hours of laboratory exercises. The lectures were common for all students and run by one lecturer, but the seminars and labs were run by several assistants, one per 8–12 students. It was decided that BL will be used, and the classical course will be supplemented with distributed materials, meaning that the number of F2F classes would not be reduced. The modernisation was divided into two phases. In the first phase, the syllabus content was updated according to the current state of knowledge. The syllabus was also structured, and its integrity was checked using Matrix Analysis (Kluz, 2002). After this phase, the course was run for two years using only the classical F2F method. The second phase included a modernisation of pedagogical approach and the implementation of BL. For the distributed part of the course, designing the didactic materials was the main challenge. There are many possibilities for the preparation of BL materials (Derntl and Motschnig-Pitrik, 2005). In our case, it was decided that the materials prepared for students for each type of class should be divided into three parts:
• Material that should be used before classes
• Material with content that was discussed during classes
• Additional tasks for the students.
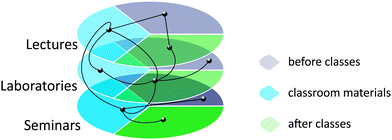
Many issues were common among various parts of the course. Those issues were connected in student materials with complex hypertext (Hammond, 1992), which created a kind of knot plane structure (Gajda et al., 2004), as presented in Fig. 1. This type of structure provides students a chance to personalise the learning process. The prepared materials played a key role in blending F2F classes with the distributed parts and the flipped classes.
Modernisation of the classes
The lectures were modernised significantly in both phases. After the first phase, the lectures were run in a classical way but according to a new syllabus. In the second phase, BL was implemented, and distributed materials and a flipped approach were used. The materials for students supporting lectures covered the following divisions:• What you should already know – a review of chemistry from the upper secondary school level that should be known before the lecture
• The lecture – highlights of the content that is introduced during the lecture, enriched with animations and other multimedia
• Test yourself – an interactive test in which students can check their knowledge covering the basic contents and the material presented during the lecture.
The original laboratory syllabus for the course included fourteen exercises covering elements of qualitative and quantitative analyses. The exercises were based on step-by-step instructions delivered directly before exercises in the form of a chemical cook-book. In the first phase, the content of the exercises was updated and contextualised (in five cases the exercises were related to industrial processes), but the way the materials were delivered and the classes were organised remained unchanged. The second phase of the modernisation covered not only the delivery of materials but also the forms of instructions for students – these classes were changed into guided and bounded inquiry (Wenning, 2005) (ten and four exercises, respectively). The classes were characterised by an increase in students' independence during exercises. However, in Poland, inquiry-based instruction is not widely used in secondary education (Bernard et al., 2012, 2015). Because of students' limited experience in that field, the inquiry process was structured, and open inquiry was not used.
The organisation of laboratory classes was also modified. Every class started with a test that was completed via clickers (Bernard et al., 2011). The test questions covered general chemical knowledge that should be known from the secondary school or lectures, and the exercises' specific issues that were introduced in the students' materials.
Didactic materials for laboratories were divided into the following sections:
• Introduction – a part including theory and problem tasks that should be learned and solved before classes
• For classes – materials that guide the students during the lab
• Report template – subsections that should be included in the report and additional problem tasks that should be solved or discussed.
Seminars in the course were focused on calculation exercises, and this approach was unchanged. In the first phase, the materials that were delivered classically were updated, and in the second phase, the blended approach was adopted. The materials for students included the following:
• Basic problems, which the students should be able to solve using their secondary-school knowledge. These problems were presented with answers and a detailed commentary.
• Advanced tasks, which were meant to be solved during the classes.
• Interactive questions covering basic and advanced levels, where the students can test their skills and knowledge.
Distribution of the materials
It should be emphasised that the process of course modernisation was started in 2005 with a pilot study. The results obtained helped in choosing a pedagogical strategy and design the materials for students. It was decided that the materials should include not only text with hyperlinks but also more advance multimedia – interactive questions, videos and animations. This approach required the selection of a method for the delivery of the prepared materials. The students from the pilot group evaluated the materials, completed a semi-structured questionnaire and gave interviews. The students declared that the best method of delivery of the content would be an independent programme that can be distributed on CDs. Simultaneously, 82% of the students declared that they have permanent access to the Internet, but they prefer CDs because of the volume of the multimedia files (Migdał-Mikuli et al., 2008). For that reason, it was decided that all materials would be gathered on a dedicated platform (based on the Authorware software) that can be distributed in various ways: on a CD, as a package that can be downloaded from the Internet and used off-line or exported as a web page with active content that can be used on-line via a web browser.Research focus
The aim of modernising the course was to develop a student-centred learning environment that motivated students, helped them to bridge the gap between upper secondary school and university and equalised their chances of successfully completing the course, regardless of prior knowledge of chemistry. The modernisation was based on a blended learning strategy that included a combination of classical classes with elements of distance education and flipped education. The modernisation of the course created an opportunity to answer the following question: ‘How does blended learning affect students' outcomes in a general chemistry course?’Methodology of the research
General background of the research
In the Polish educational system, undergraduate students choose a university and a field of study when applying for the first year. The ‘general chemistry’ course is run by the Faculty of Chemistry of JU every year for students in the following fields: chemistry (approx. 150 students), medicinal chemistry (60 students), materials chemistry (60 students), environmental protection (40 students) and biophysics (20 students). In total, more than 300 students take the class. The introductory level courses at the Jagiellonian University are separate for each field of study. Although perhaps less effective economically, this approach has many advantages over large enrolment classes for students in many fields. The student cohorts are smaller, and the course contents may be adjusted to a given profile of the studies and further development of students. In the case of biophysics, the general chemistry course is obligatory for the students, and its scope differs significantly from that of its counterparts in other fields. This course contains more advanced elements of physical chemistry, content important for biophysics students but not included in the curriculum as a separate course, as is the case with the chemistry and medicinal chemistry specialisations.Participants
The research sample consisted of 98 biophysics students, 46 males and 52 females (Table 1), who attended the general chemistry course in one of five consecutive academic years in 2008–2013. The outcomes of students who attended the course and took the final exam were analysed. The students from the first two years (A1 and A2; n = 52) took the course without using the blended (hybrid) education model and constituted a control group. The remaining students (B1, B2 and B3, n = 44) used the BL method (tested group). The results of both groups were compared.| Group | Group size | |||
|---|---|---|---|---|
| Females | Males | Total | ||
| Control (A) | A1 | 12 | 13 | 25 |
| A2 | 14 | 15 | 29 | |
| Tested (B) | B1 | 9 | 10 | 19 |
| B2 | 11 | 6 | 17 | |
| B3 | 6 | 2 | 8 | |
| Total | 52 | 46 | 98 | |
Apart from the biophysics students, this course was also taken by students in an individual education programme. However, these students were not taken into account in the research, as this course was not obligatory for them, and they could take it at various stages (years) of their studies. The results of students who started the class and completed the pre-test but withdrew from the class during the academic year and did not take the final exam were also omitted from the analysis. The number of students withdrawing during the semester was similar throughout the whole period of the research and amounted to the following numbers of people in the individual groups: A1–3, A2–2, B1–3, B2–2, B3–3.
Instruments and procedures
The research was conducted by the pre/post-test method using a questionnaire with multiple-choice and open-ended questions (Oversby, 2012). The results were collected in two stages. The first stage consisted of a verification of the competence level of the students starting the course. To this end, students were given a diagnostic test concerning chemistry on the upper secondary school level. The test consisted of 13 questions (with a total value of 26 points) prepared based on problems from the secondary school final exam, basic level. The second stage included an analysis of the results achieved by the students on the final exam. The final exam consisted of 43 questions, including 35 test questions (multiple choice, with one correct answer) and eight open-ended questions with a total value of 80 points. Every year, the order of the questions in the exam form was changed, as well as the sequence of answers and the values in the calculation problems.The research included a comparison of the results of the student groups taking the course in the classical and in the modernised form. The first stage of the analysis consisted of a comparison of the pre-test results of the consecutive groups. The goal of the analysis was to evaluate whether the knowledge and skills of students in consecutive years differed statistically at the beginning of the course. The second stage consisted of a comparison of the results achieved by the students on the final exam. The analysis was to check whether the average results achieved by the student groups taking the course in the classical form differ statistically from those of the student groups taking the course in the modified form. An analysis of the summarised results was performed (A groups taking the course in the classical form, and B groups taking the course in the modified form). Additionally, a separate analysis of the results of each year's students was conducted (A1, A2, B1, B2, and B3).
Analysis of variance (ANOVA) was used to analyse the results (Howell, 2002). Every analysis of variance was preceded by an examination of the necessary conditions of its application (King et al., 2011). This analysis determined whether the studied samples are characterised by a normal distribution (to this end, a Shapiro–Wilk test was used (Shapiro and Wilk, 1965)) and whether variances in the tested groups are homogeneous (this parameter was checked using the Levene test (Levene, 1960)). In cases where these assumptions were not met, the results of the variance analysis were verified using a non-parametric equivalent: the Mann–Whitney U test for two groups, (Mann and Whitney, 1947), or the Kruskal–Wallis test for multiple groups (Kruskal and Wallis, 1952). For the separate analysis of the results of each individual year, multiple comparisons (post hoc test) were performed using Duncan's multiple range test (Duncan, 1955). In all analyses, the significance level was assumed as α = 0.05. The statistical analysis was performed using the ‘Statistica’ software package, version 12.
The experiment reported in this study was conducted in addition to planned and institutionally approved course modernisation to establish the influence of modernisation on students' outcomes. Non-groups were intentionally taught in inadequate or out-of-date form. The research was performed in compliance with applicable law and Jagiellonian University ethical guidelines. Students participated in the study voluntarily, they were informed about the research design and the data to be collected, and they could withdraw from the research or reserve their data at any stage of the research.
Results
Analysis of the pre-test results
In Table 2, average percentage results of the pre-test for the control group (A) and for the tested group (B), collectively and separately, are presented. Apart from the average students’ results, parameters characterising the distribution of the results are shown.| Aggregate groups | Separate groups | ||||||
|---|---|---|---|---|---|---|---|
| A | B | A1 | A2 | B1 | B2 | B3 | |
| Number of students | 54 | 44 | 25 | 29 | 19 | 17 | 8 |
| Mean score [%] | 63.7 | 62.9 | 62.2 | 67.2 | 61.1 | 67.3 | 57.8 |
| Standard deviation [%] | 24.6 | 23.9 | 25.0 | 25.1 | 24.0 | 21.2 | 29.7 |
| Variance | 0.934 | 0.953 | 0.946 | 0.917 | 0.946 | 0.888 | 0.944 |
| Shapiro–Wilk test (p) | 0.005 | 0.071 | 0.208 | 0.026 | 0.333 | 0.044 | 0.652 |
For groups A2 and B2, the probability level obtained in the Shapiro–Wilk test is lower than the given level of significance (p < α); thus, a description using the normal distribution cannot be used for the results obtained by the students from these groups.
Additionally, a variance homogeneity check for the average pre-test results was performed using the Levene test. The calculated p-value for two aggregate groups was p = 0.474, and for the individual groups separately, p = 0.388. In both cases, the obtained values are higher than the given level of significance p > α; therefore, it may be assumed that the variances of the variable are homogeneous.
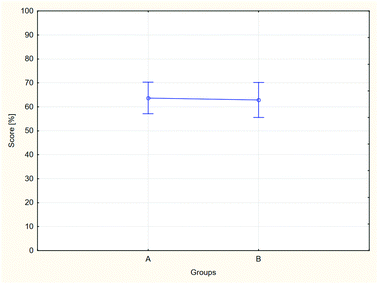
Fig. 2 and 3 present the ANOVA results. In Fig. 2, a comparison of the results of the control and tested group is shown, and in Fig. 3, a comparison of the results of all groups participating in the study is shown.
 | ||
| Fig. 3 Average pre-test results with 95% confidence interval marked for each of the groups separately. | ||
The goal of the analysis was to determine whether the average pre-test results differ statistically between the groups being compared. For two groups (Fig. 2), a probability level of p = 0.864, and for all groups separately, a probability level of p = 0.792 were obtained. In both cases, the obtained p-values are higher than the level of significance; therefore, it may be assumed that the pre-test results achieved by the groups being compared do not differ statistically (both in the aggregate approach, and for each of the groups separately).
The results of the Shapiro–Wilk test indicate that in the case of A2 and B2, the distribution of the pre-test results is not described by the normal distribution. For this reason, an alternative method, non-parametric testing, was used apart from the ANOVA analysis. A comparison of the two groups (A and B) using the Mann–Whitney U test indicated a probability level of p = 0.939, and for the constituent groups, the Kruskal–Wallis rank analysis yielded a probability level of p = 0.795. In both these tests, the probability level is higher than the assumed level of significance (p > 0.05); therefore, the obtained results confirm the results of the ANOVA analysis and allow for assuming that the differences between the average pre-test results are not statistically significant for the groups being compared.
Results of the final exam
Average results of the final exam are shown in Table 3. As was the case with the pre-test, conformance of the exam results to the normal distribution was verified using the Shapiro–Wilk test. The results are given in Table 3.| Aggregate groups | Separate groups | ||||||
|---|---|---|---|---|---|---|---|
| A | B | A1 | A2 | B1 | B2 | B3 | |
| Number of students | 54 | 44 | 25 | 29 | 19 | 17 | 8 |
| Mean score [%] | 64.4 | 70.8 | 62.4 | 65.7 | 66.9 | 73.2 | 74.5 |
| Standard deviation [%] | 11.4 | 12.8 | 11.3 | 11.7 | 13.1 | 9.8 | 16.8 |
| Variance | 0.989 | 0.968 | 0.957 | 0.961 | 0.945 | 0.969 | 0.915 |
| Shapiro–Wilk test (p) | 0.968 | 0.249 | 0.359 | 0.339 | 0.323 | 0.796 | 0.392 |
In all cases, the probability levels obtained in the Shapiro–Wilk test were higher than the given level of significance; therefore, it can be assumed that the distribution of results is consistent with the normal distribution. Moreover, the obtained variance values may be considered homogeneous because the results of the Levene test for the two groups was p = 0.271, and that for each group separately was p = 0.171.
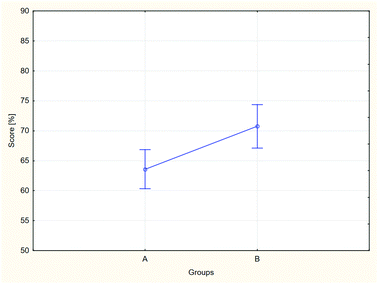
In the case of the exam results, the conditions for applicability of the ANOVA method may be considered met. The results of the variance analysis are depicted in Fig. 4 and 5.
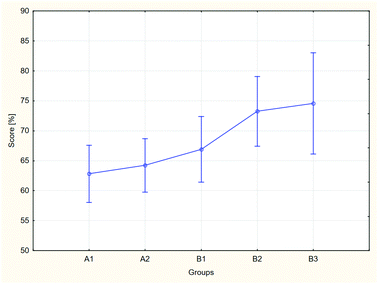
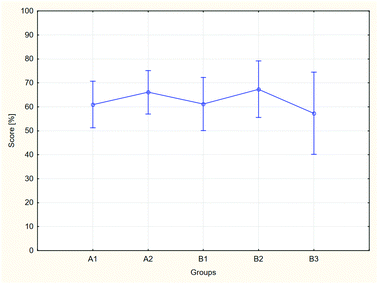
The results of the analysis, both for aggregate groups A and B, and for each of the groups separately, differ statistically, which may be assumed based on the obtained probability levels: for two groups p = 0.043, for separate groups p = 0.020. In the case of the comparison of the separate groups, a post hoc analysis was performed in order to indicate the groups between which the differences of results may be considered significant (Table 4).
| A1 | A2 | B1 | B2 | |
|---|---|---|---|---|
| A2 | 0.7427 | |||
| B1 | 0.3698 | 0.5289 | ||
| B2 | 0.0254 | 0.0463 | 0.1391 | |
| B3 | 0.0131 | 0.0261 | 0.0900 | 0.7512 |
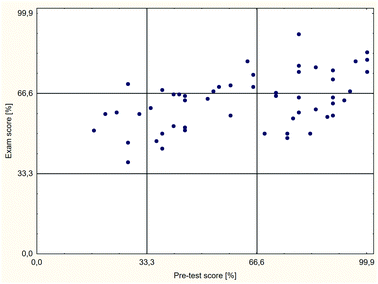
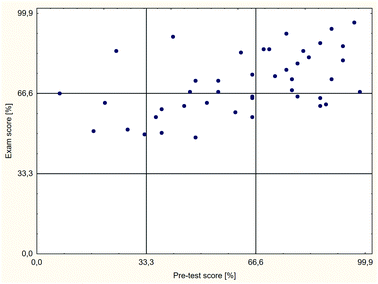
Additionally, an analysis of the relationship between the exam results and the diagnostic test results for individual students was performed. The plot in Fig. 6 shows the results for the students in the control group, and that in Fig. 7 shows those in the test group. The results in the plots are divided into three equal areas: I (0–33%) – area of low results, II (34–66%) – area of average results, and III (67–100%) – area of high results.
Discussion of results
Most of all, a distinct decline in the number of students in the Biophysics field (attending the course) in the consecutive years should be noted. This drop cannot be related to course modernisation. Rather, the decrease results from a demographic decline and a general decrease in the number of first-year students in the research time (Bernard et al., 2014b). This effect was clearly visible in less popular fields, such as biophysics and environmental protection. In this period, only very popular fields (e.g., medicine) had their admission quotas filled. Considering the reported research, there is a threat that the decrease in the number of students taking the course in following years influenced the educational process. The decrease in cohort size could increase access to teachers during F2F lectures. On the other hand, the lectures before and after modernisation were based on the didactic method, so the results should not be significantly affected by the cohort size. With the labs and seminars, where interaction between students and assistants have a major impact, the number of assistants was adjusted to the number of students (one assistant per 8–12 students) such that the overall size of the cohort did not influence the educational process.Since the results achieved by the students in the pre-test in the consecutive years do not differ statistically, it may be assumed that the groups starting the course each year had the same knowledge and skills. It should be noted that the standard deviations for the results obtained by all groups, ranging from 20% to 30%, were relatively high. This finding indicates a significant diversification of the results within the groups. In light of this fact, the lack of accordance of the pre-test result distribution with the normal distribution should not be considered surprising. However, the groups are similar, which is proved by maintaining the variance homogeneity parameter calculated by using the Levene test. Due to the existence of a standardised initial point for the consecutive groups (the pre-test results), the exam results may be compared, and conclusions about the effect of the applied didactic method for the students' results may be drawn on this basis.
The analysis allows it to be ascertained that the results achieved by the students taking the modified course were higher than the exam results achieved by the students of the control group. The results of the ANOVA analysis indicate that the observed differences are statistically significant. This dependence is visible in both the comparison of two groups, control (A) and tested (B), treated as a resultant of the constituent groups (A1, A2, B1, B2 and B3), and in an analysis of the separate constituent groups. It should be noted also that in all groups, the distribution of the exam results conforms to the normal distribution, and the standard deviations for the obtained results are clearly lower than those for the pre-test. The values range from 11% to 17%, with the highest value of ∼17% describing the B3 group. This finding is probably connected with the significantly lower number of students in this group.
The B1 group constitutes an interesting case. The post hoc analysis indicates that the exam results achieved by the students belonging to this group do not differ significantly from the results of the other groups, both control and test groups. As illustrated in Fig. 5, the results achieved by the students from this group are approximately in the middle between the results of groups A1 and A2 and between B2 and B3. Taking into account the equivalence of the groups in the beginning of the course and the statistically significant differences between the other groups, the observed effect may be connected with the process of implementing the modifications by the teachers and their ability to utilise BL. All groups participating in the research were taught by the same lecturer and assistants. These persons participated actively in planning and implementation of the course modernisation, however, the applied methods were new to them. In the B1 group, the teachers were using a flipped approach and blending remote and F2F classes for the first time. Apparently, the teachers were not able to use the full potential of the course in its modernised form. Implementation of BL may be divided into various stages (Graham et al., 2013), and this effect is visible in spite of the support provided to teachers by educators who have experience in the use of BL and who have been participating in conducting the described modernisation and research. Additionally, the continuous upward trend evident in Fig. 5 should be noted. This trend may result from acquiring more and more skill in running the classes by the teachers. It is also related to groups A1 and A2. These groups were taught using the classical method, but the course syllabus was modified earlier (the first stage of modernisation). However, the potential effects of cooperation between groups should be taken into consideration. The conclusions are based on the exam results. Although the numerical values in the problems, order of the questions and answers were changed, students of the earlier years could inform their younger colleagues about the exam's profile.
The 10% level of difference in the exam results between the control group and the test group may seem to be a slight gain in relation to the labour input necessary for the course's modernisation. However, similar values were also obtained in comparable studies (Williams et al., 2008; Bernard et al., 2014a), as well as in the case of modernisation of more specialised courses using specific multimedia tools (Antonoglou et al., 2011). Unlike other studies, in the current research, no positive influence of the modification on the dropout rate was observed (López-Pérez et al., 2011). Moreover, considering the percentage of students withdrawing from the course, this value is increasing because of the shrinking group size in the following years. The analysis of this effect was very difficult because most of the students withdrawing from the course also withdraw from the university, and contact with them is lost. The students who withdrew were observed to be not only students with low current results but also students with high achievements, who presumably lost interest in this study field.
The analysis of dependencies between the pre-test results and the exam results for the individual students, shown in Fig. 6 and 7, indicates that among students with low and average pre-test results, average exam results prevail. No significant differences between the control group (Fig. 6) and the test group (Fig. 7) were observed in this study. However, the differences among students with high pre-test results are distinct. Among these students, average exam results prevail in the control group, and high results prevail in the test group. Thus, the applied method affected students who were well-prepared for the classes positively but not necessarily students who were poorly or moderately prepared for the classes. Consequently, one of the goals of the course's modernisation, “equalise the chance to complete the course successfully regardless of prior chemistry knowledge”, was not achieved.
The form of distribution of the didactic materials selected by the students, namely on CDs, determined the selection of software for preparation of an educational platform based on the Authorware software. This selection was dictated by a significant volume of the prepared materials because multimedia files, i.e., animation and videos, were included among the materials. This distribution form enabled students to work off-line, but it precluded collection of statistical data pertaining to use of the content that was made available. For the same reason, the applied platform did not include social tools for communication either among students or between students and teachers. Social tools constitute an important element of the BL (Harasim, 2000; Rovai, 2002; Song et al., 2004; So and Brush, 2008; Wu et al., 2010), and their lack could affect the scale of the observed results. It should be added that the described course is still being taught in the BL form. In the years following the completion of this study, surveys of students about the preferred form of distributing the materials were continued. The percentage of students indicating a preference for an on-line platform increased continuously until 2015 when it reached 100% and remained at this level. This finding is related to increasingly easy access to the Internet via high-speed connections and with the introduction of a general e-learning platform at the Jagiellonian University, where materials for numerous courses are published.
Conclusions
This study allowed estimation of the influence of the BL approach on the outcomes of students taking a general chemistry course. The results showed that blending educational methods, such as flipped-classes, distance education and F2F classes, result not only in higher satisfaction of students, as was reported earlier, but also in a statistically significant increase in teaching efficiency. Moreover, an analysis of the results of the individual groups during the research indicated that this change was not occurring immediately when the method was applied. The first year after the introduction of BL should be considered as an intermediate period, during which the teachers having no experience in application of BL are adapting to the changes in the system.The purpose of modernising the course and introducing BL was to develop a student-centred learning environment which motivates students, helps them to bridge the gap between upper secondary school and university, and equalises chances of completing the course successfully, regardless of prior chemical knowledge. However, the analysis of the results shows that the applied system has only a slight influence on the results of the students who attended the course with little knowledge or skills in chemistry; the exam results of these students were similar before and after modernisation. Nevertheless, the changes markedly improved the results achieved by the students who were well-prepared for the course.
The described study was designed based on a pilot research study designed in 2005 and on the results obtained in the years 2005–2006. During the modernisation of the course, technologies to which the students in Poland had general access were used. The chosen form of the materials and lack of access to high-speed Internet resulted in preparation of the materials for distance learning in a form adapted to off-line work. This approach precluded making use of the potential of mobile technologies and social elements emerging in the following years. However, the obtained results allowed for evaluating the influence of BL by comparing the outcomes of students in a BL environment with those of students without these elements. The changes may be important for teachers in less-developed countries, in which access to fast Internet is still limited. Additionally, the results constitute an important reference point and may enable evaluation of the influence of applying these technologies on the students' outcomes in a further study.
In summary, the reported modernisation of the course can be considered as successful. The students' and lecturers' satisfaction was not measured in this study, but the general feedback was very positive. The variety of implemented changes and the experimental design used do not allow us to point to precisely which method made the difference, but we learned that the blend of methods used is coherent and creates a conducive learning environment. In the twenty-first century, we are observing the rapid growth and development of ICTs, which constantly create new educational opportunities. We can clearly see that students benefit from modern educational methods and tools but also that teachers need time and practice to use them efficiently. Considering the completed process of course modernisation, we can conclude that the modernisation of courses should be a continuous process of minor updates, rather than a product of sudden extensive change.
References
- Allenbaugh R. J. and Herrera K. M., (2014), Pre-assessment and peer tutoring as measures to improve performance in gateway general chemistry classes, Chem. Educ. Res. Pract., 15, 620–627.
- Antonoglou L. D., Charistos N. D. and Sigalas M. P., (2011), Design, development and implementation of a technology enhanced hybrid course on molecular symmetry: students' outcomes and attitudes, Chem. Educ. Res. Pract., 12(4), 454–468.
- Baepler P., Walker J. D. and Driessen M., (2014), It's not about seat time: blending, flipping, and efficiency in active learning classrooms, Comput. Educ., 78, 227–236.
- Bernard P., Broś P. and Migdał-Mikuli A., (2011), E-assessment of students based on Personal Response System, Problems of Education in the 21st Century, 35, 11–16.
- Bernard P., Maciejowska I., Odrowąż E., Dudek K. and Geoghegan R., (2012), Introduction of inquiry based science education into polish science curriculum – general findings of teachers' attitude, Chem.-Didact.-Ecol.-Metrol., 17(1–2), 49–59.
- Bernard R. M., Borokhovski E., Schmid R. F., Tamim R. M. and Abrami P. C., (2014a), A meta-analysis of blended learning and technology use in higher education: from the general to the applied, J. Comput. High. Educ., 26(1), 87–122.
- Bernard P., Migdał-Mikuli A. and Ciura K., (2014b), The Polish school – quo vadis? Chemik, 68(9), 784–793.
- Bernard P., Maciejowska I., Krzeczkowska M. and Odrowąż E., (2015), Influence of in-service teacher training on their opinions about IBSE, Procedia Soc. Behav. Sci., 177, 88–99.
- Bloomfield J., Roberts J. and While A., (2010), The effect of computer-assisted learning versus conventional teaching methods on the acquisition and retention of handwashing theory and skills in pre-qualification nursing students: a randomised controlled trial, Int. J. Nurs. Stud., 47(3), 287–294.
- Coley N. R., (1973), Prediction of success in general chemistry in a community college. J. Chem. Educ., 50(9), 613.
- Derntl M. and Motschnig-Pitrik R., (2005), The role of structure, patterns, and people in blended learning, Internet High. Educ., 8(2), 111–130.
- Driscoll M., (2002), Blended Learning: Let's Get Beyond the Hype, Consultants point of view, IBM Global Services, retrieved January 24, 2017, http://from https://www-07.ibm.com/services/pdf/blended_learning.pdf.
- Duncan D. B., (1955), Multiple Range and Multiple F Tests, Biometrics, 11(1), 1.
- Eichler J. F. and Peeples, J., (2016), Flipped classroom modules for large enrollment general chemistry courses: a low barrier approach to increase active learning and improve student grades, Chem. Educ. Res. Pract., 17(1), 197–208.
- Fies C. and Marshall J., (2006), Classroom Response Systems: A Review of the Literature, J. Sci. Educ. Technol., 15(1), 101–109.
- Freeman S., Eddy S. L., McDonough M., Smith M. K., Okoroafor N., Jordt H. and Wenderoth M. P., (2014), Active learning increases student performance in science, engineering, and mathematics, Proc. Natl. Acad. Sci. U. S. A., 111(23), 8410–8415.
- Gajda J., Juszczyk S., Siemieniecki B. and Wenta K., (2004), Edukacja medialna, Toruń: Adam Marszałek.
- Graham C. R., (2006), Blended learning systems: definition, current trends, and future directions, The Handbook of Blended Learning: Global Perspectives, Local Designs, San Francisco, CA: Pfeiffer Publishing.
- Graham C. R., Woodfield W. and Harrison J. B., (2013), A framework for institutional adoption and implementation of blended learning in higher education, Internet High. Educ., 18, 4–14.
- Hammond N., (1992), Tailoring Hypertext for the Learner. Cognitive Tools for Learning, NATO ASI Series, Series F: Computer and Systems Sciences, Berlin, Heidelberg: Springer-Verlag, vol. 81, pp. 149–160.
- Harasim L., (2000), Shift happens: online education as a new paradigm in learning, Internet High. Educ., 3(1), 41–61.
- Hofstein A., (2004), The laboratory in chemistry education: thirty years of experience with developments, implementation, and research, Chem. Educ. Res. Pract., 5(3), 247–264.
- Hofstein A. and Mamlok-Naaman R., (2007), The laboratory in science education: the state of the art, Chem. Educ. Res. Pract., 8(2), 105–107.
- Howell D., (2002), Statistical Methods for Psychology, 5th edn, Duxbury: Thomson Learning.
- King B. M., Rosopa P. J. and Minium E. W., (2011), Statistical Reasoning in the Behavioral Sciences, 6th edn, Danvers: John Wiley and Sons, Inc.
- Kluz Z., (2002), Metodologia tworzenia programów nauczania, Dydaktyka chemii, Poznań: Wydawnictwo Naukowe UAM, pp. 49–74.
- Kruskal W. H. and Wallis W. A., (1952), Use of Ranks in One-Criterion Variance Analysis, J. Am. Stat. Assoc., 47(260), 583–621.
- Levene H., (1960), Robust tests for equality of variances, Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling, Palo Alto: Stanford University Press, pp. 278–292.
- López-Pérez M. V., Pérez-López M. C. and Rodríguez-Ariza L., (2011), Blended learning in higher education: students' perceptions and their relation to outcomes, Comput. Educ., 56(3), 818–826.
- MacArthur J. R. and Jones L. L., (2008), A review of literature reports of clickers applicable to college chemistry classrooms, Chem. Educ. Res. Pract., 9(3), 187–195.
- Mann H. B. and Whitney D. R., (1947), On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other, Ann. Math. Stat., 18(1), 50–60.
- Migdał-Mikuli A., Broś P. and Bernard P., (2008), Purpose and form of realization of the blended learning system during chemistry academic courses, Problems of Education in the 21st Century, 5(5), 98–104.
- Oversby J., (2012), ASE guide to research in science education, Hatfield: The Association for Science Education.
- Rovai A. P., (2002), Sense of community, perceived cognitive learning, and persistence in asynchronous learning networks, Internet High. Educ., 5(4), 319–332.
- Russell C. B. and Weaver G. C., (2011), A comparative study of traditional, inquiry-based, and research-based laboratory curricula: impacts on understanding of the nature of science, Chem. Educ. Res. Pract., 12(1), 57–67.
- Sadler P. M. and Tai R. H., (2007), The two high-school pillars supporting college science, Science, 317(5837), 457.
- Shapiro S. S. and Wilk M. B., (1965), An analysis of variance test for normality (complete samples), Biometrika, 52(3–4), 591–611.
- Shedlosky-Shoemaker R. and Fautch J. M., (2015), Who Leaves, Who Stays? Psychological Predictors of Undergraduate Chemistry Students' Persistence, J. Chem. Educ., 92, 408–414.
- So H. J. and Brush T. A., (2008), Student perceptions of collaborative learning, social presence and satisfaction in a blended learning environment: relationships and critical factors, Comput. Educ., 51(1), 318–336.
- Song L., Singleton E. S., Hill J. R. and Koh M. H., (2004), Improving online learning: student perceptions of useful and challenging characteristics, Internet High. Educ., 7(1), 59–70.
- Tai R. H., Ward R. B. and Sadler P. M., (2006), High school chemistry content background of introductory college chemistry students and its association with college chemistry grades, J. Chem. Educ., 83(11), 1703.
- Wenning C. J., (2005), Levels of inquiry: hierarchies of pedagogical practices and inquiry processes, J. Phys. Teach. Educ. Online, 2(3), 3–12.
- Williams N. A., Bland W. and Christie G., (2008), Improving student achievement and satisfaction by adopting a blended learning approach to inorganic chemistry, Chem. Educ. Res. Pract., 9(1), 43–50.
- Wu J. H., Tennyson R. D. and Hsia T. L., (2010), A study of student satisfaction in a blended e-learning system environment, Comput. Educ., 55(1), 155–164.
| This journal is © The Royal Society of Chemistry 2017 |