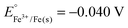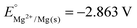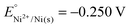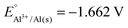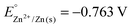Implementation of case-based instruction on electrochemistry at the 11th grade level†
Aysegul
Tarkin
 *a and
Esen
Uzuntiryaki-Kondakci
*a and
Esen
Uzuntiryaki-Kondakci
 b
b
aDepartment of Mathematics and Science Education, College of Education, Yuzuncu Yil University, 65080, Van, Turkey. E-mail: aytarkin@gmail.com
bDepartment of Mathematics and Science Education, College of Education, Middle East Technical University, 06800, Ankara, Turkey
First published on 12th May 2017
Abstract
This study aims to compare the effectiveness of case-based instruction over traditional instruction in improving 11th grade students' understanding of electrochemistry concepts, attitudes toward chemistry, chemistry self-efficacy beliefs, and motivation to learn chemistry. In total, 113 students (47 males and 66 females) from three high schools participated in this study. Two of the classes from each school were randomly assigned to be either the experimental or control group. The experimental group was instructed by case-based instruction while the control group was taught by traditionally designed instruction. The Electrochemistry Concept Test, Attitude toward Chemistry Scale, High School Chemistry Self-efficacy Scale, and Chemistry Motivation Questionnaire were applied as pre- and post-tests to students in both groups. Moreover, a feedback form was administered to students in the experimental group at the end of the study to get students' opinions about the case-based instruction. One-way Multivariate Analysis of Variance (MANOVA) revealed that case-based instruction was an effective method to improve students' understanding of electrochemistry concepts, attitude toward chemistry, and intrinsic motivation to learn chemistry. The qualitative data gathered from the feedback forms also supported the results of the inferential statistics. Students reported that chemistry lessons were more interesting and enjoyable via case-based instruction.
Introduction
Over the past twenty-five years, science educators have emphasized that learners should construct their knowledge by being actively involved in a realistic and social learning environment rather than only receiving knowledge from the teacher (Honebein, 1996; Duit and Treagust, 1998; Leonard, 2000; Barron and Darling-Hammond, 2008). Through instruction based on constructivism, teachers are more likely to increase students' interest in and attitudes toward science, enhance their motivation to learn science topics, and improve their views about the relevance of science to their life, which ultimately enhances meaningful science learning and scientific literacy (Duit and Treagust, 1998; King, 2009; Milner et al., 2011; Toraman and Demir, 2016). In addition, students' beliefs about their capability to perform science-related tasks successfully (i.e., self-efficacy)—and in turn their tendency to engage in science activities, make efforts to complete them, and persist in working when they encounter difficulties—is likely to increase as a result of constructivist teaching (Dunlap, 2005; Palmer, 2005; Mataka and Kowalske, 2015). Although many researchers have designed various instructional strategies based on constructivism and investigated their effects on students' science learning, alternative ways to teach science effectively are still within science educators' field of interest. In the last decade, an instructional strategy called case-based instruction has been used in science education. Case-based instruction creates an active learning environment that involves solving and examining real-world problems in small groups with guided instruction. Case-based instruction was firstly used in the Law and Business Schools at Harvard College around a hundred years ago (Herreid, 2005). While case-based instruction has long been used in law and business, it is increasingly used in other disciplines such as health science (Dupuis and Persky, 2008; Brown et al., 2011), nursing (Thomas et al., 2001; Kaddoura, 2011), business management (Pearce, 2002), psychology (Mayo, 2002, 2004) and educational psychology (Sudzina, 1997). For about 20 years, many instructors of various scientific disciplines have adapted case-based instruction to their courses such as environmental chemistry (e.g., Cheng, 1995), general chemistry (e.g., Jones, 1997; Hutchinson, 2000), general biology (e.g., Rybarczyk et al., 2007) anatomy and physiology (e.g., Cliff and Wright, 1996; Wilcox, 1999), and biochemistry courses (e.g., Cornely, 1998). Many of these studies presented applications of case-based instruction during undergraduate courses stated above, and students' ideas about these courses and the usefulness of case-based instruction (Cheng, 1995; Challen and Brazdil, 1996; Jones, 1997; Lantz and Walczak, 1997; Cornely, 1998; Smith and Murphy, 1998; Wilcox, 1999). In addition, some studies examined the effectiveness of case-based instruction on students' learning via one group research design (Hutchinson, 2000; Knight et al., 2008; Ayyildiz and Tarhan, 2012). These kinds of designs are weak primarily because they lack a control group and provide a weak basis for causal inference compared with a control group design. In an experimental research study with a control group the changes in the dependent variable may strongly be attributed to the treatment and generalization is more feasible. To our best knowledge, only Rybarczyk et al. (2007) conducted a two group experimental research (experimental and control group) study to investigate the effectiveness of a case-based learning approach on students' learning gain in an undergraduate course. Regarding research studies conducted in elementary and high school courses, some studies presented the way they used cases in the courses (Richmond and Neureither, 1998) similar to the other studies conducted in the undergraduate courses. Moreover, some studies investigated the effect of case-based instruction on different variables through experimental research design in the context of different courses such as science (e.g., Gabel, 1999; Adali, 2005), biology (e.g., Cakir, 2002; Saral, 2008; Skolnick, 2009), physics (e.g., Ozkan and Azar, 2005), and chemistry (e.g., Cam, 2009; Yalcinkaya, 2010; Morris, 2013). In conclusion, empirical research studies investigating the impact of case based instruction on students' learning are limited. Regarding the benefits of constructivism, case-based instruction might be effective in bringing the learning of chemistry closer to the lives and interests of students, and in using real life examples to improve students' interest in science and so enhance their understanding and scientific literacy. However, research studies investigating the impact of case-based instruction on students' chemistry learning are limited in number. Case-based instruction has had few trials among teachers in secondary science education, especially in chemistry education. In the present study, therefore, considering both the potential significance of using case-based instruction to improve science understanding and motivation, and the limited empirical studies in the area in chemistry literature, the impact of case-based instruction on promoting meaningful chemistry learning has been investigated through quasi-experimental research design. This study aims to provide empirical evidence to compare the effectiveness of case-based instruction with one group that received the treatment and another group that did not receive the treatment on the same content. More specifically, the purpose of this study is to examine whether case-based instruction is effective in enhancing 11th grade students' understanding of the electrochemistry concepts, their attitudes toward chemistry, self-efficacy beliefs, and motivation to learn chemistry.Literature review
Particularly in chemistry, students experience a lot of difficulty in understanding the concepts (Nakhleh, 1992; Harrison and Treagust, 1996; Duit and Treagust, 1998; De Jong and Taber, 2007; Sirhan, 2007). In addition, students do not see the importance and relevance of learning chemistry concepts for their life and the environment (Hutchinson, 2000). Moreover, they view chemistry as a boring subject and irrelevant to their life (Hutchinson, 2000; Soudani et al., 2000). The nature of instruction has an important role in promoting students' understanding. The findings of research studies on the topic have indicated that traditional instruction, which is mainly based on a teacher-centered approach and involves dissemination of knowledge by the teacher through verbal explanations or lectures, tends to be ineffective in engaging students in meaningful learning in different areas of science, such as biology and chemistry (Anderson and Smith, 1987; Haidar and Abraham, 1991; McDermott, 1993; Anderson and Lee, 1997; Mao and Chang, 1998; Lord, 1999; Aikenhead, 2003; Schroeder et al., 2007). Science education does not only deal with teaching theoretical knowledge but also provides ways for students to gain basic motivational and affective skills, which have been regarded as a salient factor affecting student learning in science (Pintrich and Schunk, 2002; Singh et al., 2002; Pintrich, 2003; Koballa and Glynn, 2007; Ng et al., 2012). Motivation is defined as the process that initiates and sustains goal-oriented activities. In other words, motivation stimulates individuals to start on an activity, keeps them moving, and helps them accomplish the activity (Pintrich and Schunk, 2002; Pintrich, 2003). While intrinsic motivation leads people to engage in activities because they enjoy them, extrinsic motivation directs people to do a certain task to get a desirable outcome, such as rewards or high grades (Pintrich and Schunk, 2002). Research studies indicate that intrinsic motivation is positively correlated with achievement (e.g., Bryan et al., 2011; Cerasoli et al., 2014; Taylor et al., 2014; Schumm and Bogner, 2016). In a similar vein, attitude, which can be defined as “a general and enduring positive or negative feeling about some person, object, or issue” (Petty and Cacioppo, 1981, p. 7) is another variable that has appeared as influential in science learning (e.g., Singh et al., 2002; Webster and Fisher, 2000; Willson et al., 2000). According to Raved and Assaraf (2011), one of the factors influencing students' attitudes toward science is the relevance and authenticity of the topics being studied. Unfortunately, most students are not able to see the importance and relevance of learning chemistry concepts for themselves (Hutchinson, 2000). They tend to think that learning these concepts is required only in order to reach the next step in their education (Soudani et al., 2000; Pilot and Bulte, 2006). Students generally view the scientific facts, definitions, and formulas as “school knowledge” and memorize them to pass their science exams. They could not apply their knowledge to explain real-world phenomena that they observe and experience (Roth, 1990). Many students consider the knowledge that they learned in chemistry classes as isolated from daily life, since it does not seem useful in their everyday activities (Soudani et al., 2000). Therefore, they perceive chemistry as a boring subject, irrelevant to their life (Hutchinson, 2000; Soudani et al., 2000). It appears that traditional instruction is not likely to be adequate for increasing meaningful learning and arousing interest in chemistry. Indeed, in the literature, two of the most outstanding criticisms of science education are its lack of relevance to daily life and its focus on abstract concepts beyond the interest of students (Dillon, 2009; Rannikmäe et al., 2010).Another affective variable that influences students' science learning is self-efficacy (Andrew, 1998; Britner and Pajares, 2001; Kupermintz, 2002). It is defined as “people's judgments of their capabilities to organize and execute courses of action required to attain designated types of performances” (Bandura, 1986, p. 391). Students' science self-efficacy beliefs affect their tendency to engage in science learning activities, their efforts to complete them, and their persistence in working when they encounter difficulties. Students perform the science activities that they believe they are capable of doing well and avoid tasks they believe they could not do (Bandura, 1997; Britner and Pajares, 2001). Bandura (1997) proposed that self-efficacy beliefs are shaped by four main sources of information: mastery experience, vicarious experience, social persuasion, and physiological and emotional states. Mastery experiences such as working on classroom tasks and taking responsibility for learning might provide students with opportunities to be successful, thus facilitating their self-efficacy. Students interpret the results of their previous experiences with the task and develop beliefs about their capabilities. In particular, students' successful experiences in executing a task will increase their self-efficacy, while failures will decrease it (Bandura, 1997; Linnenbrink and Pintrich, 2002). Small-group work, or collaborative work, may serve as a vicarious experience. Collaboration provides students with opportunities to see how their peers approach the learning task and solve problems. In other words, group working activities allow them to learn from peers. In addition, they get explicit feedback about their performance in the task from their peers during the collaborative process, which might contribute to students' self-efficacy beliefs. Positive affirmations and social persuasion about students' capabilities to succeed in the task will increase their self-efficacy beliefs. Moreover, positive physiological and emotional states such as happiness and exhilaration are more likely to enhance self-efficacy beliefs than negative ones such as sadness and anxiety (Bandura, 1997). Because traditional instruction does not provide students with an opportunity to be actively involved in a learning task, construct their knowledge, and work collaboratively, it is not adequate for increasing students' self-efficacy beliefs.
Case-based instruction
Case-based instruction is simply defined as using cases in instruction. Cases are described as “stories with an educational message” (Herreid, 2007, p. xiv). In other words, “Cases are well-written vignettes, usually expressed as dilemmas that allow the reader to engage ideas along emotional and intellectual dimensions” (Coppola, 1996, p. 2). Cases help learners to understand the relevance of science in society (Herreid, 2007). In a broad sense, case-based instruction involves “learning by doing, the development of analytical and decision-making skills, the internalization of learning, learning how to grapple with messy real-life problems, the development of skills in oral communications, and often teamwork” (Herreid, 2007, p. 30). In the literature, the different types of case-based instructions differ primarily in the way the instructor delivers the story in the classroom (Herreid, 1994, 1998, 2011; Cliff and Wright, 1996). The teaching methods for cases are mostly dependent on the size of the class and on time. However, when appropriate conditions exist, a small-group format is the best strategy for learning among other alternative formats (Herreid, 2011). In this format, students can learn more from each other due to the nature of team learning strategies. Hence, in the current study, case-based instruction is carried out in a small-group format.In the small-group method, collaborative or cooperative learning strategies are used with small groups. There are four formats for small-group case-based instruction: problem-based learning, interrupted case method, intimate debate method, and team learning. Problem-based learning—widely used in training medical students—is the most popular small-group case approach. In this format, teams of students work with tutors. In addition, information is provided over several class periods and students add literature research when needed. In the interrupted case method, conversely, students deal with each case in a single class period, without literature research. Cases provide all the information and data that students use while solving the problem. The intimate debate method is effective in dealing with controversial topics such as global warming or stem cell research. Groups of students prepare points on both the pro and con sides of an issue. Then, pairs of students from each group couple with pairs of students in other groups who have opposite viewpoints on the question or issue and argue their perspectives. Next, student pairs reverse the roles. Finally, they try to reach a consensus on the question. In the final format of the small-group method, team learning, students are given a reading assignment before the class session. In the class session, students first take an individual test related to the reading material and then take the test in small groups. Both the individual and group tests are scored in the classroom immediately (Herreid, 2011).
Case-based instruction allows students to construct their knowledge and puts importance on authentic, meaningful, and active learning as proposed by the constructivist approach (Guest, 2007). It enhances students' abilities to recognize a wide range of applicable social problems and concerns, and provides them with opportunities to solve them in a collaborative environment. The role of the instructor is to provide appropriate cases and guide learning by asking appropriate questions that promote analysis, discussion, and resolution for the specific problem given in the case. This helps learners to put their theoretical knowledge into practice and to see the relevance of the subject to their life, rather than merely memorizing a prescribed body of knowledge. Moreover, case-based instruction makes the classroom environment vigorous and more engaging than traditional instruction, because students are involved in trying to put ideas into their own words when studying cases (Herreid, 2005). This method also increases retention of science learning (Cornely, 1998). In science education literature, research studies have indicated that case-based instruction enhanced students' problem solving, higher order thinking, collaboration, decision making, and critical thinking skills (Herreid, 1994, 2007; Jones, 1997; Cornely, 1998; Morrison, 2001; Rybarczyk et al., 2007) and increased achievement (Gabel, 1999; Cakir, 2002; Adali, 2005; Ozkan and Azar 2005; Rybarczyk et al., 2007; Saral, 2008; Cam, 2009; Skolnick, 2009; Morris, 2013; Yalcinkaya and Boz, 2015). Case-based instruction is likely to be effective in increasing students' attitudes toward science and their motivation because it provides opportunities for students to experience or practice real-life situations and to perceive the relevance of science. The literature provides evidence that students see case-based instructional strategies as realistic, challenging, interesting, enjoyable, and encouraging learning (Bridges and Hallinger, 1992; Wassermann, 1994; Jones, 1997; Smith and Murphy, 1998; Dori and Herscovitz, 1999; Mayo, 2002, 2004; Naumes and Naumes, 2006). However, there is little empirical research on the effect of case-based instruction on students' motivation and attitude toward science, excepting the studies conducted by Cam (2009), Saral (2008), Skolnick (2009), and Yalcinkaya (2010). Their findings indicate that case-based instruction promotes intrinsic and extrinsic motivation, and that students find the learning tasks valuable more so than students instructed with traditionally designed instruction. Still, further research is warranted to provide empirical evidence for the effect of case-based instruction on students' motivation and attitudes at different grade levels and in different branches of science. In addition, case-based instruction has potential to provide mastery and vicarious experiences by engaging students in solving authentic problems and working collaboratively, and therefore is likely to promote students' science self-efficacy beliefs (Dunlap, 2005). However, to our best knowledge, there are no studies that provide empirical evidence concerning the effectiveness of case-based instruction on students' chemistry self-efficacy beliefs.
In the present study, case-based instruction was implemented in the context of chemistry, specifically in electrochemistry topics. The relevance of electrochemistry to our life and the environment is limitless; it is tied to the batteries used in many electronic devices (e.g., mobile phones, calculators, and clocks), metal plating used in industry, photosynthesis, and respiration, which involves oxidation–reduction reactions. Moreover, electrochemistry concepts explain the process of environmental events such as acid rain, corrosion of metals, water purification by chlorination, and energy production. Regardless, electrochemistry is one of the chemistry subjects which is perceived as difficult and abstract by students (Johnstone, 1980; Finley et al., 1982; Butts and Smith, 1987; Soudani et al., 2000). Mainly, students have difficulty applying their theoretical knowledge about oxidation–reduction concepts when interpreting daily life events. Soudani et al. (2000) propose some factors that may be responsible for students' difficulties in electrochemistry: teachers' focus on algorithmic problem solving rather than students' understanding of their environment; students' unawareness of the relevance of chemistry to their life and environment; students' lack of curiosity about chemistry learning; and students' exclusive focus on getting the best grades to move up into higher classes, which directs them to rote learning rather than deep understanding of concepts. Case-based instruction might help students to understand the electrochemistry concept, since activities related to daily life events will attract students' interest in and curiosity about chemistry and increase their awareness of the relevance of chemistry to their life and environment. Thus, this kind of instruction might be effective in promoting meaningful learning of electrochemistry concepts and improving students' scientific literacy.
Problem-based instruction and case-based instruction are very similar in terms of their characteristics. For example, both approaches are student-centered and collaborative, provide an authentic context for learning, and involve discussion sessions. However, they differ from each other in many points. The main difference between these two approaches is that problem-based instruction requires more course sessions to investigate each problem than case-based instruction. In case-based instruction, each case is generally investigated in two course sessions. In addition, problem-based instruction must involve an ill-structured problem which is provided in a kind of case. However, in case-based instruction, the case is not necessarily a problem. In other words, a case can be a story including a learning message such as an article from a newspaper or an anecdote from history as well as a problem. Traditionally, students read and reflect on case questions with teachers and peers by engaging in a discussion. The discussion is the important part of the learning. In contrast, in problem-based instruction, students are provided a series of artifacts and they determine the problem and propose a solution by examining the documents. The learning is embedded in the problem solving process. Students are expected to master the course objectives while working on the problem (Bridges and Hallinger, 1992; Kain, 2003). Then, a discussion session similar to case-based instruction is followed. Moreover, case-based instruction has different types which are described in the next part. In case-based instruction, the learning environment can be individual as well as collaborative, which is the essential characteristic of problem-based instruction. In contrast to problem-based instruction, case-based instruction can be conducted with large groups by using clicker cases defined in the next part. Another difference is that problem-based learning allows students to explore the knowledge needed to understand a given phenomenon whereas CBL requires the students to have a degree of prior subject matter knowledge to solve the problem given in the case (Bridges and Hallinger, 1992; Williams, 2005; Tarnvik, 2007; Allchin, 2013).
Case-based instruction has also commonalities with context-based instruction since both of them provide learning in a context. Context-based instruction is defined as “using concepts and process skills in real-world contexts that are relevant to students” (Glynn and Koballa, 2005, p. 75). As in the case-based instruction, students learn subjects in a real-world context that allows them to make connections between the subjects and their lives. In context-based instruction, a series of case studies that are based on a real-world context was developed and related to the concepts of the chemistry curriculum (Hofstein and Kesner, 2006). Pilot and Bulte (2006) stated that “contexts are meant to explicitly relate the sciences and technology to socio-scientific issues” (p. 1088). For example, by utilizing context-based instruction, organic chemistry may be introduced in the context of materials such as plastics and polymers that are familiar to the readers. Another example is that environmental context such as acid rain may be used to teach the concepts of acids, bases, and pH (Schwartz, 2006). Similar to case-based instruction, context-based instruction allows students to see “the importance and relevance of science for themselves and the application of scientific concepts and methods” (Parchmann and Luecken, 2010, p. 2). However, in context-based instruction, a unit is taught through cases based on a particular context, which generally describes a societal problem in the real world whereas a unit can be taught by forming cases based on different contexts.
Although the related literature suggests that case-based instruction would be more effective compared to the traditional instruction, the success of any teaching instruction depends on several issues. Although, major changes in the Turkish secondary chemistry education curriculum have been taking place and implemented gradually in line with constructivism since 2007, teachers and students devalued the proposed constructivist teaching, arguing that educational circumstances in Turkey are not ready to implement it under the competitive university entrance examination system. In addition, according to the new curriculum, the role of the teacher changed from the disseminator of information to the facilitator that guides students to construct their own knowledge. Teachers think that more class time is needed for students to construct knowledge, and this presents a difficulty for them since they need to complete a topic in an allocated period of time determined by the curriculum. Since teachers in Turkey are used to teaching in a traditional way, direct instruction is still dominant in our country and it would take some time for teachers to get accustomed to their new roles. Similarly, students are used to be taught by traditional instruction. It is not clear whether all teaching methods based on constructivism would be equally successful across all subject areas and for all students (Airasian and Walsh, 1997). In addition, it is misleading to consider teaching methods such as memorization and rote-learning useless. “There are, indeed, matters that can and perhaps must be learned in a purely mechanical way.” (von Glasersfeld, 1995, p. 2). Moreover, not all aspects of a subject can or should be taught in the same way or be acquired solely through student-centered activities (Airasian and Walsh, 1997). Cobern et al. (2010) also mentioned that the nature of a topic does influence the choice of the most effective method of instruction. Due to the fact that students are accustomed to more teacher control and directions, with an emphasis on correct answers and not expressing their thought processes, it is more suitable to start constructivist teaching with case-based instruction rather than problem-based instruction. Moreover, teaching with several cases in different contexts instead of a particular context might provide more opportunity for students to see the importance and relevance of science for themselves. Therefore, we thought that it would be meaningful to compare case-based instruction with traditional instruction in order to reveal which one would be more effective for the students in our country in the context of the electrochemistry topic.
Based on the literature review and the information above about the use of case-based instruction, the following research questions were created for this study in order to reveal the effects of case-based instruction on students learning and other salient variables affecting learning.
(1) Is there a significant mean difference between the groups exposed to case-based instruction and traditionally designed chemistry instruction with respect to understanding of the electrochemistry concept, attitudes toward chemistry, chemistry self-efficacy beliefs, intrinsic motivation, and perception about relevance of chemistry to personal goals at the 11th grade level?
(2) What are 11th grade students' views about case-based instruction, based on the reactions of the participants?
Methodology
Research design
This study utilized the nonequivalent pre-test/post-test control group design as a kind of Quasi-Experimental Design. In this design, the subjects are not randomly assigned to these groups; instead the already-formed groups are randomly assigned as control and experimental (Fraenkel and Wallen, 2006). For this study, existing classrooms were assigned to the treatments, rather than individual subjects. Two treatment groups, experimental and control, were pre-tested, administered a treatment, and post-tested. While the experimental group was instructed by case-based instruction, the control group was taught by traditional instruction. Table 1 presents the design of the study.| Groups | Pre-tests | Treatment | Post-tests |
|---|---|---|---|
| Note: EG: experimental group, CG: control group, ECT: Electrochemistry Concept Test, ASTC: Attitude Scale toward Chemistry, CMQ: Chemistry Motivation Questionnaire, HCSS: High School Chemistry Self-Efficacy Scale. | |||
| EG | ECT | Case-based instruction | ECT |
| ASTC | ASTC | ||
| CMQ | CMQ | ||
| HCSS | HCSS | ||
| CG | ECT | Traditional instruction | ECT |
| ASTC | ASTC | ||
| CMQ | CMQ | ||
| HCSS | HCSS | ||
Participants
The 113 participants in the study were 11th grade students (47 boys and 66 girls) from three different high schools in Ankara, Turkey. These schools were selected due to their convenient location and the willingness of their chemistry teachers. Therefore, convenience sampling was used for this study. These schools each were following the same National Chemistry Curriculum and were mostly similar in terms of school facilities. Two classes with the same teacher were chosen from each school. In each school, the classes were assigned to the experimental or control group randomly. While 59 students were instructed by case-based instruction in the experimental group, 54 students were taught by traditional instruction in the control group. The ages of the participants were 16 and 17. All the teachers who participated in the study were female and had over ten years' experience in the teaching profession. Before the study, they were given information about case-based instruction in general and how they could use this instruction in the experimental groups by providing lesson plans.Data collection
In order to determine the effect of case-based instruction on the students' understanding of electrochemistry concepts, attitudes toward chemistry, self-efficacy beliefs, and motivation to learn chemistry, four instruments were administered to all students before and after the treatment: (i) Electrochemistry Concept Test, (ii) Attitude toward Chemistry Scale, (iii) High School Chemistry Self-Efficacy Scale, and (iv) Chemistry Motivation Questionnaire. In addition, after the treatment, students' opinions about case-based instruction were gathered through a feedback form.Electrochemistry concept test (ECT)
The ECT was developed by the authors to assess students' understanding of electrochemistry concepts. The initial form of the test consisted of 29 multiple-choice items with five alternatives—one correct answer and four distracters. It was examined in detail by four chemistry educators and one chemist in terms of content validity and format. The ECT was piloted at high schools in Ankara with 131 high school students who had already learned the electrochemistry concept. To check the difficulty level of the test and determine how well it discriminates between high achievers and low achievers, item analysis was conducted through an Item and Test Analysis program (ITEMAN). According to the scale statistics calculated by the ITEMAN, the mean item difficulty was 0.52, which means that, on average, 52% of the students answered the items correctly. This result indicated that test items were neither too easy nor too difficult. Besides the difficulty level, the results of the ITEMAN revealed that the average item discrimination index was 0.45, which is within the acceptable range (Crocker and Algina, 1986; Cunningham, 2005). The reliability coefficient of the test was found to be 0.73. However, when item statistics were analyzed in terms of item difficulty and item discrimination indices for each item, it was seen that there was a need to change or revise some items. The items whose biserial correlation indices were below 0.19 or between 0.20 and 0.29 were revised or deleted (Ebel, 1965, as cited in Crocker and Algina, 1986). The final form of the test comprised 27 multiple-choice questions with five alternatives. In the study, this test was administered to students in both the experimental and the control groups before and after the treatment (see some examples of test items in Appendix A).Attitude scale toward chemistry (ASTC)
This scale was developed by Geban et al. (1994) to measure students' attitudes toward chemistry as a school subject. It is a unidimensional scale containing 15 items on a 5-point Likert-type scale, ranging from “strongly disagree” to “strongly agree.” Before using this scale in the current study, a pilot study was conducted with 387 students, also in the 11th grade, to check the validity and reliability of the scale. The evidence of construct validity was calculated in terms of “item-total score” correlation using Pearson's correlation coefficient (r). All items had moderate or high positive correlation with the total score (0.46 < r < 0.78, Hinkle et al., 1998). These results verified that all items contributed to the validity of the instrument. The Cronbach alpha reliability coefficient was found to be 0.91, which indicates a high reliability (Nunnally and Bernstein, 1994). In the main study, this scale was administered to students in experimental and control groups before and after the treatment.Chemistry motivation questionnaire
This questionnaire was originally developed by Glynn and Koballa as a “Science Motivation Questionnaire” (SMQ) to assess students' motivation to learn science (2006). It was translated into Turkish, adapted to the subject of chemistry, and re-named Chemistry Motivation Questionnaire (CMQ) by Cetin-Dindar and Geban (2010). It consists of 30 items with a 5-point Likert-type scale, as in the original version. However, unlike the original scale, it is divided into five dimensions: intrinsically motivated chemistry learning (six items), relevance of learning chemistry to personal goals (five items), self-determination for learning chemistry (seven items), confidence in learning chemistry (seven items), and anxiety about chemistry assessment (five items). The reliability coefficient estimated by Cronbach's alpha of the Turkish version was found to be between 0.75 and 0.84 for each dimension of the questionnaire. To meet the aim of the current study, two dimensions of the CMQ—intrinsically motivated chemistry learning and relevance of learning chemistry to personal goals—were utilized. This two-factor structure model was first tested in the pilot study. The questionnaire, consisting of eleven items, was administered to 417 eleventh grade students. For construct validity, Confirmatory Factor Analysis (CFA) was carried out using AMOS 21 (Analysis of Moment Structures, Arbuckle, 2012). The goodness of fit statistics showed that a two-factor structure provided a satisfactory model fit: CFI = 0.94, NFI = 0.91, and RMSEA = 0.074 (Browne and Cudeck, 1993; Kline, 1998). The Cronbach alpha reliability coefficients were found to be satisfactory (Nunnally and Bernstein, 1994) at 0.83 and 0.72, for the dimensions of relevance of learning chemistry to personal goals and intrinsically motivated chemistry learning, respectively. In the study, this questionnaire was administered to the students in the experimental and control groups before and after the treatment.High school chemistry self-efficacy scale (HCSS)
This scale was developed by Capa-Aydin and Uzuntiryaki (2009) to assess chemistry self-efficacy beliefs of high school students. The scale contains 16 items with a 9-point Likert-type response format, where 1 indicates “very poorly” and 9 indicates “very well.” This scale has two sub-dimensions: chemistry self-efficacy for cognitive skills and self-efficacy for chemistry laboratory. These sub-dimensions comprised 10 and 6 items, respectively. In this study, in order to test the factor structure of the scale via CFA, it was administered to 124 eleventh grade students in Ankara. The following fit indices, which indicated a quite satisfactory model fit, were obtained: CFI = 0.92, NFI = 0.85 and RMSEA = 0.069 (Browne and Cudeck, 1993; Kline, 1998). Moreover, Cronbach alpha values were satisfactory (Nunnally and Bernstein, 1994); they were indicated to be 0.86 and 0.95 for chemistry self-efficacy for cognitive skills and self-efficacy for chemistry laboratory, respectively. In the study, this scale was administered to the students in the experimental and control groups before and after the treatment.Feedback form regarding case-based instruction
The aim of this feedback form was to get students' opinions about the case-based instruction. In particular, the researchers aimed to determine what students thought about the case-based instruction, how they perceived the cases, whether they liked the instruction, and whether they thought they benefited from the course. In this study, therefore, this form was administered only to the students in the experimental groups. It includes seven open-ended questions adapted from the studies of Sungur (2004) and Yalcinkaya (2010). Sample questions include “When you compared case-based instruction with previous traditional chemistry instruction, what do you think about the effectiveness of case-based instruction on your learning?” and “Which characteristics of case-based instruction do you like or not?”Treatment
This study was conducted over nine weeks (two weeks for data collection, seven weeks for treatment) in three high schools. In each high school, one of the two classes was randomly assigned as the experimental and the other class was assigned as the control group. Both classes were taught by the same teacher in each school. All students followed the same national chemistry curriculum of the Ministry of National Education and were taught the same concepts, but by different instructional methods. In the national curriculum, the electrochemistry unit consists of three sub-topics: the relationship between matter and electricity, Standard Electrode Potentials, and Electrochemical Cells. These topics cover the following concepts: Faraday's laws, Redox reactions, Oxidation, Reduction, Oxidation–reduction potential, Standard electrode potential, Electrode, Half-cell, Galvanic cell, Electrolytic cell, Electrolysis, and Corrosion. The experimental group was instructed by case-based instruction, while the control group was instructed by traditionally designed chemistry instruction. The classroom instruction time was three 45 minute periods per week. Before the treatment, all teachers participating in the study were trained in case-based instruction with an emphasis on the roles of the teacher and the students. Additionally, teachers were informed about how to implement the instructional materials for the electrochemistry unit prepared by the first author. Each week, the first author came together with the teachers in their school and explained the lesson plan of that week. Before their class, the teachers were provided with a lesson plan prepared by the first author and given information about how to implement related case-based instruction step by step. During the meeting, teachers' questions related to instruction were answered, if available. Teachers were also provided answers of questions given in the case and some directing questions to guide students' discussion. In the first and last week of the study (i.e., at the beginning and the end of the treatment) the students in both groups were given ECT, ASTC, CMQ and HCSS as pre-test and post-test. The first author attended the classes of both the control and experimental groups as an observer. In order to ensure treatment verification in both groups the researcher filled in a classroom observation checklist. There were 21 items in this checklist to be scored with one of three options: yes, no, or not applicable. The items were related to the main characteristics of case-based instruction and traditional instruction. To ensure the implementation fidelity, 76% of class sessions in the experimental group and 38% of those in the control group were observed. This was enough of a period for the researchers to make the conclusion that the teachers implemented the instructions as they would be in accordance with the teacher guide. In addition to observation by the researcher, three classes from the control group and six classes from the experimental group were observed by PhD candidates in chemistry education in order to avoid bias and obtain more reliable results in the implementation process. Regarding all observers, the measured Kappa values ranged from 0.64 to 0.92, indicating a good level of agreement (Landis and Koch, 1977).Treatment in the control group
The students in the control group were instructed using traditionally designed chemistry instruction. It was teacher-centered, mainly involving lecturing. During the instruction, the teachers defined and explained the concepts of electrochemistry verbally using their notes, and wrote the formulas of the concepts on the board. The teachers' role was to transmit the facts and concepts to students. Meanwhile, students simply acted as passive listeners and took notes. During the teaching in these classes, the teacher also asked questions without creating a discussion platform. In some cases, students failed to respond to the questions. In this case, the teacher gave the answer to the question. In the control group, the students were only motivated by teacher-directed questions; there were no hands-on activities or group work in class during the teaching of electrochemistry concepts. Moreover, each teacher solved some numerical chemistry problems on the board and the students copied them into their notebooks. After that, the teacher posed new problems verbally, or wrote them on the board, and allocated certain time for the students to solve them. While the students were solving the problems, the teacher walked around the class monitoring students. Then, one of the students or the teacher solved the problem on the board. The teacher sometimes gave the students worksheets in which the students were asked to solve the questions. Instruction in the control group was based on informing students about the concepts of electrochemistry. Daily life examples similar to those presented to the experimental groups via cases were also mentioned verbally in the control groups by the teachers, but not discussed.Treatment in the experimental group
The students in the experimental group were instructed by case-based instruction in the small group format described by Herreid (2011). The same content was covered in the experimental groups as in the control groups. Cases prepared by the authors were used as an active learning material. While writing the cases, the characteristics identified by Herreid (1997) and the process defined by Wassermann (1994) were considered. First, the researcher found daily life events or problems related to the objectives of the electrochemistry unit in the national chemistry curriculum. Once the events were chosen, the scenarios were formed. Several experts in chemistry education were asked to review the cases for appropriateness of the content, grade level, and objectives. Then the researcher met with reviewers to discuss and revise the cases. In the process of preparation of the cases, case drafts underwent several cycles of review, discussion, and revision. In this study, a total of eight cases based on real-life events or socio-scientific issues were used to teach the concepts of electrochemistry. The topics of the cases were electroplated materials, cleaning tarnished silver materials, accumulators, recycling silver from old roentgenograms, fruit clocks, clean energy, electronic waste, and protection from corrosion (Table 2). After each case, teachers asked open-ended questions related to the concept. The students were supposed to read the case in groups, discuss the details, research it, and answer the questions. In other words, the role of the students was participating actively in discussions to reveal their ideas explicitly. Unlike traditional instruction, the role of teachers in case-based instruction is as a facilitator that guides students to construct their own knowledge. As in the control group, numerical chemistry problems were also provided to the students in the experimental group.| Cases | Descriptions | Concepts of electrochemistry |
|---|---|---|
| (1) Gold goods | The case presents a dialog between two cousins watching a TV program. The program introduces a house containing gold goods. Based on the program, the two cousins start to talk about those gold materials in terms of whether they are completely made of gold or electroplated by gold. They share their knowledge about the process of electroplating materials by gold with each other. | Faraday's law and the relationship among redox reaction, electric current and material changes. |
| (2) Silver materials | This case describes a dialog which takes place between two girls namely Brenda and Sindy. They are talking about their silver jewellery and the problem of silver tarnish. | Redox reactions, oxidation, and reduction |
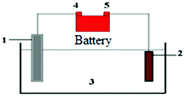
| (3) Accumulators | It describes an event that occurred while two families were having a trip in their cars. They stayed at an isolated and uninhabited place for two days. At the end of their trip, one of the cars didn’t start because of low battery voltage. There were no repair shops and residents around. | Working principles of rechargeable batteries and accumulators, galvanic cell, electrode, and half-cell |
| (4) Roentgenograms for source of money | It introduces the idea of making money by removing silver from old roentgenograms with the purpose of teaching electrolysis and the industrial application of it. | Industrial applications of electrochemistry and electrolysis |
| (5) Fruit clocks | It presents a story about a fruit clock. In the story, a girl buys a clock that worked with fruits as a present for her brother. The girl and her brother set up the clock by looking at the user guide of the clock and talk about the working principle of it. | Electrochemical cell, electrode, and half-cell |
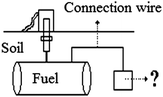
| (6) Clean energy | The case introduces news related to cars working with hydrogen fuels. | Industrial applications of electrochemistry and fuel cell |
| (7) Recycling gold from electronic waste | The case provides some information about the amount of electronic waste in Turkey and the amount of valuable metals obtained from electronic waste. Furthermore, it focuses on works of the Ministry of Environment and Forestry on the recycling process of electronic waste. | Industrial applications of electrochemistry and electrolysis |
| (8) Bridge | It presents a story about a bridge collapse in a river. | Corrosion and the protection methods of it |
Before the treatment the researcher formed small groups of five or six students in the experimental group from each school. The groups were formed based on the distribution of z-scores calculated from students' standard scores on pre-tests. The researcher aimed to form heterogeneous groups as much as possible in terms of students' chemistry achievement, attitude toward chemistry, and motivation to learn chemistry as determined by the pre-ECT, pre-ATCS, and pre-CMQ, respectively. Before the treatment, the teacher announced that the students would learn the electrochemistry topic by a new method called case-based instruction.
She provided information about the method, explaining what it is and how it is applied in classroom settings and emphasizing the roles of the students in detail.
A sample case used in the experimental groups (Silver Materials) is given in Appendix B. It describes how silver jewellery can be cleaned with a hot sodium bicarbonate solution and aluminium foil. This case was used to teach how to identify reducing and oxidizing agents in a redox reaction and how to balance redox in this case to the groups. Then, two of the students read the case to the whole class. Afterwards, each group analyzed the case and answered six questions given at the end of the case by discussing their answers with their group members. After the discussion, each group wrote down their answers. The questions were:
(1) Why do silver materials tend to lose their brightness and tarnish overtime? Could you write the chemical reaction equations that explains this situation? What are the oxidizing and reducing agents? Write oxidation and reduction half reactions.
(2) Do you think that we could polish our silver goods? Do an experiment to polish one of your silver goods.
(3) Could you write the chemical reaction equations that explain the process of polishing silver jewellery? What are the oxidizing and reducing agents? Write their half reactions.
(4) What is the function of sodium bicarbonate solution used during the cleaning process of silver jewellery? Is it important to use hot sodium bicarbonate solution? If yes, why?
(5) Why is aluminium foil used during the cleaning process of silver jewellery? Can you use another material instead of aluminium foil?
(6) Green color is produced on the surface of copper materials over time. What might be the reason for this situation? Could you write the chemical reaction equation that explains this situation? What are the oxidizing and reducing agents? Write their half reactions.
After each group answered the first question, the teacher asked them to share their answers and discuss as a class. Five minutes of discussion time were given to the students to argue how silver is tarnished, which chemical reaction occurs, and what the reducing and oxidizing agents are. Then, each group was provided with the necessary equipment and they tried to clean a tarnished silver material by employing the process explained in the case. After that, each group was asked to explain the chemical process underlying the cleaning of tarnished silver materials, to write a balanced chemical reaction equation, and to identify reducing and oxidizing agents in the reaction. Each group first wrote their answers on the paper. After finishing their answers, one of the students wrote his/her group's answers on the blackboard and explained the related chemical process. Then, the other groups discussed the answer under the guidance of the teacher and explained their answers. When necessary, the teacher provided clues to help the students answer the questions. Discussion continued until the right balanced chemical reaction equation, oxidizing and reducing agents, and half reactions were decided. Regarding the fourth and fifth questions, the students examined the cleaning process in terms of the materials used (i.e., sodium bicarbonate solution, hot water, and aluminium foil). Each group was asked what the functions of these materials were during the cleaning process. In addition, they were asked whether another material could be used instead of aluminium foil. After each group finished writing their answers, group answers were shared with the class and discussed. Finally, each group tried to answer the sixth question. Regarding this question, each group expressed their ideas about why copper materials turn green over time and which chemical reaction causes this situation. The whole class discussion continued until reaching a consensus under the teacher’s guidance. At the end of the lesson, the students worked to balance several chemical reaction equations given by the teacher. For implementation of this case, the teachers were provided an instructional guide given in Appendix C.
Analysis of data
The research questions of the study were answered through quantitative and qualitative data analysis. For the quantitative data analysis, descriptive and inferential statistics were utilized via IBM Statistical Package for the Social Sciences (SPSS) 21. As for the descriptive statistics analysis, the mean, standard deviation, skewness, and kurtosis were computed for each variable in both groups. As for inferential statistics analysis, Multivariate Analysis of Variance (MANOVA) was carried out for the pre-test and post-test scores to determine the effect of case-based instruction (independent variable of the study) on students' understanding of electrochemistry concepts, attitude toward chemistry, intrinsic motivation, perceptions regarding relevance of learning chemistry to personal goals, and chemistry self-efficacy for cognitive skills and chemistry laboratory work (dependent variables of the study). Before conducting MANOVA, the assumptions—normality, linearity, multicollinearity and singularity, and homogeneity of variance–covariance matrices—were checked. All statistical analyses were carried out at the 0.05 significance level.On the other hand, in the qualitative analysis, the data gathered from feedback forms were analyzed inductively. That is, the researcher built patterns, categories, or themes from the data by generating codes and organizing them (Creswell, 2007). Students' written responses on feedback forms for case-based instruction were categorized under three headings: students' description of case-based instruction, students' perceptions about the effectiveness of case-based instruction, and the difficulties students encountered during case-based instruction. The codes subsumed under each category are presented in Table 3.
| Categories | Codes | Sample excerpts |
|---|---|---|
| Students' descriptions of case-based instruction | Real-life issues | [It is] teaching the topic by giving examples related to the use of it in real life. |
| Doing an activity/experiment | Experiments and observations are the hearth of the instruction. | |
| Working in a group | We worked in groups. The cases were discussed and experiments were done when necessary. | |
| Dealing with a case | A text was distributed and then we discussed our opinions related to case given in the text with our teachers. | |
| Student-centered method | This instruction made us use our knowledge, think about situations, and find answers by ourselves. | |
| Students' perceptions about effectiveness of case-based instruction | Learning | Learning theoretical knowledge through daily life examples enhances our learning. |
| (i) Effective | Case-based instruction is not effective. In my view, ordinary instructions are more effective and easier. | |
| (ii) Ineffective | ||
| Enjoyment | Learning the place of chemistry in our lives was the feature that I liked most. Case-based instruction is more interesting than the instruction in the previous chemistry lesson. | |
| Difficulties students encountered during the case-based instruction | No difficulty | I did not encounter any difficulties. |
| Working in a group | Working in a group was not for me, I prefer individual working, I am more concentrated when I study alone than when I work in a group. | |
| Interpreting the cases | We had difficulties since we didn’t have sufficient knowledge about the cases. | |
| Answering the questions | It was difficult to give an answer to the questions without knowing the topic. | |
| Adopting the method | I am not used to be[ing] taught by case-based instruction. Therefore, I couldn’t adapt myself to this method. | |
Ethical issues of the study
Before the implementation of the current study, all the materials used during the treatment were reviewed and approved by the ethics committee of Middle East Technical University. In addition, legal permission from the Ministry of Education was received in order to conduct the study in schools. All the students consented to participate in the study. Regarding the issue of confidentiality, all students and teachers were informed that their names would not be reported anywhere and the accessible data would be seen only by the researcher.Results
Quantitative results
The results of the analysis of the quantitative data are presented under three headings: descriptive statistics, statistical analysis of pre-test scores, and statistical analysis of post-test scores.| Treatment | Dependent variable | Mean | Std. deviation | Max | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| Experimental group | Understanding of electrochemistry | 4.58 | 2.35 | 9.00 | −0.029 | −1.16 |
| Attitude toward chemistry | 2.82 | 0.749 | 4.73 | 0.351 | −0.072 | |
| Intrinsic motivation | 2.69 | 0.690 | 4.33 | 0.231 | −0.097 | |
| Relevance of learning chemistry | 2.89 | 0.888 | 4.80 | 0.195 | −0.644 | |
| Self-efficacy for cognitive skills | 4.95 | 1.29 | 7.90 | 0.019 | −0.117 | |
| Self-efficacy for chemistry laboratory work | 4.16 | 1.91 | 8.00 | −0.088 | −0.935 | |
| Control group | Understanding of electrochemistry | 4.44 | 2.47 | 9.00 | −0.177 | −0.917 |
| Attitude toward chemistry | 2.84 | 0.744 | 4.60 | 0.169 | −0.676 | |
| Intrinsic motivation | 2.67 | 0.762 | 4.50 | 0.115 | 0.088 | |
| Relevance of learning chemistry | 2.95 | 0.896 | 4.40 | −0.400 | −0.764 | |
| Self-efficacy for cognitive skills | 4.89 | 1.33 | 8.10 | −0.159 | 0.409 | |
| Self-efficacy for chemistry laboratory | 4.12 | 1.80 | 8.50 | 0.037 | −0.823 | |
| Treatment | Dependent variable | Mean | Std. deviation | Max | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| Experimental group | Understanding of electrochemistry | 15.27 | 4.96 | 25.00 | −0.090 | −0.624 |
| Attitude toward chemistry | 3.17 | 0.616 | 4.33 | −0.146 | −0.295 | |
| Intrinsic motivation | 2.96 | 0.621 | 4.33 | 0.009 | −0.259 | |
| Relevance of learning chemistry | 3.01 | 0.769 | 4.80 | 0.076 | −0.139 | |
| Self-efficacy for cognitive skills | 5.39 | 1.09 | 7.80 | 0.023 | −0.438 | |
| Self-efficacy for chemistry laboratory | 5.05 | 1.81 | 9.00 | −0.410 | −0.035 | |
| Control group | Understanding of electrochemistry | 12.96 | 3.50 | 21.00 | −0.157 | −0.637 |
| Attitude toward chemistry | 2.84 | 0.614 | 3.93 | −0.348 | −0.128 | |
| Intrinsic motivation | 2.61 | 0.633 | 4.00 | −0.306 | −0.081 | |
| Relevance of learning chemistry | 2.83 | 0.844 | 4.80 | −0.114 | 0.359 | |
| Self-efficacy for cognitive skills | 4.86 | 1.01 | 7.70 | 0.326 | 0.003 | |
| Self-efficacy for chemistry laboratory | 3.72 | 1.81 | 8.17 | 0.744 | 0.181 | |
| Effect | Value | F | Df | Error df | Sig. | Partial eta squared | Observed power | |
|---|---|---|---|---|---|---|---|---|
| Treatment | Pillai's Trace | 0.172 | 3.678 | 6 | 106 | 0.002 | 0.172 | 0.949 |
| Wilks' Lambda | 0.828 | 3.678 | 6 | 106 | 0.002 | 0.172 | 0.949 | |
| Hotelling's Trace | 0.208 | 3.678 | 6 | 106 | 0.002 | 0.172 | 0.949 | |
| Roy's Largest Root | 0.208 | 3.678 | 6 | 106 | 0.002 | 0.172 | 0.949 |
The results revealed that there was a statistically significant mean difference between the experimental and control groups with respect to combined dependent variables of understanding of electrochemistry, attitude, intrinsic motivation, perceptions regarding relevance of learning chemistry to personal goals, and self-efficacy for cognitive skills and chemistry laboratory work after the treatment: F(6,106) = 3.678, p < 0.05; Wilks' Lambda = 0.828. The value of partial eta squared based on Wilk's Lambda, 0.172, indicated that the magnitude of the difference between the experimental and control groups was not small. In other words, it means that 17.2% of the multivariate variance of the dependent variables could be explained by the treatment. The value of power, another important statistic, was found to be 0.949. These findings implied that the difference between the experimental and control groups arose from the treatment effect and that this difference had practical value.
Given the significance of the overall test, the univariate main effects (tests of between-subjects effects) were examined by a follow-up ANOVA. Before interpreting the result of the ANOVA, the assumption of equality of variances was checked. The result of Levene's test is displayed in Table 7.
| F | df1 | df2 | Sig. | |
|---|---|---|---|---|
| Understanding of electrochemistry | 10.354 | 1 | 111 | 0.002 |
| Attitude toward chemistry | 0.405 | 1 | 111 | 0.526 |
| Intrinsic motivation | 0.018 | 1 | 111 | 0.894 |
| Relevance of learning chemistry | 0.128 | 1 | 111 | 0.721 |
| Self-efficacy for cognitive skills | 0.490 | 1 | 111 | 0.485 |
| Self-efficacy for chemistry laboratory | 0.195 | 1 | 111 | 0.660 |
The results of Levene's test showed that each dependent variable had the same variance across groups with a significance value higher than 0.05, except for understanding of electrochemistry. Since this assumption was not met for one of the dependent variables, a more conservative critical level (0.04) was set for determining significance for that variable in the univariate F-test rather than the conventional 0.05 level (Tabachnick and Fidell, 2007). The results of follow up ANOVA were interpreted by using a Bonferroni adjusted alpha level in order to avoid Type 1 error, because a number of separate analyses would be considered (Field, 2013). For this purpose, the original alpha level of 0.04 was divided by the number of dependent variables, which was six, and the new alpha level was set as 0.0067. The follow up ANOVA using a Bonferroni adjusted alpha level of 0.0067 yielded three significant main effects for the instructional strategies on dependent variables (see Table 8).
| Source | Dependent variable | F | Sig. | Partial eta squared | Observed power |
|---|---|---|---|---|---|
| Treatment | Understanding of electrochemistry | 8.026 | 0.005 | 0.067 | 0.802 |
| Attitude toward chemistry | 7.849 | 0.006 | 0.066 | 0.793 | |
| Intrinsic motivation | 8.443 | 0.004 | 0.071 | 0.821 | |
| Relevance of learning chemistry | 1.468 | 0.228 | 0.013 | 0.225 | |
| Self-efficacy for cognitive skills | 7.035 | 0.009 | 0.060 | 0.748 | |
| Self-efficacy for chemistry laboratory | 7.455 | 0.007 | 0.063 | 0.772 |
One of the significant mean differences was observed in the students' understanding of electrochemistry: F(1,111) = 8.026, p < 0.0067. The mean scores on the electrochemistry concept post-test indicated that the students in the experimental group had significantly higher mean scores (M = 15.271, SD = 4.961) than those in the control group (M = 12.963, SD = 3.502). Another significant mean difference was found between experimental and control groups with respect to students' attitudes towards chemistry: F(1,111) = 7.849, p < 0.0067. The mean scores on the attitude toward chemistry scale indicated that students in the experimental group had significantly higher scores (M = 3.168, SD = 0.616) than those in the control group (M = 2.843, SD = 0.614). Finally, the results of the follow-up ANOVA indicated that there was a statistically significant mean difference between the experimental and control groups in terms of students' intrinsic motivation in chemistry: F(1,111) = 8.443, p < 0.0067. The students in the experimental group had a significantly higher mean score of intrinsic motivation (M = 2.957, SD = 0.621) than those in the control group (M = 2.614, SD = 0.633). The values of partial eta squared were found as 0.067, 0.066, and 0.071 for understanding of electrochemistry, attitude toward chemistry, and intrinsic motivation respectively. These values indicated that approximately 7% of multivariate variance of these dependent variables was associated with the treatment. The values of power were found as 0.802, 0.793, and 0.821 for understanding of electrochemistry, attitude toward chemistry, and intrinsic motivation respectively. These findings implied that the difference between the experimental and control groups arose from the treatment effect and had practical value.
On the other hand, although students in the experimental group had a higher mean score on the relevance of learning chemistry to personal goals (M = 3.013, SD = 0.769) than those in the control group (M = 2.830, SD = 0.844), the mean difference between the groups was not significant: F(1,111) = 1.468, p > 0.0067. In addition, there was no statistically significant mean difference between the experimental and control groups in terms of students' chemistry self-efficacy for cognitive skills (F(1,111) = 7.035, p > 0.0067) and self-efficacy for chemistry laboratory work (F(1,111) = 7.455, p > 0.0067). However, the mean score of the experimental group on these dependent variables was higher than the control group.
Briefly, as can be seen from Table 5, the mean score of the experimental group on each dependent variable was higher than that of the control group; but only the scores on the electrochemistry concept test, attitude toward chemistry, and intrinsic motivation were significant.
Qualitative results
After the treatment, the experimental group students' opinions about case-based instruction were determined through a feedback form in order to answer the second question of the study. Analysis of students' responses resulted in three main categories: students' description of case-based instruction, students' perceptions about the effectiveness of case-based instruction, and difficulties that students encountered during case-based instruction. Each category with representative responses of students is presented below. During the translation of participants' ideas into English, although we aimed to provide the best possible representation and understanding of their ideas, the translation process may have developed some limitations on their meaning.Another key element of case-based instruction as stated by the students was doing an activity/experiment (47.1%). One of the students emphasized the importance of the activities in case-based instruction as “Experiments and observations are the hearth of the instruction.” Another student thought that it was the experiments that helped them learn the topic. The students also made a connection between the cases and the experiments, based on the following excerpt: “We explained cases considering the results of experiments.” Besides working on daily life issues and doing experiments, the students pointed to working in a group (27.9%) and dealing with a case (25%) as other characteristics of case-based instruction. The clearest feedback for this category was detailed and accurate:
[During the instruction], groups were formed. A text was distributed and then we discussed our opinions related to the case given in the text with our teachers and did experiments.
Another student explained that “We worked in groups. The cases were discussed and experiments were done when necessary.”
Some of the students concluded that case-based instruction was a student-centered method. For instance, it was stated that “It provided us with an opportunity to express our opinions and thoughts.” Likewise, some students emphasized their efforts during class to find the knowledge they needed to explain the case. For example, one of the students wrote that “All students were actively involved in learning activities by observing and doing.” Another student expressed that “This instruction made us use our knowledge, think about situations, and find answers by ourselves.” These ideas supported students' view of case-based instruction as student-centered instruction.
In summary, the students viewed case-based instruction as a student-centered teaching method including implementation of an activity/experiment, working in a group, and dealing with real-life examples.
Case-based instruction is an effective method because visual examples/materials are permanent/long-lasting in the mind. Learning through visuals provides meaningful learning instead of rote learning. When we see similar situations, we can make a logical interpretation by figuring out the previous case in our minds.
In addition, the students expressed their ideas about the effectiveness of incorporating daily life events and using visuals on chemistry learning by comparing their previous instruction with the case-based instruction: “Beforehand, knowledge was presented directly not by cases and therefore, understanding chemistry was quite difficult. Now, both observing and relating the topics with real-life events are more helpful for us to understand the topic.” They emphasized the benefit of cases in their learning; the cases help them realize the importance of studying chemistry:
Beforehand, when formulas and names of the compounds were written on the board, none of the examples emerged in my mind. There was no explanation about why we were doing/learning this. However, this method makes chemistry more illuminating for me since it teaches chemistry by providing cases and using visual materials. It teaches not only formulas for university exams but also chemistry which we need in our lives.
Furthermore, some students stated that they learned the chemistry topics easily through case-based instruction. For example, one of the students started to think that chemistry was not difficult any more. S/he stated that “We primarily understood that chemistry can be learned.” Conversely, a few students (9%) thought that the case-based instruction was ineffective because they were not used to being taught that way and doing activities without knowing the topic. For example, one student stated that “In my opinion, case-based instruction is not an effective method since the instruction by which we were taught for years required rote learning (and we want to get knowledge directly).” Similarly, another one expressed that “Case-based instruction is not effective. In my view, ordinary instructions are more effective and easier.”
Regarding enjoyment, 40.3% of the students indicated that the case-based instruction was interesting and enjoyable. For instance, one student stated that “Chemistry lessons became livelier and less boring due to experiments and reading texts/cases. I learned chemistry in this class better than in previous lessons.” Similarly, another student thought that “Chemistry became more enjoyable through case-based instruction.” Generally, students described case-based instruction as amusing, enjoyable, and interesting. One also expressed that “It increases our interest in chemistry since it answers the question: where do we use this chemistry knowledge in our lives?” One of the reasons students find case-based instruction enjoyable is that they realize the relationship between chemistry and real life. For instance, one of the students stated that “Lessons were more enjoyable. My interest to chemistry increased. I performed some experiments at home. I tried to clean my tarnished silver ring (lemon juice, carbonate) and I succeeded. I liked it very much. I understood that chemistry is embedded in our lives.” Another student also expressed a similar idea: “the feature of case-based instruction that I liked most was its emphasis on the relationship between chemistry and our lives; and thus we understand the importance of chemistry in our life. Thus, my interest in chemistry learning increased.” Some students highlighted that their attitude toward chemistry improved, as can be concluded from the following statement: “Learning the place of chemistry in our lives was the feature that I liked most. Case-based instruction is more interesting than the instruction in the previous chemistry lesson. I liked chemistry a bit more.” Students also enjoyed the active learning process: “I enjoyed the lesson because the teacher and the students draw conclusions together during the lesson. In addition, making us think and draw conclusions based on our previous knowledge was good.”
In my opinion, previous lessons were more effective. From primary school to now, teachers transferred knowledge to us directly without conducting any experiments. Learning is easier since we were accustomed to learn in that way. I had difficulty in interpreting cases and observations during experiments.
Another student considered the experience as similarly problematic:
I certainly had difficulties because I hadn't been taught with this method before. Since I didn't know what I would do exactly I found it difficult at the beginning. Probably, I am not used to be[ing] taught by case-based instruction. Therefore, I couldn't adapt myself to this method. I thought that I didn't learn well. However, I overcame my deficiencies by my own effort.
Discussion
The purpose of this study was to explore the effect of case-based instruction on 11th grade students' understanding of electrochemistry concepts, attitude toward chemistry, motivation to learn chemistry, and chemistry self-efficacy beliefs compared to traditional chemistry instruction. MANOVA results indicated that the students who were instructed through case-based instruction acquired electrochemistry concepts better, developed more positive attitudes toward chemistry, and improved their intrinsic motivation more than the students who were taught with traditional instruction. However, the results demonstrated no significant effect of case-based instruction on students' perceptions regarding relevance of learning chemistry to personal goals and their chemistry self-efficacy beliefs as compared to traditional chemistry instruction.Students who were taught by case-based instruction demonstrated significantly higher scores on the electrochemistry concept test than those who were taught traditionally. In other words, this study indicated that the case-based instruction was more effective than traditional instruction in terms of promoting meaningful understanding of electrochemistry concepts. Therefore, the current study provides further empirical support for the previous studies in science education showing the effectiveness of case-based instruction over traditional instruction (e.g., Cakir, 2002; Ozkan and Azar, 2005; Rybarczyk et al., 2007; Saral, 2008; Cam, 2009; Skolnick, 2009; Morris, 2013; Yalcinkaya and Boz, 2015). The probable underlying reasons that the case-based instruction was effective in improving students' understanding of electrochemistry concepts can be tied to the characteristics of the instruction. Leonard (2000) pointed out that when students are actively involved (physically, emotionally, and mentally) in a learning process, they will have a deeper understanding of concepts and retain that understanding longer than when the learning experience is passive. In the literature, it is clear to science educators that active learning environments based on a constructivist approach have a crucial impact on students' meaningful learning (Barron et al., 2008; Duit and Treagust, 1998; Mayer, 1999). In the experimental group of this study, the case-based instruction created an active learning environment that involved students in solving and examining real-world problems in small groups with guided instruction. Small group and whole class discussions directed the students to think about the situations and encouraged them to express their ideas. Thus, the case-based instruction allowed the students to construct their knowledge in an authentic and active learning environment. On the other hand, the students in the control group were passive during their traditional chemistry instruction. The knowledge was transmitted from the teacher to the students. The active learning environment during the case-based instruction may have provided the students with better understanding of electrochemistry concepts compared to traditional instruction.
Furthermore, dealing with real-life examples might have played a role in the difference between the experimental group and control group students' acquisition of the concepts. The learning tasks that emphasize the relevance and meaningfulness of the content promote students' interest in learning and thus enhance students' learning (Kortland, 2007). In this study, the content of the cases reflected daily life situations, which helped students gain an insight into the role of chemistry in their life and thus see the importance and relevance of it for themselves. Students in the experimental group also stressed on the feedback form that visual materials, experiments, and daily life examples were helpful in learning chemistry because those tools allowed them to create links to real life instead of focusing on simple memorization. In addition, the use of cases during instruction helped them realize the importance of chemistry learning. In general, electrochemistry is one of the chemistry subjects perceived as difficult by students (Johnstone, 1980; Finley et al., 1982; Butts and Smith, 1987; Soudani et al., 2000). Soudani et al. (2000) proposed that one of the factors responsible for students' difficulties in electrochemistry was their unawareness of the relevance of chemistry to their life and environment. Students generally see the scientific facts, definitions, and formulas as school knowledge and memorize them just to pass their chemistry exams. They do not see the importance and relevance of learning chemistry concepts for themselves (Hutchinson, 2000). However, for meaningful learning it is suggested that the learning material should be relevant to students' lives; when this is the case, students view the content they are learning as useful and learn the topic more meaningfully (Ames, 1992; Zusho et al., 2003; Glynn et al., 2007). Regarding this point, the present study reveals that the case-based instruction provided an effective learning environment that increases students' attention and helps them see the relevance and importance of chemistry to their lives (rather than merely memorizing a prescribed body of knowledge) and thus enhances their understanding of the topic.
The present study provided empirical evidence for the positive effect of case-based instruction not only on chemistry learning but also on the development of positive attitudes toward chemistry as a school subject. This result is parallel with the findings of other studies that utilized case-based instruction to promote students' attitudes toward science, such as Cam (2009), Cakir (2002), Ozkan and Azar (2005), Gallucci (2007), and Yalcinkaya (2010). In addition, analysis of students' written responses on the feedback form also supported this result—the students instructed with the case-based instruction reported positive opinions about the chemistry lesson and chemistry learning, and they found chemistry lessons more interesting and enjoyable via case-based instruction when compared to their previous traditional chemistry instruction. In addition, they pointed out that realizing the importance and the relevance of chemistry to their lives increased their interest in learning chemistry. This finding supported the fact that the relevance and authenticity of the topics being studied is one of the factors influencing students' attitudes toward science, as stated by Raved and Assaraf (2011) and Movahedzadeh (2011). In general, the findings of the present study supported the results of previous studies that revealed that the students find case-based instruction realistic, challenging, interesting, enjoyable, and encouraging for learning (Bridges and Hallinger, 1992; Wassermann, 1994; Jones, 1997; Smith and Murphy, 1998; Dori and Herscovitz, 1999; Mayo, 2002, 2004; Naumes and Naumes, 2006; Herreid, 2007; Ayyildiz and Tarhan, 2012). The case-based instruction provided opportunities for the students to experience and practice real life situations and thus perceive the relevance of science. Since chemistry is seen as a boring subject and irrelevant to life (Hutchinson, 2000; Soudani et al., 2000), case-based instruction is more likely to contribute to an increase in student interest in chemistry and improve their views about the relevance of chemistry to their lives, which enhances their attitudes toward chemistry. The literature indicated that active participation of students in the learning process is also a main characteristic of effective instructions that promote a positive attitude toward science (Fouts and Myers, 1992; Wong et al., 1997; Oliver-Hoyo and Allen, 2005). Since the students instructed using the case-based instruction were provided with opportunities to be actively involved in and take responsibility for their learning, they might have had more positive attitudes toward chemistry compared to students taught traditionally.
Regarding the motivation variable, after the treatment the students instructed by the case-based instruction had higher intrinsic motivation to learn chemistry than those taught traditionally as concluded from both MANOVA results and written responses. These findings are inconsistent with some of the results of previous studies conducted in science education. For example, Yalcınkaya (2010) investigated the effectiveness of case-based instruction on students' motivation in chemistry. The results of her study revealed that there was no significant mean difference in students' perceived intrinsic motivation. Similarly, a study by Saral (2008) detected no significant mean difference in students' perceived intrinsic motivation in biology after case-based instruction, although their scores were higher than those of students taught traditionally. Opposite to those results, the present study provides empirical evidence for the effectiveness of case-based instruction on students' motivation to learn science, particularly chemistry. The case-based instruction provided opportunities for the students to deal with cases involving authentic examples. This characteristic of case-based instruction may have increased students' curiosity and interest, thus the students in the experimental group were more inherently motivated to learn chemistry than those in the control group. Moreover, Herreid (2005) stated that case-based instruction makes the classroom environment vigorous and more engaging than traditional instruction because students are involved in trying to put ideas into their own words while studying cases. Students are intrinsically motivated when they engage in activities (Wigfield et al., 1998). In the literature, in addition to the relevance of the content to one's life, encouraging students' active participation in the learning process through small group work activities or leading discussions is seen as useful for promoting motivation to learn (Ryan and Deci, 2000; Linnenbrink and Pintrich, 2002; Glynn and Koballa, 2006; Kusurkar et al., 2011; Vaino et al., 2012). Due to the fact that the students instructed with case-based instruction worked in small groups and discussed their ideas with group members and the whole class, this kind of learning environment might have increased their intrinsic motivation at the end of the treatment.
Surprisingly, the results of the study demonstrated no significant effect of the case-based instruction on students' perceptions regarding relevance of learning chemistry to personal goals compared to traditional chemistry instruction. In other words, there was no significant difference between experimental and control groups in terms of their willingness to engage in chemistry learning for reasons such as their future careers, goals, and lives. One of the reasons for this non-significant result might be the limited implementation period of the case-based instruction. Seven weeks might not be enough to significantly change students' perceptions about the relevance of learning chemistry to their personal goals. In addition, implementation of the case-based instruction was limited to one unit (electrochemistry) in the high school chemistry curriculum. It is difficult to consider all students' future goals and careers while designing the case-based instruction only on the topic of electrochemistry. In this study, the topics of the cases differed from each other. Therefore, the topics of the cases might not have all been related to students' future goals, or one related topic might not be sufficient for students to relate chemistry learning to their future careers. Designing the case-based instruction based on students' interests and future goals might be more effective in promoting their perceptions regarding the relevance of learning chemistry to their personal goals. In addition, having a longer implementation period of case-based instruction on different topics of chemistry may yield greater change in students' perceptions. Still, although we could not find a statistically significant difference, it is interesting to note that students' perceptions about the relevance of learning chemistry decreased after receiving traditional instruction for the topic of electrochemistry, while they increased after the case-based instruction.
Regarding the last affective variable of the study, no significant mean difference was detected in either students' self-efficacy for cognitive skills or for chemistry laboratory work across the experimental and control groups after the treatment. However, it is worthwhile to say that in both these categories the scores of the students instructed with the case-based instruction were higher than those instructed traditionally. One of the possible reasons for this result might again be related to the short duration of the treatment. Since many authors have argued that beliefs are highly resistant to change (Bandura, 1986, 1997; Pajares, 1992), the limited period of implementation of case-based instruction might not have been sufficient for the students to improve their chemistry self-efficacy beliefs. Having more time for implementation of case-based instruction on various chemistry topics may result in greater changes in students' chemistry self-efficacy. Moreover, although students in the experimental group did some experiments, observed demonstrations, and reported and discussed their results during the case-based instruction, the instruction was not completely based on laboratory activities. This might be one reason for not determining a significant rise in the students' self-efficacy for chemistry laboratory work.
Conclusion and implications
In conclusion, this study confirms and broadens many findings related to the effectiveness of a relatively new method, case-based instruction, on student learning in the context of chemistry. This study also provides a body of evidence that case-based instruction promotes students' attitude toward chemistry and motivation to learn chemistry. Accordingly, the results of this study are likely to broaden the knowledge of science educators as to what kind of instructional strategies can enhance students' meaningful chemistry learning, attitude toward chemistry, and motivation to learn chemistry.This study has several implications for chemistry educators and researchers. In the literature, it is clear that the nature of chemistry instruction has an important role in promoting students' meaningful learning. Instructions based on a constructivist approach emphasizing active learning are more effective in promoting meaningful learning than traditional instruction. In terms of effective chemistry teaching, although many researchers have already designed various instructional strategies providing active learning environments and explored their effects on students' learning, new teaching methods are still within chemistry educators’ interest. Case-based instruction, as a student-centered method, encourages students to involve themselves in the learning process actively through working on cases. Based on the findings presented in this study, case-based instruction offers a more effective learning environment than traditionally designed chemistry instruction. Therefore, this study can serve as a guide to chemistry teachers in designing effective chemistry instruction, particularly on the topic of electrochemistry.
Chemistry educators do not only deal with students' performance in the cognitive domain, but also put emphasis on students' development in the affective domain. Affective variables such as attitude and motivation impact students' learning. Chemistry lessons should be enjoyable and interesting to encourage students to improve their attitude toward chemistry and motivation to learn chemistry. In light of the findings of this study, chemistry teachers can use case-based instruction in their classes to improve students' attitudes toward chemistry and their intrinsic motivation to learn the subject. Due to the fact that students found the real-life events presented during the case-based instruction effective, enjoyable, and interesting, chemistry teachers should enrich their instructions with real-life context to improve students' learning, attitude toward chemistry, and motivation to learn chemistry.
Finally, future researchers might explore the effects of case-based instruction on students' learning in other chemistry subjects, or on other variables (such as higher order thinking and problem-solving skills) to broaden findings related to the effectiveness of case-based instruction. In addition, qualitative studies can be conducted in order to explore the effect of case-based instruction on students' chemistry learning, attitude toward chemistry, motivation to learn chemistry, and chemistry self-efficacy beliefs. In this study, implementation of case-based instruction, including real-world application, group work, and discussion, was found to be more effective than traditional instruction. In other words, the synergistic effect of dealing with real-life events, working in a group, and discussing ideas as a class enhanced students' learning, attitudes toward chemistry, and motivation to learn chemistry. However, it is uncertain which of these particular characteristics of the case-based instruction influenced students. Therefore, in future studies, some of the characteristics of the case-based instruction, such as group work, could be isolated and their influence on students' learning, attitudes toward chemistry, and motivation to learn chemistry could be examined. Moreover, further studies may investigate the effectiveness of using cases in various forms: as an in-class activity, out of class assignment, or an online assignment.
Appendix A
(1) It is known that a Zn (zinc) electrode is an anode and a Cr (chrome) electrode is a cathode in a galvanic cell formed by Zn and Cr electrodes. Which of the following statements is true for this battery?(A) Zn gives electrons.
(B) The standard reduction potential of the Zn electrode is higher than the standard reduction potential of the Cr electrode.
(C) If you want to make a galvanic cell from Zn and Cr electrodes, the Zn electrode should be placed on the left side of the cell.
(D) Oxidation reaction takes place at the Cr electrode.
(E) Zn is a stronger oxidizing agent.
(2) Ege likes a project called “Silver Tree” that he saw during a science festival event which he attended. In this project, a tree prepared using copper wire was immersed in a silver nitrate solution and the following image was obtained.
Which of the following statements describes this situation? 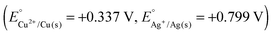
(A) When the copper passes through the solution by reducing, the silver ions in the solution are oxidized to metallic form on the copper wire.
(B) As the copper passes through the solution by oxidizing, the silver ions in the solution are reduced to metallic Ag on the copper wire.
(C) Copper oxidizes silver by acting as a cathode.
(D) Copper has formed a complex with silver.
(E) The reaction is Cu(s) + AgNO3(aq) → Cu(NO3)(aq) + Ag(s) + 2e−
(3) In the chemistry lab course, Sibel needs to set up an experiment to cover a copper material with silver. Sibel has the following materials. Which materials should Sibel connect to the poles of the battery and which solutions should she use in order to set up the experiment correctly?
Sibel has the following materials: copper material to be coated (Cu), silver bar (Ag), 0.1 M Cu (NO3)2 solution, and 0.1 M AgNO3 solution
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| (A) | Ag | Cu | Cu(NO3)2 | − | + |
| (B) | Ag | Cu | Cu(NO3)2 | + | − |
| (C) | Ag | Cu | AgNO3 | + | − |
| (D) | Cu | Ag | AgNO3 | + | − |
| (E) | Cu | Cu | AgNO3 | − | + |
(4) Pipelines, ships, bridges, tanks, chemical containers, reinforced concrete bars, water pipes, refineries and oil pipelines can be protected from corrosion by cathodic protection. In order to protect an underground gasoline tank made from iron from corrosion, which of the following metals cannot be put in the place shown by the question mark?
(A) Mg
(B) Ni
(C) Al
(D) Zn
(E) Cu
(5) Which of the following statements about the event shown below is true?
(A) Fe atoms in the key are reduced by taking electrons from the power source.
(B) The mass of the copper rod increases over time.
(C) The Cu+2 ions in the solution are collected on the copper rod by taking electrons.
(D) Copper atoms accumulate on the iron key over time.
(E) The copper rod acts as a cathode.
(6) In some of the experiments listed below, which iron nail or nails rust after a while?
(A) Only I
(B) Only II
(C) Only III
(D) I and III
(E) I, II and III
Appendix B
Silver materials
(Sindy likes wearing silver jewelry and has many silver bracelets, cuffs and bracelets. However, Sindy complains about the loss of the brightness of this silver jewelry over time. One day she shared her problem with silver jewelry with her friend Brenda.)Brenda: I do not have any silver jewelry, but we have silver items at home, and the bright, shiny surface of them gradually darkens and becomes less shiny over time.
Sindy: Do you know why it turns dark?
Brenda: No, I do not know.
Sindy: What do you do when your silver items get dark?
Brenda: There is a shop selling silver items. We take them to the shop and make them shiny again.
Sindy: How is it cleaned? Will my silver jewelry be cleaned and shiny like new?
Brenda: Of course, your jewelry will also shine. If you want, we can go to the shop where we polish our silver items.
Sindy: Yes, I'd like to go very much. Yippee, my silver jewellery will shine like new again.
(Brenda and Sindy go to the shop and ask the shopkeeper whether he can clean her silver jewellery.)
Shopkeeper: Of course, I can polish them. Your silver jewelry will be like the first day.
Sindy: How do you do that?
Shopkeeper: Do you want to see how I do it?
Sindy: Yes, I would like to see.
Shopkeeper: If you want to come to my side, you can see how I do it.
Sindy: OK.
(The shopkeeper put the silver pieces in a container covered with aluminum foil and poured hot sodium liquid containing sodium bicarbonate on them. After leaving the silver items in the liquid for a while, he takes out them from the container and shows them to Sindy.)
Sindy: Yippee! They shine very well. Thank you.
Shopkeeper: But after a while, the brightness of them goes again and they get dark again. When it does happen again, come back here. I will polish them again.
Sindy: OK.
(Sindy and Brenda leave the shop and walk to their homes.)
Sindy: Actually, maybe we can polish silver items in our homes.
Brenda: How can we do it?
Sindy: Let's search!
Appendix C
Teacher guide
This guide will help you to implement case-based instruction in your class by using the case called “Silver Materials”. Please follow the steps below.– First, students form pre-defined groups.
– Second, the case material including text and questions are distributed to the groups.
– Third, the case representing a dialog among three persons is potrayed by three students. These students will read the text aloud to the whole class.
– Fourth, students are asked to write their answers on the paper by discussing the questions at the end of the text in groups.
– Finally, the answers given by all the groups to the questions are then taken one by one in turn and then discussed as a class. The following information will guide you through the discussion.
• In the first question, give five minutes for group discussion to argue how silver is tarnished, which chemical reaction occurs, and what the reducing and oxidizing agents are. Students may think that silver reacts with oxygen. But oxidation of silver is very difficult. If there are students who think so, ask them whether it is easy to oxidize silver. Once students think about it for a while, you can tell them that silver is very easily affected by sulfur in the weather, so it is tarnished. You can share the following information with your students.
Silver is tarnished under the influence of airborne sulfur, hydrogen sulfide (H2S) and sulfur dioxide gases, or when it is contacted with a sulfur-rich substance (egg yolk, sulfur rubber). The gases emitted from the exhaust gases of cars, fumes coming out from stones, and even the natural gas including sulfur affect the silver. Ask your students to write the reaction on their papers. (During the group work, you can give your students the clue that silver is tarnished because of the sulfur in the air environment. You can also give reactants and ask them to write the reaction).
Ag(s) + O2(g) + H2S(g) → Ask the groups, what kind of substances will form.
| Unbalanced reaction: Ag(s) + O2(g) + H2S(g) → Ag2S(s) + H2O(g) |
Then ask your students to balance the reaction.
| Balanced reaction: 4Ag(s) + O2(g) + 2H2S(g) → 2Ag2S(s) + 2H2O(g) |
Ask your students what is reduced and oxidized. (Silver (Ag) is oxidized, oxygen is reduced.)
Ask students to write half reactions on their paper. Then, ask a student to write half reactions on the board.
| Oxidation half-reaction: 2Ag → 2Ag+ + 2e− |
| Reduction half-reaction: O2 + 4e− → 2O2− |
• Regarding the second question, each group will be provided with the necessary equipment and they will try to clean a tarnished silver material by employing the process explained in the case.
Each group will carry out a cleaning experiment as indicated in the case. Before, the experiment, ask your students how to get the sodium bicarbonate solution needed for the experiment. Which substance we use in daily life includes sodium bicarbonate? (Answer: dough baking powder).
A silver material that has lost its brightness is thrown into hot sodium bicarbonate solution (dough baking powder) and left for a while. The silver material is then removed from the solution, washed with water (due to the sodium bicarbonate salt on it), then dried and displayed to the students. (The silver material is expected to be brighter than the old one).
• For the third question, each group is asked to explain the chemical process underlying the cleaning of tarnished silver materials, to write a balanced chemical reaction equation, and to identify reducing and oxidizing agents in the reaction. Each group first will write their answers on the paper. After finishing their answers, one of the students will write his/her group's answers on the blackboard and explain the related chemical process. Then, the other groups will discuss the answer under the guidance of the teacher and explain their answers. When necessary, the teacher provided clues to help students answer the questions. Discussion will continue until the right balanced chemical reaction equation, oxidizing and reducing agents, and half reactions are decided.
Students can give the following answer. In this case, ask students whether this reaction is written correctly or not.
| Ag2S(s) + Al(s) → Ag(s) + Al2S3(aq) |
Ask students to balance the reaction.
| Balanced reaction: 3Ag2S(s) + 2Al(s) → 6Ag(s) + Al2S3(aq) |
Also, ask students to balance the reaction by showing the electron exchange on their paper. Ask a student to do it on the board and then ask the class whether the student's work is correct or not.
Demonstrate how to balance the redox reaction with the class by using the half-reaction method.
The correct reaction is as follows (students may not write this equation):
| Ag2S(s) + Al(s) → Ag(s) + Al3+(aq) + H2S(g) (basic medium) |
Say that the above reaction actually takes place during the cleaning process (by writing the actual reaction on the board). Then, ask students to balance the reaction with their groups.
| Balanced reaction: 3Ag2S(s) + 2Al(s) + 6H2O(l) → 6Ag(s) + 2Al3+(aq) + H2S(g) + 6OH−(aq) |
Ask groups to show how they have balanced the reaction with the half-reaction method. Check the group work and guide them when necessary. By choosing a student from a random group, ask him/her to balance the reaction on the board by using the half-reaction method. If s/he was doing it wrong, ask another student to help him.
• Regarding the fourth and fifth questions, students will examine the cleaning process in terms of the materials used (i.e., sodium bicarbonate solution, hot water, and aluminum foil). Each group is asked what the functions of these materials are during the cleaning process. In addition, they are asked whether another material could be used instead of aluminum foil. After each group finished writing their answers, group answers will be shared with the class and discussed.
Sodium bicarbonate solution acts as an electrolyte. (Electrolyte definition can be given here). It provides the basic environment for the reaction. The warmth of the solution allows the reaction to take place faster, which allows the tarnished silver to be cleaned more quickly. (The students can be reminded of the effect of temperature on reaction rate.)
Because aluminum is a more active metal than silver, it separates silver from sulfide, and the aluminum ion in the aluminum foil reduces the silver ion (Ag+) in Ag2S to metallic silver.
We can use a metal that is more active than silver. The more active it is, the easier it will be to separate from the sulfide. (If necessary, students can be informed about activity, and reminded of previous information).
• Finally, each group will try to answer the sixth question. Regarding this question, each group will express their ideas about why copper materials turn green over time and which chemical reaction causes this situation. The whole class discussion will continue until reaching a consensus under teacher guidance.
Pure copper in yellowish pink color turns reddish brown when it is oxidized due to oxygen in the air. Ask your students to write the oxidation reaction of copper, and the half-reaction equations and to identify reduced and oxidized species.
| 2Cu(s) + O2(g) → 2CuO(s) (Copper is oxidized, oxygen is reduced) |
As the copper metal continues to be in contact with air, the surface of the metal gradually becomes green. The copper oxide reacts with CO2 and SO2 in the air and forms copper carbonate (Cu2CO3(OH)2) or copper sulfate (Cu4SO4(OH)6). The formed layer, called copper rust or patina, protects the underlying metal from other chemical agents, even though it is a very thin layer.
Note: If copper oxide is exposed to hydrogen gas, it will gain its old brightness again. (This information is given to the students and they are asked to write the reaction, to determine the reduced and oxidized substances, and to show half reactions.)
Copper ions are reduced and hydrogen is oxidized.
| CuO(s) + H2(g) → Cu(s) + H2O(g). |
References
- Adali B., (2005), Effect of case-based method on 5th grade elementary school students academic achievement and attitudes toward science while teaching “viruses, bacteria, fungi and protists” topic, Unpublished master's thesis, Mustafa Kemal University, Hatay.
- Aikenhead G., (2003), Review of research on humanistic perspectives in science curricula, paper presented at the European Science Education Research Association (ESERA) 2003, August, Noordwijkerhout, The Netherlands, retrieved March 24, 2013, from http://www.usask.ca/education/people/aikenhead/ESERA_2.pdf.
- Airasian P. W. and Walsh M. E., (1997), Constructivist cautions, Phi Delta Kappan, 78(6), 444.
- Allchin D., (2013), Problem- and case-based learning in science: an introduction to distinctions, values, and outcomes, CBE Life Sci. Educ., 12(3), 364–372.
- Ames C., (1992), Classrooms: goals, structures, and student motivation, Journal of Educational Psychology, 84, 261–271.
- Anderson C. W. and Lee O., (1997), Will students take advantage of opportunities for meaningful science learning? The Phi Delta Kappan, 78(9), 720–724.
- Anderson C. W. and Smith E. L., (1987), Teaching science, in Richardson-Koehler V. (ed.), Educators' handbook: a research perspective, New York: Longman, pp. 84–111.
- Andrew S., (1998), Self-efficacy as a predictor of academic performance in science, J. Adv. Nurs., 27, 596–603.
- Arbuckle J. L., (2012), IBM SPSS Amos 21 User's Guide, Amos Development Corporation.
- Ayyildiz Y. and Tarhan L., (2012), Effect of case studies on primary school teaching students' attitudes toward chemistry lesson, Hacet. Univ. J. Educ., 43, 62–70.
- Bandura A., (1986), Social foundations of thought and action: a social cognitive theory, Englewood Cliffs, NJ: Prentice Hall.
- Bandura A., (1997), Self-efficacy: the exercise of control, New York: W.H. Freeman.
- Barron B. and Darling-Hammond L., (2008), Teaching for meaningful learning: a review of research on inquiry-based and cooperative learning, in Darling-Hammond L., Barron B., Pearson D., Schoenfeld A., Stage E., Zimmerman T., Cervetti G. and Tilson J. (ed.), Powerful learning: what we know about teaching for understanding, San Francisco: Jossey-Bass, pp. 11–70.
- Bridges E. M. and Hallinger P., (1992), Problem-based learning for administrators, ERIC Clearinghouse on Educational Management, University of Oregon.
- Britner S. L. and Pajares F., (2001), Self-efficacy beliefs, motivation, race, and gender in middle school science, J. Women Minor. Sci. Eng., 7, 271–285.
- Brown K., Commandant M., Kartolo A., Rowed C., Stanek A., Sultan H. and Wininger V., (2011), Case based learning teaching methodology in undergraduate health sciences, Interdiscipl. J. Health Sci., 2(2), 47–65.
- Browne M. W. and Cudeck R., (1993), Alternative ways of assessing model fit, in Bollen K. A. and Long J. S. (ed.), Testing structural equation models, Newbury Park, CA: Sage, pp. 136–162.
- Bryan R. R., Glynn S. M. and Kittleson J. M., (2011), Motivation, achievement, and advanced placement intent of high school students learning science, Sci. Educ., 95(6), 1049–1065.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17(1), 192–201.
- Cakir O. S., (2002), The development, implementation and evaluation of a case-based method in science education, Unpublished doctoral dissertation, Middle East Technical University, Ankara.
- Cam A., (2009), Effectiveness of case-based learning instruction on students' understanding of solubility equilibrium concepts, Unpublished doctoral dissertation, Middle East Technical University, Ankara.
- Capa-Aydin Y. and Uzuntiryaki E., (2009), Development and psychometric evaluation of the high school chemistry self-efficacy scale, Educ. Psychol. Meas., 69(5), 868–880.
- Cerasoli C. P., Nicklin J. M. and Ford M. T., (2014), Intrinsic motivation and extrinsic incentives jointly predict performance: a 40 year meta-analysis, Psychol. Bull., 140(4), 980.
- Cetin-Dindar A. and Geban O., (2010), The Turkish adaptation of the Science Motivation Questionnaire, in Tasar M. F. and Cakmakci G. (ed.), Contemporary science education research: pre-service and in-service teacher education, Ankara, Turkey: Pegem Akademi, pp. 119–128.
- Challen P. and Brazdil L. C., (1996), Case studies as a basis for discussion method teaching in introductory chemistry courses, Chem. Educ., 1(5), 1–13.
- Cheng V. K., (1995), An environmental chemistry curriculum using case studies, J. Chem. Educ., 72(6), 525–527.
- Cliff W. H. and Wright A. W., (1996), Directed case study method for teaching human anatomy and physiology, Adv. Physiol. Educ., 15(1), 19–28.
- Cobern W. W., Schuster D. G., Adams B., Applegate B., Skjold B., Undreiu A., Loving C. C. and Gobert J. D., (2010), Experimental comparisons of inquiry and direct instruction in science, Research in Science & Technological Education, 28(1), 81–96.
- Coppola B. P., (1996), Progress in practice: teaching and learning with case studies, Chem. Educ., 1(4), 1–13, DOI: http://10.1333/s00897960050a.
- Cornely K., (1998), Use of case studies in an undergraduate biochemistry course, J. Chem. Educ., 75(4), 475–478.
- Creswell J.W., (2007), Qualitative inquiry and research design: choosing among five approaches, 2nd edn, Thousand Oaks, CA: Sage.
- Crocker L. and Algina J., (1986), Introduction to Classical and Modern Test Theory, Philadelphia: Harcourt Brace Jovanovich College Publishers.
- Cunningham G. K., (2005), Assessment in the classroom: constructing and interpreting texts, London: The Falmer Press.
- De Jong O. and Taber K. S., (2007), Teaching and learning the many faces of chemistry, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 631–652.
- Dillon J., (2009), On scientific literacy and curriculum reform, Int. J. Environ. Sci. Educ., 4(3), 201–213.
- Dori Y. J. and Herscovitz O., (1999), Question posing capability as an alternative evaluation method: analysis of an environmental case study, J. Res. Sci. Teach., 36(4), 411–430.
- Duit R. and Treagust D. F., (1998), Learning in science—From behaviourism towards social constructivism and beyond, in Fraser B. J. and Tobin K. (ed.), International Handbook of Science Education, Dordrecht, The Netherlands: Kluwer Academic Publishers, pp. 3–25.
- Dunlap J. C., (2005), Problem-based learning and self-efficacy: how a capstone course prepares students for a profession, Educ. Technol. Res. Dev., 53(1), 65–83.
- Dupuis R. E. and Persky A. M., (2008), Use of case-based learning in a clinical pharmacokinetics course, Am. J. Pharmaceut. Educ., 72(2), 29, 1–7.
- Field A., (2013), Discovering statistics using IBM SPSS statistics, 4th edn, Thousand Oaks, California: SAGE Publications.
- Finley F. N., Stewart J. and Yarroch W. L., (1982), Teachers' perceptions of important and difficult science content, Sci. Educ., 66(4), 531–538.
- Fouts J. T. and Myers R. E., (1992), Classroom environments and middle school students' view of science, J. Educ. Res., 85(6), 356–361.
- Fraenkel J. R. and Wallen N. E., (2006), How to design and evaluate research in education, 6th edn, Boston: McGraw-Hill.
- Gabel C., (1999), Using case studies to teach science, paper presented at the Annual Meeting of the National Association for Research in Science Teaching, Boston, MA.
- Gallucci K. K., (2007), The case method of instruction, conceptual change and student attitude, Doctoral dissertation, North Carolina State University, ProQuest Dissertations Publishing, 3293616.
- Geban O., Ertepinar H., Yilmaz G., Altin A. and Sahbaz F., (1994), Bilgisayar destekli eğitimin öğrencilerin fen bilgisi başarılarına ve fen bilgisi ilgilerine etkisi. I. Ulusal Fen Bilimleri Eğitimi Sempozyumu: Bildiri Özetleri Kitabı, İzmir: Dokuz Eylül Üniversitesi, pp. 1–2.
- Glynn S. and Koballa T. R., (2005), The contextual teaching and learning instructional approach, in Yager R. E. (ed.), Exemplary Science: Best Practices In Professional Development, Arlington, VA: National Science Teachers Association Press, pp. 75–84.
- Glynn S. M. and Koballa Jr. T. R., (2006), Motivation to learn college science, in Mintzes J. J. and Leonard W. H. (ed.), Handbook of college science teaching, Arlington, VA: National Science Teachers Association Press, pp. 25–32.
- Glynn S. M., Taasoobshirazi G. and Brickman P., (2007), Nonscience majors learning science: a theoretical model of motivation, J. Res. Sci. Teach., 44(8), 1088–1107.
- Guest J. M., (2007), The effectiveness of using case-based instruction in an online course, Unpublished doctoral dissertation, University of South Alabama, Mobile, AL.
- Haidar A. and Abraham M., (1991), A comparison of applied and theoretical knowledge of concept based on the particulate nature of matter, J. Res. Sci. Teach., 28(10), 919–938.
- Harrison A. G. and Treagust D. F., (1996), Secondary students' mental models of atoms and molecules: implications for learning chemistry, Sci. Educ., 80(5), 509–534.
- Herreid C. F., (1994), Case studies in science: a novel method of science education, J. Coll. Sci. Teach., 23, 221–229.
- Herreid C. F., (1997), What makes a good case? J. Coll. Sci. Teach., 27(3), 163–165.
- Herreid C. F., (1998), Sorting potatoes for Miss Bonner: bringing order to case study methodology through a classification scheme, J. Coll. Sci. Teach., 27(4), 236–239.
- Herreid C. F., (2005), Science education needs case studies, Scientist, 19(4), 10.
- Herreid C. F., (2007), Start with a story: the case study method of teaching college science, Arlington, VA: NSTA Press.
- Herreid C. F., (2011), Case study teaching, New Dir. Teach. Learn., 31–40, DOI: http://10.1002/tl.466.
- Hinkle D. E., Wiersma W. and Jurs S. G., (1998), Applied statistics for the behavioral sciences, 4th edn, Boston: Houghton Mifflin Company.
- Hofstein A. and Kesner M., (2006), Industrial Chemistry and School Chemistry: Making chemistry studies more relevant, International Journal of Science Education, 28(9), 1017–1039.
- Honebein P. C., (1996), Seven goals for the design of constructivist learning environments, in Wilson B. G. (ed.), Constructivist Learning Environments, Case Studies in Instructional Design, Englewood Cliffs, New Jersey: Educational Technology Publications, Inc., pp. 11–24.
- Hutchinson J. S., (2000), Teaching introductory chemistry using concept development case studies: interactive and inductive learning, Univ. Chem. Educ., 4(1), 3–9.
- Johnstone A. H., (1980), Chemical education research: facts, findings and consequences, Chem. Soc. Rev., 9(3), 365–380.
- Jones M. A., (1997), Use of a classroom jury trial to enhance students' perception of science as part of their lives, J. Chem. Educ., 74(5), 537.
- Kaddoura M., (2011), Critical thinking skills of nursing students in lecture-based teaching and case-based learning, Int. J. Scholarsh. Teach. Learn., 5(2), 1–18.
- Kain D. L., (2003), Problem-based learning for teachers, grades 6-12, Boston: Allyn and Bacon.
- King D., (2009), Teaching and Learning in a Context-based Chemistry Classroom, Unpublished doctoral dissertation, Queensland University of Technology, Australia.
- Kline R. B., (1998), Principles and practice of structural equation modeling, New York: Guildford.
- Knight J. D., Fulop R. and Marquez-Magana L., (2008), Investigative cases and students outcomes in an upper-division cell and molecular biology laboratory course at a minority-serving institution, CBE Life Sci. Educ., 7, 382–393.
- Koballa T. R. and Glynn S. M., (2007), Attitudinal and motivational constructs in science learning, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 75–102.
- Kortland J., (2007), Context-based science curricula: exploring the didactical friction between context and science content, paper presented at the ESERA Conference, Malmo, Sweden.
- Kupermintz H., (2002), Affective and conative factors as aptitude resources in high school science achievement, Educ. Assess., 8, 123–137.
- Kusurkar R. A., Croiset G. and Ten Cate O. T. J., (2011), Twelve tips to stimulate intrinsic motivation in students through autonomy-supportive classroom teaching derived from self-determination theory, Med. Teach., 33(12), 978–982.
- Landis J. R. and Koch G. G., (1977), The measurement of observer agreement for categorical data, Biometrics, 33, 159–174.
- Lantz J. M. and Walczak M. M., (1997), The elements of a chemistry case: teaching chemistry using the case discussion method, Chem. Educ., 1(6), 1–22.
- Leonard W., (2000), How do college students best learn science? J. Coll. Sci. Teach., 29, 385–388.
- Linnenbrink E. A. and Pintrich P. R., (2002), Motivation as an enabler for academic success, Sch. Psychol. Rev., 31(3), 313–327.
- Lord T. R., (1999), A comparison between traditional and constructivist teaching in environmental science, J. Environ. Educ., 30(3), 22–27.
- Mao S. L. and Chang C. Y., (1998), Impacts of an inquiry teaching method on earth science students' learning outcomes and attitudes at the secondary school level. Proc. Natl. Sci. Counc. ROC(D), 8(3), 93–101.
- Mataka L. M. and Kowalske M. G., (2015), The influence of PBL on students' self-efficacy beliefs in chemistry, Chem. Educ. Res. Pract., 16, 929–938, DOI: 10.1039/C5RP00099H.
- Mayer R., (1999), Designing instruction for constructivist learning, in Reigeluth C. M. (ed.), Instructional-design theories and models: Vol. 2. A new paradigm of instructional theory, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 141–160.
- Mayo J. A., (2002), Case-based instruction: a technique for increasing conceptual application in introductory psychology, J. Construct. Psychol., 15(1), 65–74.
- Mayo J. A., (2004), Using case-based instruction to bridge the gap between theory and practice in psychology of adjustment, J. Construct. Psychol., 17, 137–146.
- McDermott L. C., (1993), Guest Comment: How we teach and how students learn—a mismatch? Am. J. Phys., 61, 295–298.
- Milner A. R., Templin M. A. and Czerniak C. M., (2011), Elementary science students' motivation and learning strategy use: constructivist classroom contextual factors in a life science laboratory and a traditional classroom, J. Sci. Teach. Educ., 22, 151–170, DOI: http://10.1007/s10972-010-9200-5.
- Morris J. A., (2013), How does the use of case studies, as an instructional strategy, affect the perception of relevance of science in a high school conceptual science class? Unpublished doctoral dissertation, Montana State University, Bozeman, Montana.
- Morrison T., (2001), Actionable learning: a handbook for capacity building through case based learning, Tokyo: Asian Development Bank Institute, retrieved January 29, 2013, from http://adbi.adb.org/files/2001.06.books.actionable.learning.pdf.
- Movahedzadeh F., (2011), Improving students' attitude toward science through blended learning, Sci. Educ. Civ. Engagem., 3(2), 13–19.
- Nakhleh M., (1992), Why some students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69(3), 191–196.
- Naumes W. and Naumes M. J., (2006), The art & craft of case writing, Armonk, NY: M. E. Sharpe.
- Ng K. T., Lay Y. F., Areepattamannil S., Treagust D. F. and Chandrasegaran A. L., (2012), Relationship between affect and achievement in science and mathematics in Malaysia and Singapore, Res. Sci. Technol. Educ., 30(3), 225–237.
- Nunnally J. C. and Bernstein I. H., (1994), Psychometric theory, New York, NY: McGraw-Hill.
- Oliver-Hoyo M. T. and Allen D., (2005), Attitudinal effects of a student-centered active learning environment, J. Chem. Educ., 82(6), 944–949.
- Ozkan M. and Azar A., (2005), Örnek olaya dayalı öğretim yönteminin dokuzuncu sınıf öğrencilerinin ders başarısı ve derse karşı tutumlarına olan etkisinin incelenmesi, Milli Eğitim Dergisi, 33(168), retrieved January 22, 2017, from http://dhgm.meb.gov.tr/yayimlar/dergiler/milli_egitim_dergisi/168/index3-ozkan.htm.
- Pajares F., (1992), Teachers' beliefs and educational research: cleaning up a messy construct, Rev. Educ. Res., 62(3), 307–332.
- Palmer D., (2005), A motivational view of constructivist-informed teaching, Int. J. Sci. Educ., 27(15), 1853–1881, DOI: http://10.1080/09500690500339654.
- Parchmann I. and Luecken M., (2010), Context-based learning for students and teachers: Professional development by participating in school innovation projects, Paper presented at the International Seminar on Professional Reflections, National Science Learning Centre, York, February, 2010.
- Pearce R. J., (2002), Case-based structured conflict: a means for enhancing classroom learning, J. Manage. Educ., 26, 732–744.
- Petty R. E. and Cacioppo J. T., (1981), Attitude and persuasion: classic and contemporary approaches, Dubuque, IA: Wm. C. Brown.
- Pilot A. and Bulte A. M., (2006), The use of “contexts” as a challenge for the chemistry curriculum: its successes and the need for further development and understanding, Int. J. Sci. Educ., 28(9), 1087–1112.
- Pintrich P. R., (2003), A motivational science perspective on the role of student motivation in learning and teaching contexts, J. Educ. Psychol., 95, 667–686.
- Pintrich P. R. and Schunk D. H., (2002), Motivation in education: theory, research, and applications, 2nd edn, Upper Saddle River, NJ: Prentice Hall.
- Rannikmäe M., Teppo M. and Holbrook J., (2010), Popularity and relevance of science education literacy: using a context-based approach, Sci. Educ. Int., 21(2), 116–125.
- Raved L. and Assaraf O. B., (2011), Attitudes towards science learning among 10th-grade students: a qualitative look, Int. J. Sci. Educ., 33(9), 1219–1243.
- Richmond G. and Neureither B., (1998), Making case for cases, Am. Biol. Teach., 60(5), 335–340.
- Roth K., (1990), Developing meaningful conceptual understanding in science, in Jones B. F. and Idol L. (ed.), Dimensions of thinking and cognitive instruction, Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 139–175.
- Ryan R. M. and Deci E. L., (2000), Intrinsic and extrinsic motivations: classic definitions and new directions, Contemp. Educ. Psychol., 25(1), 54–67.
- Rybarczyk B. J., Bainess A. T., McVey M., Thompson J. and Wilkins H., (2007), A case-based approach increases student learning outcomes and comprehension of cellular respiration concepts, Biochem. Mol. Biol. Educ., 35(3), 181–186.
- Saral S., (2008), The effect of case-based learning on tenth grade students' understanding of human reproductive system and their perceived motivation, Unpublished master's thesis, Middle East Technical University, Ankara, Turkey.
- Schroeder C. M., Scott T. P., Tolson H., Huang T. Y. and Lee Y. H., (2007), A meta-analysis of national research: effects of teaching strategies on student achievement in science in the United States, J. Res. Sci. Teach., 44, 1436–1460.
- Schumm M. F. and Bogner F. X., (2016), The impact of science motivation on cognitive achievement within a 3-lesson unit about renewable energies, Stud. Educ. Eval., 50, 14–21.
- Schwartz A. T., (2006), Contextualized Chemistry Education: The American experience, International Journal of Science Education, 28(9), 977–998.
- Singh K., Granville M. and Dika S., (2002), Mathematics and science achievement: effects of motivation, interest, and academic engagement, J. Educ. Res., 95(6), 323–332.
- Sirhan G., (2007), Learning difficulties in chemistry: an overview, J. Turkish Sci. Educ., 4(2), 2–20.
- Skolnick R., (2009), Case study teaching in high school biology: effects on academic achievement, problem solving skills, teamwork skills, and science attitudes, Unpublished doctoral dissertation, TUI University, Cypress, California.
- Smith R. A. and Murphy S., (1998), Using case studies to increase learning & interest in biology, Am. Biol. Teach., 60(4), 265–268.
- Soudani M., Sivade A., Cros D. and Medimagh M. S., (2000), Transferring knowledge from the classroom to the real world: Redox concepts, Sch. Sci. Rev., 82(298), 65–72.
- Sudzina M. R., (1997), Case study as a constructivist pedagogy for teaching educational psychology, Educ. Psychol. Rev., 9(2), 199–218.
- Sungur S., (2004), The implementation of problem based learning in high school biology courses, Unpublished doctoral dissertation, Middle East Technical University, Ankara, Turkey.
- Tabachnick B. G. and Fidell L. S., (2007), Using Multivariate Analysis, 5th edn, Boston: Allyn and Bacon.
- Tarnvik A., (2007), Revival of the case method: a way to retain student-centred learning in a post-PBL era, Med. Teach., 29(1), e32–36.
- Taylor G., Jungert T., Mageau G. A., Schattke K., Dedic H., Rosenfield S. and Koestner R., (2014), A self-determination theory approach to predicting school achievement over time: the unique role of intrinsic motivation, Contemp. Educ. Psychol., 39(4), 342–358.
- Thomas M. D., O'Connor F. W., Albert M. L., Boutain D. and Brandt P. A., (2001), Case-based teaching and learning experiences, Issues in Mental Health Nursing, 22(5), 517–531.
- Toraman C. and Demir E., (2016), The effect of constructivism on attitudes towards lessons: a meta–analysis study, Eurasian J. Educ. Res., 62, 115–142, DOI: 10.14689/ejer.2016.62.8.
- Vaino K., Holbrook J. and Rannikmäe M., (2012), Stimulating students' intrinsic motivation for learning chemistry through the use of context-based learning modules, Chem. Educ. Res. Pract., 13(4), 410–419.
- von Glasersfeld E., (1995), Radical constructivism: A way of knowing and learning, Washington, DC: Falmer Press.
- Wassermann S., (1994), Introduction to case method teaching: a guide to the galaxy, New York: Teachers College Press.
- Webster B. J. and Fisher D. L., (2000), Accounting for variation in science and mathematics achievement: a multilevel analysis of Australian data Third International Mathematics and Science Study (TIMSS), School Effectiveness and School Improvement, 11(3), 339–360.
- Wigfield A., Eccles J. S. and Rodriguez D., (1998), The development of children's motivation in school contexts, in Iran-Nejad A. and Pearson P. D. (ed.), Review of research in education, Washington, DC: American Educational Research Association, vol. 23, pp. 73–118.
- Wilcox K. J., (1999), The case method in introductory anatomy & physiology: using the news, Am. Biol. Teach., 61(9), 668–671.
- Williams B., (2005), Case based learning—a review of the literature: is there scope for this educational paradigm in prehospital education? Emerg. Med. J., 22(8), 577–581.
- Willson V. L., Ackerman C. and Malave C., (2000), Cross-time attitudes, concept formation, and achievement in college freshman physics, J. Res. Sci. Teach., 37(10), 1112–1120.
- Wong A. F. L., Young D. J. and Fraser B. J., (1997), A multilevel analysis of learning environments and student attitudes, Educ. Psychol., 17, 449–468.
- Yalcinkaya E., (2010), Effect of case based learning on 10th grade students' understanding of gas concepts, their attitude and motivation, Unpublished doctoral dissertation, Middle East Technical University, Ankara, Turkey.
- Yalcinkaya E. and Boz Y., (2015), The effect of case-based instruction on 10th grade students' understanding of gas concepts, Chem. Educ. Res. Pract., 16, 104–120.
- Zusho A., Pintrich P. R. and Coppola B., (2003), Skill and will: the role of motivation and cognition in the learning of college chemistry, Int. J. Sci. Educ., 25(9), 1081–1094.
Footnote |
| † This study is a part of the first author's doctoral dissertation and was presented at the 2014 NARST Annual International Conference at the Wyndham Grand Pittsburgh, PA, USA on March 30–April 2, 2014. |
| This journal is © The Royal Society of Chemistry 2017 |