A cross-cultural study of CKCM efficacy in an undergraduate chemistry classroom
Muammer
Çalik
 *a and
William W.
Cobern
*a and
William W.
Cobern
 b
b
aFatih Faculty of Education, Karadeniz Technical University, 61335 Trabzon, Turkey. E-mail: muammer38@hotmail.com; muammer38@ktu.edu.tr
bThe Mallinson Institute for Science Education, Western Michigan University, Kalamazoo, MI, USA. E-mail: bill.cobern@wmich.edu
First published on 7th June 2017
Abstract
The aim of this study was to cross-culturally investigate the instructional efficacy of the Common Knowledge Construction Model (CKCM) with college students learning about ‘factors affecting solubility’ focusing on students’ conceptual understanding, attitudes and scientific habits of mind. Even though the CKCM is a decade old, there has been little study of its effectiveness in different cultural contexts, which is unfortunate because having effective studies from different cultural contexts can increase the strength of generalization. In order to add to the cultural context variation in which the CKCM has been studied, the study reported in this paper investigated the effectiveness of a CKCM undergraduate chemistry unit taught in Turkey and the USA for pre-service teachers. The study was quasi-experimental with the CKCM and country as the independent variables; and conceptual understanding, scientific habits of mind and attitudes towards chemistry as the dependent variables. Data were collected using a pre/post-test administration of the Factors Affecting Solubility Test (FAST), the Scientific Habits of Mind (SHOM) survey, and the Chemistry Attitudes and Experiences Scale (CAES). The data were analyzed using an independent samples t-test, paired samples t-test, and ANCOVA. Statistically significant differences were found between contexts, generally favoring the Turkish pre-service teachers. This is an interesting result in that the findings of the study suggest that the CKCM may be more effective in its adapted setting than in its original (American) setting.
Introduction
Abd-El-Khalick and BouJaoude (1997) argue that a scientifically literate person should have a proficient understanding of the concepts, principles, theories, and processes of science; and an awareness of the complex relationship among science, technology, society, and the environment. Scientific literacy is multidimensional, consisting of conceptual understanding, nature of science, socio-scientific issues, and scientific habits of mind, among other things (Coll et al., 2009). A scientifically literate person can respond to the questions of “what, how and why of science?” (Ebenezer et al., 2010). To develop this multidimensional understanding of scientific literacy, Ebenezer and Connor (1998) have suggested the Common Knowledge Construction Model (CKCM) of teaching and learning. Unfortunately, even though the CKCM idea is a decade old, there has been little research on its effectiveness on scientific literacy. Ebenezer et al. (2004, 2010) are two small-scale experimental studies that have focused on conceptual change using the first two phases of the CKCM. Bakırcı et al. (2017), Kiryak and Çalik (2017) and Wood et al. (2013) have included the third phase of the CKCM that pertains to the social relevance of science; however, they have studied only one dimension of scientific literacy. Thus, the study at hand tested CKCM efficacy on the multidimensionality of scientific literacy as described above. Furthermore, the current study used an international comparison (i.e., different cultural contexts) as a basis for strengthening the generalizability of the findings (Cronbach, 1975).Pre-service teachers in Turkey, an economically developing country, and the USA, a developed country, provided the two cultural contexts for this study. While Turkish and American classrooms have different cultures, there are similarities. Pre-service teachers in both countries, for example, have similar attitudes (see Titrek and Cobern, 2011). Both teacher education systems emphasize inquiry-based learning. In both countries, pre-service teachers today are quite familiar with technology. On the other hand, there are significant differences. The majority of Turkish pre-service teachers are Muslim, but American pre-service teachers are more religiously diverse and secular. We have worked in both countries and consistently find that Turkish pre-service teachers tend to accept lecturer authority; whereas, American pre-service teachers are more out-spoken. We have also found that Turkish pre-service teachers are willing to participate in group work, curious to find answers to scientific questions, and sensitive to socio-scientific issues, but also make hasty decisions. That is, in making decisions, Turkish pre-service teachers depend on feelings rather than reasoning.
Given the aforementioned differences, this cross-cultural study extends the scope of CKCM research. Moreover, because the CKCM was developed in the USA, the current study compares CKCM efficacy in its original setting and an adopted setting in Turkey. Such an international comparison might provide insights on addressing the issues of equity and excellence in learning from the perspective of the CKCM.
Even though teachers teach as they were taught, the CKCM studies have not focused on pre-service elementary teachers yet. For this reason, there is a need to get pre-service elementary teachers to experience the CKCM instruction during their teacher education programs. Hence, they may have opportunities to develop their limited science knowledge, and generate positive attitudes towards science/science teaching. Since low science teaching self-confidence explicitly affects their science teaching (i.e., Bursal, 2008), science educators should look for any alternative pedagogical intervention (i.e., the CKCM) challenging their negative dispositions towards deeper conceptual understanding and attitudes towards learning science/chemistry (e.g., Appleton, 1995; Bursal, 2008).
Even though there have been tremendous studies on student learning of solution chemistry topics and different teaching strategies to attain conceptual change (see Çalik et al., 2005, for a review of solution chemistry studies), few intervention studies of the factors affecting solubility have reported some improvements in conceptual change, conceptual understanding, and scientific process skills (e.g., Çalık et al., 2010; Tosun and Taskesenligil, 2013). Nevertheless, the current solution chemistry studies have not investigated the effectiveness of their interventions on pre-service elementary teachers' chemistry attitudes and scientific habits of mind. Hence, a need for any alternative pedagogical intervention that would be more effective for teaching solution chemistry (particularly, the factors affecting solubility) appeared in the current study. Phrased differently, the present study attempted to test an alternative teaching and learning approach (i.e., the CKCM) by examining unexplored learning factors (i.e., chemistry attitudes, scientific habits of mind) using the factors affecting solubility.
Theoretical framework of the study
The CKCM is grounded in phenomenography (Marton, 1981) and relational conceptual change (Lybeck et al., 1988; Ebenezer et al., 2010) (For a through description of the CKCM see Ebenezer et al., 2010, Wood et al., 2013). Phenomenography asserts that the world is multifaceted and open to interpretive variations. That is, individuals interpret a natural phenomenon in qualitatively different ways. In other words, phenomenography focuses on describing the possible conceptual variations held by individuals for a particular phenomenon. Based on phenomenography or variation theory of learning, students should be encouraged to deploy intellectual tools in appropriate contexts in order to enhance conceptual change. This procedure requires students to discern contexts of meaning through conceptual frameworks. For example, students should be enabled to distinguish a subject-specific, cultural context from daily life experience. Students should be encouraged to use intellectual tools (i.e., scientific habits of mind) within various contexts in the study of a socio-scientific issue such as road salting. A multifaceted framework of phenomenography may improve students' attitudes toward learning chemistry. For example, someone with an optimistic attitude may view a phenomenon or an issue very differently from a pessimistic person. The aforementioned concerns may be alleviated because the CKCM is an amalgam of different perspectives: phenomenography or variation theory of learning, intellectual empathy, the nature of science, and the nature of reasoning that underpins socio-scientific issues (Ebenezer and Connor, 1998; Ebenezer and Haggerty, 1999). To accommodate the multi-voices in science education, the CKCM is identified with four interactive phases of teaching and learning: Exploring and Categorizing, Constructing and Negotiating, Translating and Extending, and Reflecting and Assessing.The first phase of the CKCM, exploring and categorizing, employs phenomenography as an inquiry tool facilitating learner-centered generation of functional and sensible conceptions of natural phenomena. This phase enables students to explore their ideas using one or two related simple tasks. Students observe the phenomena under investigation, and make records of their observations in notebooks, and propose interpretations of their observations. Students simply share their ideas and interpretations while the teacher drops their ideas on the board without any judgment.
In the second phase, constructing and negotiating, the peers together with the teacher evaluate the merits of the personal ideas in an open forum. The teacher acts as a mediator guiding teacher-to-student and peer-to-peer discourse to develop explanatory models.
In the third phase, extending and translating, students exploit their conceptualizations of scientific ideas gained in the second phase in order to scaffold problems of a socio-scientific inquiry; thus improving their awareness of the complex interactions among science, technology, society and environment through a critical thinking disposition (see Solomon and Aikenhead, 1994; Çalik and Coll, 2012).
In the final phase, reflecting and assessing, the teacher measures how students explore, expose, revise or reject their conceptions based on evidence and explanation. The teacher tracks students' conceptual changes, and determines how effective the teaching has been. Focus is on conceptual change and identifying what concepts need to be further explored, and how students use their learning to design, conduct, and evaluate scientific and socio-scientific inquiries that have personal and societal relevance.
The theoretical framework of the study also shaped the current study's research questions. For example, the fact that Turkish and American classroom cultures may influence pre-service elementary teachers' interpretive varied views of the factors affecting solubility emerged as the first research question of the current study. Further, phenomenography or variation theory of learning, which encourages students to deploy intellectual tools in appropriate contexts, yielded Research Questions 2, 3 and 5. Also, the fact that students may improve their own attitudes towards chemistry given a multifaceted framework of phenomenography (e.g., an optimistic attitude or a pessimistic attitude) appeared as the first and fourth research questions of the study.
Earlier studies of the CKCM
The CKCM as described above has been the object of efficacy research. Ebenezer et al. (2004, 2010), working with 7th graders, found that a partial CKCM approach used in an excretion unit improved science achievement and relational conceptual change. Similarly, Kiryak and Çalik (2017), also working with 7th graders, found that a CKCM unit significantly improved student conceptual understanding of ‘water pollution’ and substantially replaced students' alternative conceptions related to water pollution. In addition, Kiryak and Çalik (2017) reported the improved use of scientific language over everyday language. Wood et al. (2013) studied the effectiveness of a CKCM unit on acids and bases taught at an alternative high school. A qualitative analysis of students' prior- and post-intervention conceptions of acids and bases using phenomenography found: (a) changes in the number of descriptive categories of students’ conceptions of acids and bases; (b) a shift in language use from everyday talk to more chemical talk; and (c) development of chemical knowledge hierarchy. Quantitative results from the Acids and Bases Achievement Test showed that students in the experimental group (n = 17) achieved significantly higher scores (p < 0.003) than those in the control group (n = 22). Also, Bakırcı et al. (2017) explored the effect of a Common Knowledge Construction Model (CKCM)-oriented instruction on grade 6 students' views on the Nature of Science (NOS) and compared it with the existing learning model (i.e., 5Es learning model in the control group). They found that the control group's views of the NOS, except for the empirical aspect, were at a transitional level whilst those of the experimental group were at the informed level.These CKCM findings are promising. To date, however, the CKCM studies have not been conducted with pre-service elementary teachers, who might use the CKCM once they become teachers. It is commonly known that teachers teach as they were taught. That being the case, it could be a good idea for teachers to experience CKCM instruction during their teacher education programs. Because pre-service elementary teachers possess a limited science background, and some have negative attitudes towards science/science teaching and low science teaching self-confidence (e.g., Appleton, 1995; Bursal, 2008), any improvement in their views of science teaching/learning explicitly influences their science teaching (i.e., Bursal, 2008). This means that there is a need to challenge their negative dispositions towards deeper conceptual understanding and attitudes towards learning science/chemistry. Hence, pre-service elementary teachers were chosen as subjects for the current study.
Why solution chemistry?
Chemistry is replete with abstract concepts, concepts such as dissolution, particulate nature of matter, and chemical bonding, that are fundamental to learning chemistry (Nakhleh, 1992; Abraham et al., 1994; Kaya and Geban, 2012). Solution chemistry was chosen as the science content for this study because it is a cornerstone for advanced chemistry learning, i.e., acid–base chemistry, rate of reaction, electrochemistry, and chemical equilibrium. Solution chemistry concepts underpin important chemical/industrial processes and life systems that involve a series of complex processes occurring in solutions. Solution chemistry is crucial for making sense of our daily life consumptions of commodities based on solutions such as beverages, air, and household ammonia. Moreover, solution chemistry is included in elementary science education, and pre-service elementary teachers will eventually have the opportunity to teach it in their classrooms. For these reasons, solution chemistry was selected for embedding the related concepts/features of the factors affecting solubility in the CKCM.Chemistry education research has examined student learning of various solution chemistry topics (e.g., dissolution, solubility, solutions and their components, and the concentration of solutions) and different teaching strategies (e.g., the use of a hypermedia environment, dual situated learning model, inquiry-based activities, a learning study with variation theory, problem-based learning, constructivist-based teaching model—4Es) to bring about conceptual change (see Çalik et al., 2005, for a review of solution chemistry studies). Studies with different graders have found that comprehension of solution chemistry (especially factors affecting solubility) is difficult (e.g., Blanco and Prieto, 1997; Pinarbasi and Canpolat, 2003; Çalık, 2005; Ozden, 2009; Pinarbasi et al., 2009; Adadan and Savasci, 2012). However, some intervention studies of the factors affecting solubility have successfully improved conceptual change, conceptual understanding, and scientific process skills (e.g., Çalık et al., 2010; Tosun and Taskesenligil, 2013). Nevertheless, these researchers recognize a need for alternative teaching strategies that would be more effective for teaching solution chemistry (particularly, the factors affecting solubility). In addition, current solution chemistry research studies have not looked at pre-service elementary teachers' chemistry attitudes and scientific habits of mind that would impact the effectiveness of their instruction. The present study took up the challenge of investigating an alternative teaching and learning approach (i.e., the CKCM) while concurrently examining pre-service elementary teachers' chemistry attitudes and scientific habits of mind.
The aim of the study
The aim of this cross-cultural study was to investigate the instructional efficacy of the Common Knowledge Construction Model (CKCM) based on pre-service elementary teachers' learning about the ‘factors affecting solubility.’ The study foci were pre-service elementary teachers' conceptions, attitudes toward chemistry, and scientific habits of mind. The following research questions guided the study:(1) Is there any significant difference between Turkish and American pre-service elementary teachers' pretest scores on the FAST, SHOM Survey and CAES?
(2) Is there a significant difference between Turkish and American pre-service elementary teachers' conceptual understanding of ‘factors affecting solubility’ based on the pretest and post-test mean scores on the Factors Affecting Solubility Test (FAST)?
(3) To what extent, and how similarly, does instruction using the CKCM influence the Turkish and American pre-service elementary teachers' pretest and post-test mean scores on the Scientific Habits of Mind (SHOM) survey, within the context of a unit on ‘factors affecting solubility’?
(4) Is there any statistically significant difference between Turkish and American pre-service elementary teachers' pretest and post-test mean scores on the Chemistry Attitudes and Experiences Scale (CAES)?
(5) Does the CKCM foster the Turkish and American pre-service elementary teachers' relational development levels?
Methodology
Study setting and sample
The pre-service elementary teachers were recruited for the study from introductory chemistry courses specifically offered for elementary teacher education at a large Midwestern, American public university and at a large North-Eastern Black Sea, Turkish public university. The pre-service elementary teachers were informed that the researchers would like to use their course assessments as data for a research study if they agreed. Sixty of 72 pre-service elementary teachers in three American course sections, and 68 of 81 pre-service elementary teachers in two Turkish course sections signed consent forms. The researchers emphasized that the course instructors would never know whether or not pre-service elementary teachers gave consent. However, 13 pre-service elementary teachers in the USA and 12 in Turkey were dropped from the study for not having completed the post-test. The American sample consisted of 47 pre-service elementary teachers (aged 18 to 45 years; mean age: 22.6; 5 males and 42 females) of varied ethnicity (43 Whites, 3 African-Americans, and one Hispanic-American). The Turkish sample consisted of 56 pre-service elementary teachers (aged 17 to 23 years; mean age: 19.1; 16 males and 40 females) of more homogenous ethnicity (48 Turks and 3 Caucasians, that is, from Turkmenistan). The courses in both countries included topics such as: nature of science, models and modeling, description of chemistry, matter-micro and macro, chemical vs. physical properties, mixtures, development of the atomic model, Periodic Table, subatomic particles (protons, neutrons, and electrons), chemical bonding, the mole and stoichiometry, collision theory and rates of reaction, acids and bases, heat and temperature, and factors affecting solubility.Data collection
A Factors Affecting Solubility Test (FAST) with six open-ended questions was designed to probe pre-service elementary teachers' conceptual understanding (see Appendix 1). The FAST was validated by a panel of independent experts (three science educators and three chemistry educators), who confirmed its content validity. Its reliability co-efficient was found to be 0.75 for the American setting and 0.71 for the Turkish setting. Both exceeded the acceptable value suggested by Hair et al. (2006).The SHOM survey (Çalik and Coll, 2012) was used to investigate the effect of the CKCM on pre-service elementary teachers' Scientific Habits of Mind, as reflected by socio-scientific issues and nature of science in the SHOM survey (see Appendix 2). The SHOM survey was validated and found to be reliable. Its Cronbach's alpha coefficient was 0.75 for the American setting and 0.74 for the Turkish setting, both of which were slightly higher than the acceptable value (0.70) suggested by Hair et al. (2006).
To measure pre-service elementary teachers' attitudes towards chemistry, the researchers used an adapted version of the Chemistry Attitude and Experience Questionnaire developed by Dalgety et al. (2003). Çalık et al. (2015) adapted this questionnaire and dubbed it as the Chemistry Attitude and Experience Survey (CAES) that contains 15, seven-point Likert items (see Appendix 3). The CAES Cronbach alpha co-efficient was 0.89 for the American setting and 0.83 for the Turkish setting, both of which exceeded the acceptable value (0.70). All instruments were administered as pre-tests one week before instruction. After instruction, the same instruments were immediately re-administered as post-tests. Moreover, prediction–explanation–observation–explanation (PEOE) tasks embedded worksheets were employed as a formative assessment. That is, after each lesson, the researchers collected the worksheets and gave feedback about the student responses.
The data collection continuum began with the course instructors recording the assessments for course purposes and then making photocopies for the research. The photocopies of both pre- and post-tests for those not giving consent were separated. Also, the student names on the matched pre- and post-tests were then removed so as to make the instruments completely anonymous.
Data analysis
Data were analyzed using a non-equivalent, quasi-experimental design (e.g., Borg and Gall, 1989; Robson, 1998). The FAST responses were labeled according to the following criteria: Sound Understanding (4 points) that included all components of the validated response, Partial Understanding (3 points) that included at least one of the components of the validated response, but not all the components, Partial Understanding with Specific Alternative Conception (2 points) that showed understanding of the concept, but also made a statement that demonstrated a misunderstanding, Specific Alternative Conceptions (1 point) that included illogical or incorrect information and No Understanding (zero point), which included an irrelevant or unclear response or blank (Abraham et al., 1994). Moreover, responses classified under ‘partial understanding with specific alternative conception’ and ‘specific alternative conception’ were re-examined to identify percentages of their alternative conceptions in the pre- and post-tests of the FAST. Then, types of alternative conceptions were counted and percentages calculated: (number of the participants in each type of alternative conception/the total participant number) × 100. Two bilingual researchers independently analyzed the FAST responses with an inter-rater consistency of 77%. Disagreements were resolved through negotiation.Responses to the SHOM survey items were made using a four-point Likert scale. Pretest scores were subjected to independent samples t-tests. Post-test scores were subjected to an analysis of covariance (ANCOVA) with the intervention as the independent variable and pretest scores as covariates. The statistically significant differences on the FAST pretest, and the pretest subscale scores for ‘Skepticism, Rationality, Suspension of Belief, Objectivity’ on the SHOM survey, and the ‘Chemistry Research’ subscale of the CAES, called for an ANCOVA statistical analysis. The PEOE embedded worksheets were analyzed using three criteria:
• Positive Relational Development: Responses improved from partial understanding in the ‘prediction’ to sound understanding in the ‘explanation;’ from partial understanding with specific alternative conceptions in the ‘prediction’ to partial/sound understanding in the ‘explanation.’
• Negative Relational Development: Responses deteriorated through the PEOE (e.g., from sound/partial understanding in the ‘prediction’ to partial understanding with specific alternative conceptions in the ‘explanation;’ from sound/partial understanding in the ‘prediction’ to partial understanding with specific alternative conceptions in the ‘explanation’).
• No Change: Almost the same responses throughout the PEOE (e.g., partial understanding or partial understanding with specific alternative conceptions from the ‘prediction’ and the ‘explanation’).
Similarly, responses to the FAST were analyzed using the foregoing formula to yield positive conceptual change, negative conceptual change, and no change categories.
The treatment
The final version of the CKCM lesson sequence (see Table 1) for implementation was developed after consultation with both American and Turkish experts. Subsequently, the CKCM lesson sequence (i.e., the treatment) was first implemented in the American classrooms and later in the Turkish classrooms. The first researcher taught the lessons in both the American and Turkish classrooms to eliminate teacher-based differences.| Lessons based on participants' conceptions | Activities | CKCM strategies | Features of scientific habits of mind directly emphasized | Features of chemistry attitudes and experiences directly emphasized |
|---|---|---|---|---|
| Phase 1: exploring and categorizing | ||||
|
Lesson 1
Exploration and categorization of participants' conceptions of factors affecting solubility |
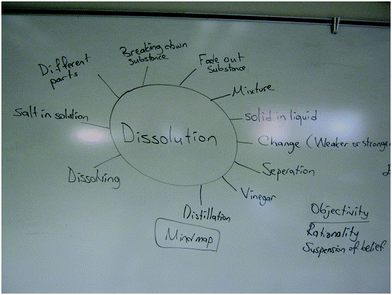
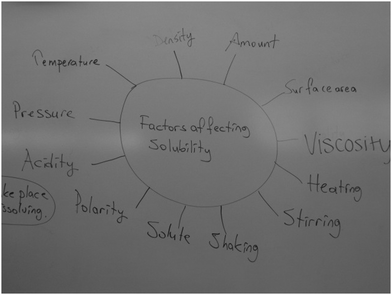
The instructor explored participants' conceptions of factors affecting solubility through brainstorming and mind map (see Fig. 1 and 2) to employ variation and relational theory of learning. He classified and categorized them for the next teaching stages. | Exploration and categorization | Open-mindedness and curiosity for varied ideas in mind map and brainstorming |
Chemists that are socially aware (Item 2), flexible in their ideas (Item 4) and care about the effects of their results (Item 5)
Chemistry research (as a part of chemistry education) that helps people (participants in this case) (Item 8) and solves problems (Item 10). |
|
Lesson 2
Participants' awareness of their conceptions |
The instructor discussed the descriptive categories of the factors affecting solubility concept with participants. | Awareness of participants' conceptions of the factors affecting solubility was revealed through discussion | Open-mindedness, curiosity, suspension of belief for varied ideas in mind map and brainstorming |
Chemists that are socially aware (Item 2), flexible in their ideas (Item 4) and care about the effects of their results (Item 5)
Chemistry research (as a part of chemistry education) that helps people (participants in this case) (Item 8) and solves problems (Item 10). |
| Phase 2: constructing and negotiating | ||||
|
Lesson 3
Investigating effects of temperature and pressure on dissolution of gases into liquids |
Participants predicted, observed, and explained as they engaged in the injector and heating (i.e., Çalık et al., 2007) activities with carbonated (fizzy) drinks (Worksheets 1–2). Further, they took notes on specially designed worksheets. |
Predict, Explain, Observe and Explain (PEOE) conceptual change inquiry strategy
Flexible grouping to facilitate student peer discourse Student–student, student–instructor discourse |
Rationality (consistency or inconsistency with prediction, explanation, observation and explanation), skepticism (inconsistency between prediction, explanation, observation and explanation), objectivity (repeating activities at least twice), curiosity (inquisitiveness for factors affecting solubility) |
Chemists that are imaginative (Item 6) and patient (Item 7). Also, chemists, as a chemistry job, produce carbonated (fizzy) drinks.
Chemistry research investigates alternative ways to replace nitrogen gas in a scuba tube with inert gas (i.e., helium) Chemistry jobs that are varied (Item 12) (i.e., industrial chemistry). |
|
Lesson 4
Investigating the effect of temperature on dissolution of solids into liquids |
Participants predicted, observed, and explained as they engaged in hands-on activities (i.e., Çalık et al., 2006) with different solid chemicals (e.g. KNO3, NaCl) (Worksheet 3). Further, they took notes on specially designed worksheets. |
PEOE conceptual change inquiry strategy
Flexible grouping to facilitate student peer discourse Student–student, student–instructor discourse |
Chemists that are imaginative (Item 6) and patient (Item 7) | |
|
Lesson 5
Examining the effects of surface area and stirring on dissolution rate/process |
Participants predicted, observed, and explained as they engaged in hands-on activities (i.e., Çalık et al., 2009) with sugar cube analogies (Worksheets 4–5). Further, they took notes on specially designed worksheets and filled in the analogical mapping of the activities. |
PEOE conceptual change inquiry strategy
Flexible grouping to facilitate student peer discourse Student–student, student–instructor discourse Analogical reasoning |
Chemists that are imaginative (Item 6) and patient (Item 7) | |
| Phase 3: extending and translating | ||||
|
Lesson 6
Exploration of participants’ conceptions of freezing point depression |
Participants predicted, explained, observed, and explained as they engaged in a freezing point depression activity (Worksheet 6). Further, they took notes on specially designed worksheets and argued pros and cons (environmentally, economically and ecologically) of road salt through the studies and news (i.e., Wegner and Yaggi, 2001; Çoban, 2004; Bereket, 2013; Kadıoğlu, 2013). |
PEOE conceptual change inquiry strategy
Student–student, student–instructor discourse |
Suspension of belief (Holding abeyance for alternative chemicals in road salt), Mistrust of Arguments From Authority (a comparison or evaluation of trustworthiness of alternative chemicals in road salt), rationality (revising their ideas in the light of evidence and argument about pros and cons of road salt) (NaCl), skepticism (a provisional approach to alternative chemical claims that will be replaced with table salt) and curiosity (arousing inquisitiveness for exploration and discovery concerning freezing point depression) |
Chemists that are socially aware (Item 2) and environmentally aware (Item 3) (i.e., road salt and its effects to the environment)
Chemistry research that helps people (Item 8), improves quality of life (Item 9), solves problems (Item 10) and advances society (Item 11) (i.e., seeking alternative ways to minimize the effect of alternative salt chemicals on the environment and our life) Chemistry jobs that are varied (Item 12) (i.e., environmental chemistry—effect of road salt on environment, industrial chemistry and geochemistry that produce or look for alternative chemicals for road salt), interesting (Item 13) (i.e., chemistry includes several sub-disciplines or integrated disciplines) |
| Phase 4: reflecting and assessing | ||||
|
Lesson 7
Exploration of participants' conceptions of factors affecting solubility Final test |
Instructor explored participants' conceptions of factors affecting solubility through the worksheets that they filled in.
Instructor deployed Factors Affecting Solubility Test (FAST), Scientific Habits of Mind (SHOM) survey (Çalik and Coll, 2012) and Chemistry Attitudes and Experiences Survey (CAES) (Dalgety et al., 2003) |
Formative assessment
Post-test |
||
For the American setting, because the instruction relevant to this study took place during the regularly scheduled classes of all three CHEM 2800 sections, the week before the treatment, the course instructors introduced the first researcher as the instructor for the next three class sessions and that the topic was to be the factors affecting solubility. The course instructors advised pre-service elementary teachers that the instruction on the factors affecting solubility was part of the course curriculum and that the assignments were assessed for the grade book (the researchers had no input on grades). For the Turkish setting, the instruction relevant to this study also happened during the regularly scheduled classes of two CHEM (SINO) 104 sections. The first researcher disclosed to the pre-service elementary teachers that the instruction on the factors affecting solubility was part of the course curriculum and had no input on grades.
Because the treatment was firstly implemented in the American classrooms, the researchers initially improved the CKCM lesson sequence concerning the factors affecting solubility for the American setting given the related literature and documents. Then, they sent the CKCM lesson sequence and related worksheets to the course instructor (as well as her teaching assistants) and laboratory specialist for their consideration. After having a look at the documents, the researchers organized a meeting with them to negotiate the CKCM lesson sequence. They confirmed the readability and comprehensibility of pre-service elementary teachers and suggested some minor revisions about: (1) reminding Boyle's Laws in Worksheet 1, (2) adding a chart in case pre-service elementary teachers lacked graphing skill in Worksheets 3 and 6, (3) starting brainstorming with “dissolution” concept (see Fig. 1 and 2). All suggestions were taken into account in revising the worksheets and learning sequence. For the Turkish setting, the CKCM lesson sequence concerning the factors affecting solubility and related guide materials (i.e., worksheets) were translated from English into Turkish. A group of experts (one chemistry educator and two science educators) looked over the relevant documents and ensured the readability and comprehensibility for pre-service elementary teachers. Further, they suggested some typographical errors and minor translation revisions. The final version of the CKCM sequence concerning the factors affecting solubility is outlined in Table 1.
The current study drew the ‘factors affecting solubility’ topic from the regular general chemistry curriculum for pre-service elementary teachers. Hence, the CKCM lesson sequence deployed the topic as only content knowledge. Because the CKCM had an amalgam structure, it covered the ‘factors affecting solubility’ topic like an umbrella. This means that any direct effectiveness is directly pertaining to the CKCM lesson sequence as an actual treatment rather than the content/topic.
At the beginning of the treatment, the first researcher had pre-service teachers suggest words they thought related to dissolution and the factors affecting solubility through brainstorming and mind mapping (see Fig. 1 and 2). Wood et al. (2013) explored students' conceptions of acids–bases by showing a picture of a factory with gases coming out of the smokestacks in a rainy day. The teacher asked ‘second-order’ questions based on the following scenario: What sense do you make of this picture? Can you see what is happening?” Similarly, Ebenezer et al. (2004, 2010) explored 7th standard students' ideas of how waste was produced in human systems, by directing their thoughts to a meal of the day. Hence, their purpose was to enable the teacher to understand the first phase of the CKCM lesson sequence. Thus, in our procedure, the instructor asked follow-up questions to explore their reasons and how these words are related to dissolution and factors affecting solubility, and then categorized and classified the words and conceptions for the next teaching stages. Later, the instructor discussed the descriptive categories of the ‘factors affecting solubility’ with pre-service elementary teachers. At that point, he posed questions that activated features of the SHOM survey and CAES. For example, why do we have different ideas about the same concepts? How would these different views shape learning of the factors affecting solubility topic? Do you think that chemists also have various views of the same phenomena/issues? Do you think that chemists are aware of other views? Who would guide/help you to learn this topic in order for you to meet your learning needs?
Afterwards, the instructor handed out a related worksheet asking for predictions and reasons for those predictions. For instance, Worksheet 1 (Effect of Pressure on Dissolution of Gases into Liquid) asked:
What happens to the carbonated drink when the injector's pump is pulled from the first phase to second (see Figure A)? Please explain your prediction.
Pre-service elementary teachers then conducted the activities twice as directed and wrote their observations. Later, they explained how their predictions were consistent or inconsistent with their observations, and why they thought they were directed to do the activities twice.
To clarify the features of the SHOM, the instructor asked such questions: How did you decide consistency and/or inconsistency through the PEOE (e.g., rationality, skepticism)? How did you meet inquisitiveness for the factors affecting solubility (i.e., Curiosity)? Why did you repeat the activities in Worksheet 1 at least twice (i.e., Objectivity)? Moreover, to stress the features of the CAES, the instructor asked provocative questions such as what professions link macroscopic observations with sub-microscopic levels, or what professions produce carbonated (fizzy) drinks. What research group investigates alternative ways to replace nitrogen gas in a scuba tube with inert gas (i.e., Helium)? Pre-service elementary teachers constructed and negotiated their views with peers and the instructor. By giving instructor feedback at the end of each lesson, pre-service elementary teachers were more conscious about their conceptions and/or progression. Overall, the aforementioned treatment continuum depicts that the features of the SHOM survey and CAES were intertwined with the ‘factors affecting solubility’ topic.
Findings
As seen in Table 2, the independent samples t-test for the pre-test results indicate statistically significant differences on the FAST, the ‘Skepticism, Rationality, Suspension of Belief, and Objectivity’ subscales of the SHOM survey, and the ‘Chemistry Research’ subscale of CAES. The differences favored the Turkish participants (p < 0.05) (see Table 2). In contrast to the given common habits of Turkish and American participants, the Turkish participants’ pretest scores on the ‘rationality and suspension of belief’ subscale of the SHOM survey were significantly higher than those for the American participants (see Table 2). That is, Table 2 denotes that the Turkish participants' common habit of making a hasty decision seems to have meaningfully affected their rationality and suspension of belief of socio-scientific issues. There were no differences between the American and Turkish participants’ pretest mean scores on the rest of the SHOM survey and CAES survey subscales (p > 0.05). The significant differences shown in Tables 2–4 indicate that the Turkish and American participants interpreted the issues under investigation differently; whereas scientific literacy levels between the groups are similar.| Instrument | Pre-test | t | Sig. (2-tailed) | American | Turkish | Mean difference | Std. error difference | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | ||||||
| *p < 0.05. **p < 0.01. | |||||||||
| FAST | −2.14 | 0.04* | 8.30 | 3.07 | 9.82 | 3.98 | −1.52 | 0.71 | |
| SHOMS | Mistrust of arguments from authority | −1.27 | 0.21 | 9.76 | 1.90 | 10.21 | 1.69 | −0.45 | 0.35 |
| Open-mindedness | −1.62 | 0.11 | 16.74 | 2.17 | 17.46 | 2.31 | −0.72 | 0.44 | |
| Skepticism | −5.10 | 0.00** | 11.11 | 2.26 | 13.27 | 2.04 | −2.16 | 0.42 | |
| Rationality | −4.82 | 0.00** | 10.57 | 1.65 | 12.07 | 1.50 | −1.50 | 0.31 | |
| Suspension of belief | −3.41 | 0.00** | 11.83 | 2.53 | 13.48 | 2.38 | −1.65 | 0.48 | |
| Objectivity | −5.77 | 0.00** | 12.64 | 2.04 | 14.73 | 1.65 | −2.09 | 0.36 | |
| Curiosity | 0.16 | 0.88 | 13.12 | 2.11 | 13.05 | 2.56 | 0.07 | 0.47 | |
| CAES | Chemists | −1.29 | 0.20 | 36.00 | 5.59 | 37.54 | 6.39 | −1.54 | 1.19 |
| Chemistry research | −3.89 | 0.00** | 21.76 | 4.61 | 24.82 | 3.34 | −3.06 | 0.79 | |
| Chemistry jobs | −1.49 | 0.14 | 20.10 | 4.58 | 21.53 | 5.05 | −1.43 | 0.96 | |
As seen in Table 3, the ANCOVA results showed statistically significant differences between the American and Turkish participants' post-test mean scores on the FAST, and on the ‘Mistrust of Arguments From Authority, Skepticism, Rationality, Suspension of Belief and Objectivity’ subscales of the SHOM survey. Additionally, the ‘Chemists and Chemistry Jobs’ subscales of the CAES favored the Turkish participants (p < 0.05). The partial eta squared (η2) values for the post-tests ranged between zero (for ‘open-mindedness’ subscale) and 0.34 (for ‘chemists’ subscale). The closer this (i.e., η2) is to 1, the stronger the relationship between the factors. This means that the partial eta squared (η2) values for the post-tests indicate a small-size effect.
| Dependent variable (post-test) | Covariate (pre-test) | Type III sum of squares | Mean square | F | Sig. | Partial eta squared |
|---|---|---|---|---|---|---|
| *p < 0.05. **p < 0.01. | ||||||
| Post FAST | Pre FAST | 43.38 | 43.38 | 6.33 | 0.01* | 0.06 |
| Post-mistrust of arguments from authority | Pre-mistrust of arguments from authority | 35.52 | 35.52 | 10.39 | 0.00** | 0.09 |
| Post-open-mindedness | Pre-open-mindedness | 1.04 | 1.04 | 0.18 | 0.67 | 0.00 |
| Post-skepticism | Pre-skepticism | 53.22 | 53.22 | 12.08 | 0.00** | 0.11 |
| Post-rationality | Pre-rationality | 11.37 | 11.37 | 5.26 | 0.02* | 0.05 |
| Post-suspension of belief | Pre-suspension of belief | 47.87 | 47.87 | 9.90 | 0.00** | 0.09 |
| Post-objectivity | Pre-objectivity | 23.57 | 23.57 | 6.94 | 0.01* | 0.07 |
| Post-curiosity | Pre-curiosity | 4.04 | 4.04 | 0.54 | 0.46 | 0.01 |
| Post-chemists | Pre-chemists | 1036.66 | 1036.66 | 51.06 | 0.00** | 0.34 |
| Post-chemistry research | Pre-chemistry research | 10.25 | 10.25 | 0.90 | 0.35 | 0.01 |
| Post-chemistry jobs | Pre-chemistry jobs | 295.88 | 295.88 | 18.12 | 0.00** | 0.15 |
Both Turkish and American participants' familiarity with inquiry-based learning resulted in better conceptual understanding as determined by the FAST and PEOE results (see Tables 4–6). But, the CKCM lesson sequence was more influential with the Turkish participants' conceptual understanding (see Table 3) due to its competitive framework. Even though the Turkish and American participants' reaction to the subject/course relies on the lecturer's behavior, chemistry attitudes improved, though in different ways. Table 4 shows that the American participants improved more on the CAES ‘Chemistry Research’ subscale; whereas the Turkish participants improved more on the ‘Chemists and Chemistry Jobs’ subscale. Further, the Turkish participants' habit of ‘Trust of argument from authority’ (e.g., trusting the instructor as an authority) significantly changed towards ‘mistrust of arguments from authority’ vis-à-vis the American ones (see Tables 3 and 4). Although the American participants were already more out-spoken than the Turkish participants, the Turkish participants' ‘objectivity’ level significantly improved through the CKCM based instruction (see Table 3). Similarly, a higher consciousness of the American participants' secular understanding did not influence the scores on the ‘skepticism and suspension of belief’ subscales of the SHOM survey (see Table 4). However, Table 3 indicates that the Turkish participants significantly improved on the ‘skepticism and suspension of belief’ subscales of the SHOM survey as compared to the American ones.
| Group | Instruments | Subscales | Pre-test | Post-test | Paired differences (from pretest to post-test) | t | df | Sig. (2-tailed) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Mean | Std. deviation | Mean | Std. deviation | Std. error mean | ||||||
| *p < 0.05. **p < 0.01. | ||||||||||||
| The American participants | FAST | 8.30 | 3.07 | 15.36 | 3.21 | −7.06 | 3.40 | 0.50 | −14.23 | 46 | 0.00** | |
| CAES | Chemists | 36.00 | 5.59 | 35.89 | 5.56 | 0.11 | 6.88 | 1.00 | 0.11 | 46 | 0.92 | |
| Chemistry research | 21.76 | 4.62 | 23.53 | 4.11 | −1.77 | 4.59 | 0.67 | −2.64 | 46 | 0.01* | ||
| Chemistry jobs | 20.11 | 4.58 | 19.96 | 4.72 | 0.15 | 5.39 | 0.79 | 0.19 | 46 | 0.85 | ||
| SHOM | Mistrust of arguments from authority | 9.76 | 1.90 | 9.40 | 1.99 | 0.36 | 2.46 | 0.36 | 1.01 | 46 | 0.32 | |
| Open-mindedness | 16.75 | 2.17 | 16.98 | 2.41 | −0.23 | 2.76 | 0.40 | −0.58 | 46 | 0.56 | ||
| Skepticism | 11.11 | 2.26 | 11.32 | 2.33 | −0.21 | 2.78 | 0.41 | −0.52 | 46 | 0.60 | ||
| Rationality | 10.57 | 1.65 | 10.68 | 1.71 | −0.11 | 1.93 | 0.28 | −0.38 | 46 | 0.71 | ||
| Suspension of belief | 11.83 | 2.53 | 12.34 | 2.40 | −0.51 | 2.99 | 0.44 | −1.17 | 46 | 0.25 | ||
| Objectivity | 12.64 | 2.04 | 13.17 | 2.21 | −0.53 | 1.86 | 0.27 | −1.96 | 46 | 0.06 | ||
| Curiosity | 13.13 | 2.11 | 12.36 | 2.74 | 0.77 | 3.03 | 0.44 | 1.73 | 46 | 0.09 | ||
| The Turkish participants | FAST | 9.82 | 3.98 | 14.45 | 2.37 | −4.63 | 3.91 | 0.52 | −8.85 | 55 | 0.00** | |
| CAES | Chemists | 37.53 | 6.39 | 42.66 | 3.80 | −5.13 | 6.12 | 0.82 | −6.27 | 55 | 0.00** | |
| Chemistry research | 24.82 | 3.35 | 25.27 | 3.17 | −0.45 | 3.93 | 0.53 | −0.85 | 55 | 0.40 | ||
| Chemistry jobs | 21.54 | 5.05 | 23.79 | 3.77 | −2.25 | 5.31 | 0.71 | −3.17 | 55 | 0.00** | ||
| SHOMS | Mistrust of arguments from authority | 10.20 | 1.70 | 10.71 | 1.81 | −0.51 | 2.03 | 0.27 | −1.86 | 55 | 0.07 | |
| Open-mindedness | 17.38 | 2.25 | 16.93 | 2.46 | 0.45 | 2.65 | 0.36 | 1.27 | 55 | 0.21 | ||
| Skepticism | 13.24 | 2.05 | 13.51 | 2.03 | −0.27 | 2.31 | 0.31 | −0.88 | 55 | 0.38 | ||
| Rationality | 12.02 | 1.46 | 11.62 | 1.24 | 0.40 | 1.82 | 0.25 | 1.63 | 55 | 0.11 | ||
| Suspension of belief | 13.51 | 2.40 | 14.02 | 2.06 | −0.51 | 2.82 | 0.38 | −1.34 | 55 | 0.19 | ||
| Objectivity | 14.73 | 1.66 | 15.15 | 1.80 | −0.42 | 2.16 | 0.29 | −1.44 | 55 | 0.16 | ||
| Curiosity | 13.00 | 2.55 | 12.73 | 2.76 | 0.27 | 3.25 | 0.44 | 0.62 | 55 | 0.54 | ||
As can be seen in Table 4, the results of the paired samples t-test showed positive statistically significant differences between the American participants' the FAST pre- and post-test mean scores, and the CAES ‘Chemistry Research’ subscale (p < 0.05). The Turkish participants showed positive significant, statistical differences on the FAST, and the CAES ‘Chemists’ and ‘Chemistry Jobs’ subscales. However, there were no significant differences between pre- and post-test mean scores for the other CAES and SHOM survey subscales.
The American and Turkish participants' curiosity level was consistent due to non-significant differences between the SHOM survey pre- and post-test mean scores (see Table 4). The CKCM-based instruction did not lead the Turkish or American participants to be significantly more open-minded or improve ‘objectivity’ (SHOM survey). The American participants' scores significantly improved on the CAES ‘Chemistry Research’ subscale (see Table 4).
Table 5 represents the degree to which the CKCM sequence achieved conceptual change. As observed in Table 5, there were decreased percentages for all alternative conceptions, except for two: ‘Overgeneralizing the effect of temperature on dissolution of a gas into a liquid to that of a solid into a liquid’ (American participants) and ‘Misunderstanding the effect of temperature on dissolution of a solid into a liquid’ (Turkish participants). This means that the CKCM lesson sequence helped the participants to replace some alternative conceptions with more scientific conceptions; however, the impact varied between the American and Turkish participants. For instance, the Turkish participants improved in interpreting related concepts to explain the effects of temperature and pressure on dissolution of a gas into a liquid, applying related concepts to imply freezing point depression, and the effect of the stirring process on solubility. On the other hand, the American participants improved their understanding of the effect of temperature on dissolution of a solid into a liquid; but they still overgeneralized the effect of temperature on dissolution of a solid into a liquid, which resulted in alternative conceptions of the effect of temperature on dissolution of a gas into a liquid as a negative conceptual change.
| Alternative conceptions | Sample responses | The American participants | The Turkish participants | ||||
|---|---|---|---|---|---|---|---|
| Pretest | Post-test | CC | Pretest | Post-test | CC | ||
| CC: conceptual change; +: shows positive conceptual change; −: shows negative conceptual change. | |||||||
| Misinterpretation of interrelated concepts to explain the effect of temperature on dissolution of a gas into a liquid | Cold water is less soluble and does not evaporate like hot water in that it is less dense than warm water. | 6.4 | 4.3 | +2.1 | 5.4 | — | +5.4 |
| Overgeneralizing the effect of temperature on dissolution of a gas into a liquid to that of a solid into a liquid | There is less solubility in colder water so there is less oxygen. | 8.5 | 12.8 | −4.3 | 16.1 | 12.5 | +3.6 |
| Misunderstanding of related concepts to defend the effect of pressure on dissolution of a gas into a liquid | Pressure of blood affects solubility in this situation because if a diver goes up too fast, the blood pressure will be too much as a consequence of dissolution of nitrogen gas in blood and so it causes veins to burst. | 10.6 | 2.1 | +8.5 | 5.4 | — | +5.4 |
| Making inverse relationship between pressure and dissolution of a gas into a liquid | As a scuba diver goes deeper, the amount of nitrogen dissolved in his blood decreases. If a scuba diver reaches the surface too rapidly, the amount of nitrogen does not have time to adjust or come back from the dissolution process. | 14.9 | 4.3 | +10.6 | 7.1 | 1.8 | +5.4 |
| Misunderstanding the effect of temperature on dissolution of a solid into a liquid | They would dissolve at the same rate because they have the same solubility and the same species. | 48.8 | 27.7 | +21.1 | 16.1 | 30.4 | −14.3 |
| Misusing interrelated concepts to state the effect of temperature on dissolution of a solid into a liquid | The same amount of sugar will eventually dissolve in both but with the temperature the sugar will melt first in the hot tea because there is more solubility. The cold tea has less solubility and needs to be stirred to get it to dissolve. | 6.3 | — | +6.3 | 26.8 | 8.9 | +17.9 |
| Use of unrelated concepts to imply freezing point depression | Washing a plane with ethyl alcohol reduces the solubility of the falling precipitation because ethyl alcohol reacts with ice as a catalyst. Hence, ethyl alcohol does not freeze so it decreases the likelihood that ice will build upon the plane | 53.2 | 6.4 | +46.8 | 25.0 | — | +25.0 |
| Inability to grasp the effect of stirring process on solubility | When the salt is not stirred, the salt sits in the bottom and does not dissolve. Stirring the salt increases solubility | 51.1 | 8.5 | +42.6 | 26.8 | 21.4 | +5.4 |
| Use of unrelated concepts to depict the effect of stirring process on solubility | By stirring the water you create a chemical reaction and a chance for the atoms in the salt and water to connect making chemical bonds | 23.4 | 8.5 | +14.9 | 12.5 | — | +12.5 |
| Misunderstanding the effect of surface area on solubility | The smaller the structure of a molecule or atom or compound the easier it is to fit into a mixture with other molecules, atoms, etc. The crushed salt has higher solubility than the uncrushed salt because of surface area. So water easily absorbs the crushed salt. | 21.3 | 6.4 | +14.9 | 14.3 | 7.1 | +7.1 |
| Making inverse link between surface areas in crushed and uncrushed salt | The uncrushed salt dissolves faster because the surface area is much larger. | 38.3 | 6.4 | +31.9 | 14.3 | 8.9 | +5.4 |
Tables 5 and 6 present the findings for the participants’ relational conceptual change from pre-existing knowledge and/or prediction. The results reveal that the participants deployed intellectual tools differently in various contexts to yield conceptual change. Higher percentages of ‘alternative conceptions’ in the pretest of the FAST point to the participants' limited science background (see Table 5).
As seen in Table 6, between 41.1 and 100% of the participants' responses were categorized as Positive Relational Development. For example, all American participants' responses to Worksheets 3 and 6 fell into the Positive Relational Development category. Percentages of the American participants' responses categorized as Negative Relational Development were 14.9 for Worksheet 1, 12.8 for Worksheet 2, 25.5 for Worksheet 4 and 36.2 for Worksheet 5. For the Turkish participants, the percentages were 46.4, 5.4, 21.4 and 1.8, respectively. As for the No Change category, the American participants' responses were 25.5 for Worksheet 1 and 19.1 for Worksheet 2; whereas those for the Turkish participants were 12.5 for Worksheet 1, 12.5 for Worksheet 2, 14.3 for Worksheet 3, 21.4 for Worksheet 4, 51.8 for Worksheet 5, and 17.9 for Worksheet 6.
| Name and number of the worksheet | Categories | The American participants | The Turkish participants | Sample responses | |
|---|---|---|---|---|---|
| Prediction → | Explanation | ||||
| S: Pre-service elementary teacher. | |||||
| Worksheet 1. Effect of pressure on dissolution of gases into liquids | Positive relational development | 59.6 | 41.1 | Pressure on carbonated drink is lessened and gas will move more freely (S23) | As the pressure decreases the solubility of CO2 also decreases. As the pressure increased the solubility of the CO2 also increases (S23). |
| Negative relational development | 14.9 | 46.4 | There will be 40 cc of air pulled into the pump. The drink will become more bubbly (S26). | When pressure rises, it is harder for gases to dissolve into a liquid (S26). | |
| No change | 25.5 | 12.5 | When it will be pulled by pressure, it will be fizzy (S32) | When the pressure pulls on the carbonation, it causes the fizz (S32). | |
| Worksheet 2. Effect of temperature on dissolution of gases into liquids | Positive relational development | 68.1 | 82.1 | The carbonate drink will lose its carbon molecules as it is heated up (S48) | The more temperature increases the more kinetic energy of the particles increases. So an increase in temperature decreases dissolution of gases into liquid (S48) |
| Negative relational development | 12.8 | 5.4 | The heated liquid is well bubbled and the carbonated drink releases the carbon from the liquid causing volume to decrease (S53) | The temperature causes the dissolution of gasses to happen at a quicker rate when heated (S53). | |
| No change | 19.1 | 12.5 | If heat is increased on the carbonated drink, then CO2 will escape at a faster rate, decreasing dissolution (S1). | Increased temperature decreases the dissolution of gas in a liquid due to allowing CO2 to escapes at a faster rate (S1). | |
| Worksheet 3. Effect of temperature on dissolution of solids into liquids | Positive relational development | 100.0 | 85.7 | The heated one will dissolve quicker and create a quicker reaction. The amount of solute in each will be the same, no matter the temperature (S50). | The temperature caused the solutes to dissolve quicker. With an increase in the temperature, the KNO3 has a higher ppm at the end and dissolves more (S50). |
| Negative relational development | — | — | — | — | |
| No change | — | 14.3 | The water quickly dissolves the solute but the amount of solute in each will be the same (S37). | No matter the temperature, the water quickly dissolves the solute and the amount of solute in each will be the same (S37). | |
| Worksheet 4. Effect of surface area on dissolution process | Positive relational development | 74.5 | 57.1 | If sugar is crushed, creating more surface area, it will interact/dissolve in water faster than an uncrushed sugar cube (S1). | More accessibility to solute particles speeds up dissolution. A change in surface area does not change the amount of solute, only affects the dissolution rate. Because it will interact more surface area, accessibility of particles increases and speeds up dissolution (S1). |
| Negative relational development | 25.5 | 21.4 | Crushed sugar dissolves faster because of less surface area (S22). | The lower the surface area the quicker it dissolves, the more surface area the slower it dissolves. The more solute the quicker it dissolves (S22). | |
| No change | — | 21.4 | Crushed sugar dissolves faster and the amount of dissolved sugar is the same in both beakers (S30). | The amount of sugar dissolved is the same in both beakers but crushed sugar dissolves faster than the uncrushed one (S30). | |
| Worksheet 5. Effect of stirring on the dissolution process | Positive relational development | 63.8 | 46.4 | The one that is stirred vigorously will dissolve quicker than the other beaker. Stirring makes it easier for the solute to dissolve (S38). | The stirring process affects the dissolution process because the more stirring that is done, the quicker the dissolution process. The stirring process breaks down the amount of solute but the amount of solute dissolved in the stirred and un-stirred ones eventually stays the same (S38). |
| Negative relational development | 36.2 | 1.8 | The beaker that is stirred will dissolve faster because the particles will be forced to collide more and faster (S24). | Stirring the solute makes the particles collide more often and more frequently causing them to dissolve into solution faster than an unstirred solute (S24). | |
| No change | — | 51.8 | The stirred one will dissolve quicker than the other beaker because stirring makes dissolution of the solute into the solution easier (S47). | Stirring speeds up the dissolution process. For this reason, the stirred one dissolves quicker (S47). | |
| Worksheet 6. Freezing point depression | Positive relational development | 100 | 82.1 | When salt is added to ice the ice will melt because the salt lowers its freezing temperature (S13) | Adding salt to the ice lowers the freezing point of the ice it is added to. In this way, the temperature of the water can be below water's normal freezing point but still be in liquid form (S13). |
| Negative relational development | — | — | — | — | |
| No change | — | 17.9 | When salt is poured onto ice, dissolution of salt into the ice takes place. Hence, it lowers the ice's freezing temperature. Such a process slows down the melting phenomenon (S22). | Because salt is dissolved into the ice, this process causes freezing point depression and slows down the melting phenomenon (S22). | |
Discussion
Statistically significant differences on the FAST pretest, and the pretest subscale scores for ‘Skepticism, Rationality, Suspension of Belief, Objectivity’ on the SHOM survey, and the ‘Chemistry Research’ subscale of the CAES favoring the Turkish participants (p < 0.05) (Research Question 1) may be attributed to the science curriculum differences between Turkey and the USA (see Table 2). Moreover, higher percentages of ‘alternative conceptions’ on the FAST pretest may be viewed as an indicator of the participants’ limited science background (see Table 5). Even though the Turkish science curricula are general adaptations of American curricula, the Turkish participants' constructions and interpretations of the issues under investigation were phenomenologically different from the American participants. In other words, this means that pre-service elementary teachers tended to interpret the factors under investigation in different ways (Research Question 1). Phrased differently, the pre-service elementary teachers had interpretive variations of their views influenced by Turkish and American classroom cultures (Research Question 1).Given a definition of effectiveness as the performance needed to accomplish a goal, this paper uses relational conceptual change and improvements in the SHOM survey and CAES as effectiveness measures of the CKCM lesson sequence (Research Questions 2–4). After the teaching intervention, there were statistically significant differences between the American and Turkish participants' post-test mean scores on the FAST, the ‘Mistrust of Arguments From Authority, Skepticism, Rationality, Suspension of Belief and Objectivity’ subscales of the SHOM survey, and the ‘Chemists and Chemistry Jobs’ subscales of the CAES in favor of the Turkish participants (p < 0.05). This means that the CKCM lesson sequence seems to have resulted in better gains for the Turkish participants in comparison with the American ones. This is an interesting result in that the CKCM seems to be more effective in its adapted setting than the originally launched American setting. Significant differences on the FAST may come from Turkey's more competitive education system. Moreover, an evaluation of the use of road salt as a socio-scientific issue, and features directly emphasized by the CAES (see Table 1), seem to have resulted in better gains by the Turkish participants (as compared with the American participants) on the ‘Mistrust of Arguments from Authority’ (in contrast to trusting an authority, e.g., lecturer, chemistry) and the ‘Chemists and Chemistry Jobs’ subscales of the CAES. No significant difference in terms of ‘chemistry research’ (see Table 3) may result from their first-hand experiences with chemistry tasks and/or their familiarity with inquiry-based learning. On the other hand, the CKCM lesson sequence seems to have equally impacted both American and Turkish participant attitudes toward ‘chemistry research.’ Despite the fact that American participants are more secular than the Turkish participants, the CKCM lesson sequence resulted in more improvements in the Turkish participants’ levels of ‘skepticism and suspension of belief’ subscales of the SHOM survey (see Table 3). Indeed, given the partial eta squared (η2) values for post-tests, the CKCM lesson sequence possessed small-size effects on the post-tests (see Table 3). Because the purpose of the CKCM lesson sequence (including Phenomenography or variation theory of learning) was to encourage students to deploy intellectual tools in appropriate contexts (Research Questions 2, 3 and 5) and their own attitudes towards chemistry (Research Questions 1 and 4), the results of the current study supported the multifaceted framework of phenomenography.
As seen in Table 4, the American and Turkish participants’ the FAST pre- and post-test mean scores were significantly different (Research Questions 2). These differences denote that the CKCM lesson sequence facilitates students’ conceptions of the factors affecting solubility. Furthermore, because of regional public snow removal and road salting, these examples in the CKCM lesson sequence resulted in statistically significant differences for the American participants’ conceptual understanding as measured by the FAST, and on the ‘Chemistry Research’ subscale of the CAES (see Table 4) (Research Questions 2–4). Faced with the pros- and cons- of road salting in the CKCM lesson sequence, the American participants had the opportunity to apply scientific knowledge as responsible citizens. This claim concurs with earlier studies (Zeidler, 1984; Driver et al., 2000; Kolstø, 2001; Sadler, 2004; Anagun, 2011). This suggests that the CKCM lesson sequence gave pre-service teachers an opportunity to capture the idea that ‘science is related to and part of their lives; not something confined to classroom learning or school work,’ as mentioned by Ültay and Çalik (2012). Moreover, the fact that Pheonomenography or variation theory of learning in the CKCM lesson sequence requires students to discern contexts of meaning through conceptual frameworks may have caused pre-service elementary teachers to distinguish a subject-specific, cultural context from daily life experience and to use intellectual tools (Research Questions 2–4). Furthermore, varied improvements on ‘alternative conceptions’ and the subscales of the SHOM survey and CAES reveal that the CKCM lesson sequence drove the participants to learn various features of the FAST, SHOM survey and CAES levels (Research Questions 2–4). Thus, the findings support the CKCM lesson sequence undergirded by phenomenography (Ebenezer and Connor, 1998; Ebenezer and Haggerty, 1999). For example, even though the same chemistry instructor (the first author) taught the CKCM lesson to the American and Turkish participants, there was a significant improvement for the Turkish participants on the ‘chemists’ subscale (see Tables 3 and 4). This improvement may have been influenced by the fact that the instructor was likely the only chemist the Turkish pre-service elementary teachers knew because he is the only person teaching chemistry in their department. Thus, the Turkish participants may have generalized from his personal characteristics when responding this subscale, whereas the American participants more likely had experience with other chemists. This may come from the multi-voices in the CKCM lesson sequence that promotes pre-service elementary teachers to employ intellectual tools within various contexts via their own worldviews (Research Questions 2–4).
Furthermore, the CKCM lesson sequence with the CAES may have shaped pre-service elementary teachers' varied attitudes toward learning chemistry (Research Question 4). Similarly, varied attitudes towards chemistry may be viewed as an outcome of a multifaceted framework of phenomenography, which interpret any issue or phenomenon in different ways with regard to common attitudinal traits (i.e., optimistic, pessimistic) (Research Questions 1 and 4). Likewise, Wong and Fraser (1996) depicted a positive correlation between student enjoyment of the chemistry lesson and the chemistry laboratory tasks. A lack of improvement across the CAES subscales may have been in part due to the more sensitive 7-point Likert response scale (as opposed to a 4 or 5-point scale) (e.g., Geban et al., 1994; Kurbanoglu, 2014; Çalık et al., 2015). It may also come from specific CAES subscales (e.g., chemists, chemistry research and chemistry jobs) rather than general attitudes towards chemistry (i.e., I like chemistry; I enjoy studying chemistry) or limited intervention period. Non-significant results between the CAES and SHOM survey pre- and post-test mean scores may shed more light on ‘objectivity’ or consistent replication of the SHOM survey.
As seen in Table 5, the CKCM lesson sequence tended to help the participants replace their alternative conceptions with the scientific one (Research Question 2). Likewise, the results of PEOE embedded worksheets (Research Question 5) support this issue and indicate a pivotal role of the CKCM lesson sequence in improving the participants' conceptions of the factors affecting solubility. This may be explained with ‘soft-core’ beliefs that tend to change after the intervention (Lakatos, 1970). In other words, the CKCM lesson sequence presented good reasons and logical arguments for overcoming the participants' alternative conceptions. Also, this process (with good reasons and logical arguments) improved their views on the ‘rationality’ of the SHOM survey. Further, these results addressed varied interpretations and constructions of the factors affecting solubility (Research Questions 2 and 5). The fact that various intellectual tools in the CKCM lesson sequence constructed different learning outcomes of the foregoing issues fostered variation theory of learning.
However, even though the CKCM lesson sequence involved hands-on activities and pictorial representations, some participants' alternative conceptions remained robust:
• Overgeneralizing the effect of temperature on dissolution of a gas into a liquid to that of a solid into a liquid (American participants);
• Misunderstanding the effect of temperature on dissolution of a solid into a liquid (Turkish participants).
The stubbornness of these two alternative conceptions (Research Question 2) may stem from an inability to link macroscopic chemistry knowledge with sub-microscopic one (see Ebenezer, 2001; Çalik et al., 2005). The resistance to change may also result from a lack of differentiating dissolution of a gas into a liquid with that of a solid into a liquid (see Pinarbası and Canpolat, 2003; Adadan and Savasci, 2012). In fact, despite the fact that the CKCM lesson sequence illustrated how the particles behaved over an increase in temperature, this seems to have differently impacted the Turkish and American participants. This issue at least highlights the importance of pictorial representations of chemical phenomena in communicating and developing ideas (Akaygun and Jones, 2014). On the other hand, resistance to change may come from the participants' limited science backgrounds. Low or limited prior knowledge may make it difficult for pre-service teachers to develop a conceptual understanding of abstract scientific knowledge and multiple external scientific representations (Corradi et al., 2014). It may also be that interactions between alternative conceptions hinder the development of conceptual change (i.e., Çalık, 2005). For example, the alternative conception “Misusing interrelated concepts to state the effect of temperature on dissolution of a solid into a liquid” seems to have interacted with the one “Overgeneralizing the effect of temperature on dissolution of a gas into a liquid to that of a solid into a liquid” and resulted in a negative conceptual change. Theoretical framework of the CKCM lesson sequence may have resulted in interpretive varied views of the factors affecting solubility (Research Questions 1 and 2).
The results of the PEOE embedded worksheets indicate some negative relational development (Research Question 5). The Negative Relational Development responses with alternative conceptions (see Table 6) may be an indicator of ‘hard-core’ beliefs that are deeply rooted in the participants' cognitive structures (Lakatos, 1970). On the other hand, this may come from the belief that ‘the worst decision is better than indecision’ that challenges the SHOM survey ‘suspension of belief’ sub-scale. Also, this may be seen as an interpretive variation in the theoretical framework of the CKCM lesson sequence.
Given the close relationship amongst pre-service teachers' achievement, pedagogical content knowledge, content skills, and instructional practices, the CKCM lesson sequence study gave an opportunity for the participants to re-think, build and model their capacities regarding related issues under investigation (Research Question 5). This claim relates to earlier studies (e.g., Çalik et al., 2005). Hence, the CKCM lesson sequence enabled the participants to engage in stimulating intellectual, reasoning and social growth via learning about socio-scientific issues, hands-on activities, and the study of the nature of science. Also, the results point to an amalgam structure of the CKCM lesson sequence (i.e., Phenomenography or the Variation Theory of Learning, and the nature of science) and illustrate its usability, feasibility and fidelity in two different cultural contexts.
Given the improvements in the Turkish and American participants (Research Questions 2–5), it can be deduced that the CKCM lesson sequence promotes equity and excellence in learning to some extent. Moreover, because pre-service elementary teachers will have a pivotal role in fostering students’ attitudes toward chemistry (science) and students’ understanding at an early age (Titrek and Cobern, 2011), the results of the current study are promising with respect to initially shaping and scaffolding their own conceptual understanding and their interests towards chemistry. This promise has also been claimed by Titrek and Cobern (2011). The current study also reveals that the CKCM lesson sequence seems to have potential to overcome alternative conceptions, and anti-chemistry attitudes as well as low levels of the SHOM survey.
Implications
Based on the study's results, the CKCM not only enhances learning possibilities by including various pedagogies and/or techniques (i.e., relational conceptual change, hands-on and minds-on activities, PEOE strategy, student–student discourse), but also facilitates learning chemistry. Even though the CKCM meets requirements and expectations of the current science curriculum (i.e., scientific literacy, scientific habits of mind, and socio-scientific issues), its design requires much more time in thinking about the topic/unit as a whole. For example, the first phase of the CKCM, Exploring and Categorizing, requires extra-time to deploy phenomenography and relational conceptual change. Similarly, the third phase of the CKCM, Translating and Extending, asks for a proper socio-scientific issue and/or a science–technology–society–environment cycle to be found, which may be difficult for all science/chemistry topics. Hence, the applicability of the CKCM seems to be dependent on science/chemistry topic. Given these issues, the current study handled the factors affecting solubility (as a whole unit) and freezing point depression with table salt (NaCl) (as a socio-scientific issue). That is, the theoretical perfectness of the CKCM somewhat reflects in practicum. However, further studies with all phases of the CKCM may give an opportunity for researchers and teachers to gain insight into its practical reflections.Regarding future research, because the current study uniquely embedded the scientific habits of mind and chemistry attitudes and experiences within the CKCM lesson sequence, further study should be undertaken to compare their explicit instruction with implicit ones. Even though the current study demonstrates the feasibility of the CKCM lesson sequence in two cross-cultural contexts, it was limited by the instruction period. Further studies should be conducted using the CKCM lesson sequence over a longer period of instruction. To generalize the viability of the CKCM lesson sequence, a follow-up study ought to be conducted with the participants from un-developing countries. Because this current study deployed the PEOE task embedded worksheets, which are activities developing scientific argumentation skills (Bağ and Çalık, 2017), future study should explore how the CKCM lesson sequence affects the scientific argumentation skills. Given Çalik and Coll's (2012) idea ‘socioscientific issues and SHOM survey are valuable ways to teach the nature of science, using classroom discourse and argumentation, and case-based issues (p. 1910)’, this study supplies ‘activities that work’ (Appleton, 2006) or ‘teaching strategies’ (Pinarbasi et al., 2009) for elementary teacher education. However, it is restricted with the factors affecting solubility and post-test analysis. For this reason, it should be extended to other science topics using delayed-post tests. Because science education is multifaceted and open to interpretative variations, how to accommodate the multi-voices in science education should be investigated and discussed in depth. Also, any alternative pedagogical approach supporting variation theory of learning or phenomenography or relational conceptual change ought to be critically integrated into science classes and exemplified in teacher education programs. Hence, they may practically experience how to recruit intellectual tools in science education via varied worldviews.
Appendix 1. Factors affecting solubility test (FAST)
(1) The number of fish living in colder waters is more than what live in warmer waters. Which factor(s) affecting solubility can address this situation? Please defend your response.(2) If a scuba diver strives to reach the surface too rapidly, the diver risks a kind of paralysis called caisson disease. Which factor(s) affecting solubility can explain this phenomenon? Please defend your response given the dissolution of nitrogen gas in blood.
(3) Erdem and Zerrin like tea with sugar. However, Erdem prefers drinking ice tea while Zerrin would rather drink hot tea with sugar. They both start with hot tea but Erdem puts ice into his tea and then adds sugar while Zerrin adds sugar to her hot tea. If they have the same amount of tea, will the same amount of sugar dissolve in both Erdem's and Zerrin's tea? Please defend your answer by taking into consideration the factors affecting solubility.
(4) In the winter, planes are washed with ethyl alcohol before taking off. This process is called de-icing. How is this process related to the solubility? Please defend your response.
Please respond to Items 5–6 with regard to the following information
In pickling, an egg is sometimes used to decide whether water is sufficiently salty. If an egg sinks, the water is not salty enough. If the egg floats, there is enough salt in the water for the pickling process.
(5) For pickling purposes, an equal amount of table salt is dropped into containers of equal amounts of water at the same temperature. However, only one of the containers is stirred. The other is just left to stand. How does the stirring in one container affect the solubility of the salt? Please defend your answer.
(6) For pickling purposes, equal amounts of crushed and uncrushed salt are added to two containers with equal amounts of water at the same temperature. How does the type of salt (crushed and uncrushed) affect the solubility in each container? Please defend your answer.
Appendix 2. Scientific habits of mind (SHOM) scale
Directions: Please indicate the answer you think MOST CLOSELY REPRESENTS your opinion about the following statements. It is important to understand that there is no right or wrong answer. We are just interested in your views. Thanks for your help.Demographic data (for data analysis purposes only). Please tick ALL that apply to you:
□Male □Female □Ethnicity (please specify)____________
Please indicate your age: …………
| I believe that this is | ||||
|---|---|---|---|---|
| Almost certainly true | Quite likely to be true | Quite likely to be untrue | Almost certainly untrue | |
| 1. Modern medical science is dismissive of traditional Chinese approaches to curing illnesses. | □ | □ | □ | □ |
| 2. Because the National Radiation Research Institute reports that the radiation emitted by digital cell phones is not hazardous, we should believe this. | □ | □ | □ | □ |
| 3. The Ministry of Health should be believed when it says that the benefits of a mass public vaccination program outweigh individual risks of side effects. | □ | □ | □ | □ |
| 4. The National Association of Dentists should be believed, when it says that the use of fluoride in municipal water improves dental health. | □ | □ | □ | □ |
| 5. If scientific evidence is produced that homeopathic medicines have an effect beyond that of a placebo, it is reasonable to consider using them. | □ | □ | □ | □ |
| 6. It is reasonable to consider using colloidal silver medicines to cure serious illnesses, if scientific evidence is produced that proves this. | □ | □ | □ | □ |
| 7. If scientific research revealed a relationship between overheard power lines and increased rates of cancer, it is sensible to consider living away from power lines. | □ | □ | □ | □ |
| 8. It is reasonable to consider not vaccinating children, if new scientific studies produced evidence that mass vaccination programs result in harmful side effects such as autism. | □ | □ | □ | □ |
| 9. If new scientific studies produced evidence that use of fluoride in municipal water causes defects in tooth enamel, it is reasonable to consider the use of non-fluoridated water. | □ | □ | □ | □ |
| 10. It is reasonable to reconsider concerns about climate change, if new scientific studies reported that long-term average global temperatures have both increased and decreased at various times. | □ | □ | □ | □ |
| 11. We need to see more scientific evidence before we should consider the use of Yoga and meditation to treat serious illness. | □ | □ | □ | □ |
| 12. Herbal medicines are claimed to be a better way to treat illnesses because they have fewer side effects; but we need to see more scientific evidence before we consider their use. | □ | □ | □ | □ |
| 13. We need to see more scientific evidence before being convinced the extra cost of underground power lines compared with overhead power lines can be justified on safety grounds. | □ | □ | □ | □ |
| 14. National mass vaccination programs to prevent Swine flu seem to have reduced the effects of the pandemic, but we need more long-term scientific studies to be sure such programs are worth the cost and trouble. | □ | □ | □ | □ |
| 15. Reducing human-produced carbon dioxide is probably a good way to prevent the potential effects of global warming, but there are so many factors to be considered we need more scientific studies before we consider changing our environmental or business practices. | □ | □ | □ | □ |
| 16. The use of colloidal silver may lead to ill-health such as kidney damage, because it contains a lot of silver ions that are deposited in our organs. | □ | □ | □ | □ |
| 17. It is reasonable to conclude that underground power lines reduce the risk for illness like leukaemia, because radiation passes through air more easily than through soil. | □ | □ | □ | □ |
| 18. A higher concentration of atmospheric carbon dioxide may affect the biological systems of the oceans, because oceans may become more acidic as a result of absorbing additional carbon dioxide. | □ | □ | □ | □ |
| 19. Early studies indicate that use of cellphones may cause brain tumours, however, we don't know enough to be sure. | □ | □ | □ | □ |
| 20. We don't know enough to be sure that greenhouse gas emissions play a key role in climate change. | □ | □ | □ | □ |
| 21. There is insufficient evidence to think that a focus on the whole person makes any difference when treating serious human illness, compared with trying to cure a specific illness. | □ | □ | □ | □ |
| 22. There is insufficient evidence to seriously consider the integration of herbal treatment with modern medicine. | □ | □ | □ | □ |
| 23. There is little evidence about the effect of overheard power lines on leukaemia in children. | □ | □ | □ | □ |
| 24. Credible research requires the use of scientific methods. | □ | □ | □ | □ |
| 25. The only convincing medical research is that which employs double-blind, clinical trials. | □ | □ | □ | □ |
| 26. Scientists must make sure they do not get emotionally involved with their research, if their findings are to be believed. | □ | □ | □ | □ |
| 27. To be confident of the impact of any research, we need to make sure we control for variables as much as practically possible. | □ | □ | □ | □ |
| 28. Good research is research that has undergone independent peer review of the methods, findings, and interpretation of the findings. | □ | □ | □ | □ |
| 29. Money spent on research about unusual and interesting creatures found in the deep ocean is wasted. | □ | □ | □ | □ |
| 30. It's a waste of money doing research about ways to improve our understanding of the brain. | □ | □ | □ | □ |
| 31. It's a waste of money doing research about other planets and star systems. | □ | □ | □ | □ |
| 32. Research about the fundamental forces in nature is hard to justify. | □ | □ | □ | □ |
Appendix 3. Chemistry attitudes and experiences survey (CAES) (Adapted from Dalgety et al., 2003, p. 663)
For each entry below (1–15), place a mark closer to the term or phrase that best reflects your view of the term in the center column.For example, if you feel chemistry is mostly about the study of natural substances, and only a little bit about the study of synthetic substances, then you might answer like this:
| Please indicate what you think about the following | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemists | |||||||||
| 1 | Unfit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Athletic |
| 2 | Socially unaware | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Socially aware |
| 3 | Environmentally unaware | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Environmentally aware |
| 4 | Fixed in their ideas | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Flexible in their ideas |
| 5 | Only care about their results | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Care about the effects of their results |
| 6 | Unimaginative | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Imaginative |
| 7 | Impatient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Patient |
| Chemistry research | |||||||||
| 8 | Harms people | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Helps people |
| 9 | Decreases quality of life | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Improves quality of life |
| 10 | Creates problems | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Solves problems |
| 11 | Causes society to decline | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Advances society |
| Chemistry jobs | |||||||||
| 12 | Repetitive | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Varied |
| 13 | Boring | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Interesting |
| 14 | Unsatisfying | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Satisfying |
| 15 | Tedious | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Exciting |
Acknowledgements
The study was funded by The Scientific and Technological Research Council of Turkey (TUBITAK). We would like to thank Dr Brandy Pleasants, Ms Jacinta Mutembuki, Ms Kathy Eaton, Ms Betty Adams and Dr Selçuk Şahingöz from Western Michigan University and Assistant Professor Muhammed Ertaç ATILA from Erzincan University for their kind assistance. Also, we are thankful to pre-service elementary teachers from Fatih Faculty of Education, Karadeniz Technical University and the Mallinson Institute for Science Education, Western Michigan University due to their voluntarily participation and efforts.References
- Abd-El-khalick F. and Boujaoude S., (1997), An exploratory study of the knowledge base for science teaching, J. Res. Sci. Teach., 34, 673–699.
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross-age study of the understanding five concepts, J. Res. Sci. Teach., 31, 147–165.
- Adadan E. and Savasci F., (2012), An analysis of 16–17-year-old students' understanding of solution chemistry concepts using a two-tier diagnostic instrument, Int. J. Sci. Educ., 34(4), 513–544.
- Akaygun S. and Jones L. L., (2014), Words or pictures: a comparison of written and pictorial explanations of physical and chemical equilibria, Int. J. Sci. Educ., 36(5), 783–807.
- Anagun S. S., (2011), The impact of teaching-learning process variables to the students' scientific literacy levels based on PISA 2006 results, Educ. Sci., 36(162), 84–102.
- Appleton K., (1995), Student teachers' confidence to teach science: Is more science knowledge necessary to improve self-confidence? Int. J. Sci. Educ., 17(3), 357–369.
- Appleton K., (2006), Science pedagogical content knowledge and elementary school teachers, in Appleton K. (ed.), Elementary Science Teacher Education: International Perspectives on Contemporary Issues and Practice, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 31–54.
- Bağ H. and Çalık M., (2017), A thematic review of argumentation studies at the K-8 level, Educ. Sci., 42(190), 281–303, DOI:10.15390/EB.2017.6845.
- Bakırcı H., Çalık M. and Çepni S., (2017), The effect of the common knowledge construction model-oriented education on sixth grade pupils' views on the nature of science, J. Balt. Sci. Educ., 16(1), 43–55.
- Bereket G., (2013), Tuzlanan yol araç ömrünü kısaltıyor! (Road salting reduces vehicle life). Retrieved October 22, 2013 from http://otomobil.haber7.com/otogundem/haber/972299-tuzlanan-yol-arac-omrunu-kisaltiyor.
- Blanco A. and Prieto T., (1997), Pupils' views on how stirring and temperature affect the dissolution of a solid in a liquid: A cross-age study (12 to 18), Int. J. Sci. Educ., 19, 303–315.
- Borg W. R. and Gall M. D., (1989), Educational research: An introduction, New York: Longman.
- Bursal M., (2008), Changes in Turkish pre-service elementary teachers' personal science teaching efficacy beliefs and science anxieties during a science method course, J. Turk. Sci. Educ., 5(1), 99–112.
- Çalık M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- Çalik M. and Coll R. K., (2012), Investigating socioscientific issues via scientific habits of mind: Development and validation of the Scientific Habits of Mind Survey, Int. J. Sci. Educ., 34(12), 1909–1930, DOI:10.1080/09500693.2012.685197.
- Çalik M., Ayas A. and Ebenezer J. V., (2005), A review of solution chemistry studies: Insights into students' conceptions, J. Sci. Educ. Technol., 14(1), 29–50.
- Çalık M., Ayas A. and Coll R. K., (2006), A constructivist-based model for the teaching of dissolution of gas in a liquid, Asia-Pacific Forum on Science Learning and Teaching, 7(1), 4.
- Çalık M., Ayas A., Coll R.K., Ünal S. and Coştu B., (2007), Investigating the effectiveness of a constructivist-based teaching model on student understanding of the dissolution of gases in liquids, J. Sci. Educ. Technol., 16(3), 257–270.
- Çalık M., Ayas A. and Ebenezer J. V., (2009), Analogical reasoning for understanding solution rates: Students' conceptual change and chemical explanations, Res. Sci. Technol. Educ., 27(3), 283–308.
- Çalık M., Ayas A. and Coll R. K., (2010), Investigating the effectiveness of usage of different methods embedded with four-step constructivist teaching strategy, J. Sci. Educ. Technol., 19(1), 32–48.
- Çalık M., Ültay N., Kolomuç A. and Aytar A., (2015), A cross-age study of science student teachers' chemistry attitudes, Chem. Educ. Res. Pract., 16, 228–236, 10.1039/c4rp00133h.
- Çoban A., (2004), Tuzlamanın zararı, yararından çok (Disadvantages of road salting is more than its advantages). Retrieved March 30, 2013 from http://arsiv.ntvmsnbc.com/news/299952.asp?cp1=1.
- Coll R. K., Taylor N. and Lay M. C., (2009), Scientists' habits of mind as evidenced by the interaction between their science training and religious beliefs, Int. J. Sci. Educ., 31(6), 725–755.
- Corradi D. M. J., Elen J., Schraepen B. and Clarebout G., (2014), Understanding possibilities and limitations of abstract chemical representations for achieving conceptual understanding, Int. J. Sci. Educ., 36(5), 715–734.
- Cronbach L. J., (1975), Beyond the two disciplines of scientific psychology, Am. Psychol., 30(2), 116–127.
- Dalgety J., Coll R. K. and Jones A., (2003), Development of chemistry attitudes and experiences questionnaire, J. Res. Sci. Teach., 40(7), 649–668.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84(3), 287–312.
- Ebenezer J., (2001), A hypermedia environment to explore and negotiate students' conceptions: Animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–91.
- Ebenezer J. V. and Connor S., (1998), Learning To Teach Science: A Model for the 21st Century, Upper Saddle River, New Jersey: Prentice-Hall, Inc., Simon and Schuster/A. Viacom Company.
- Ebenezer J. V. and Haggerty S., (1999), Becoming secondary school science teachers: Preservice teachers as researchers, Upper Saddle River, New Jersey: Prentice-Hall, Inc., Simon and Schuster/A. Viacom Company.
- Ebenezer J., Chacko S. and Immanuel N., (2004), Common knowledge construction model for teaching and learning science: Applications in the Indian context. An international conference to review research on Science, Technology and Mathematics Education International Centre (epiSTEME-1), Dona Paula, Goa, India.
- Ebenezer J., Chacko S., Kaya O. N., Koya S. K. and Ebenezer D. K., (2010), The effects of common knowledge construction model sequence of lessons on science achievement and relational conceptual change, J. Res. Sci. Teach., 47(1), 25–46.
- Geban Ö., Ertepınar H., Yılmaz G., Altan A. and Tahpaz F., (1994), Bilgisayar destekli eğitimin öğrencilerin fen başarılarına ve fen bilgisi ilgilerine etkisi (Effects of computer aided instruction on students' science achievement and interests towards science), paper presented at First National Symposium of Science Education, Dokuz Eylul University, İzmir, Turkey (In Turkish).
- Hair J. F., Jr, Black W. C., Babin B. J., Anderson R. E. and Tatham R. L., (2006), Multivariate Data Analysis, 6th edn, Mahwah, NJ: Prentice-Hall.
- Kadıoğlu M., (2013), Tuzlamalı ama ne kadar, ne zaman ve nereyi? (Road salting but how much, when and where?), retrieved May 13, 2013 from http://www.yerdurumu.org/makaleler/documents/tuzlamak_ama_nereyi_nezaman.asp.
- Kaya E. and Geban O., (2012), Facilitating conceptual change in rate of reaction concepts using conceptual change oriented instruction, Educ. Sci., 37(163), 216–225.
- Kiryak Z. and Çalik M., (2017), Improving grade 7 students' conceptual understanding of water pollution via the common knowledge construction model, Int. J. Sci. Math. Educ., DOI:10.1007/s10763-017-9820-8.
- Kolstø S. D., (2001), Scientific literacy for citizenship: tools for dealing with the science dimension of controversial socioscientific issues, Sci. Educ., 85, 291–310.
- Kurbanoğlu N. I., (2014), Investigation of the relationships between high school students' chemistry laboratory anxiety and chemistry attitudes in terms of gender and types of school, Educ. Sci., 39(171), 199–210.
- Lakatos I., (1970), Falsification and the methodology of scientific research programmes, in Lakatos I. and Musgrave A. (ed.), Criticism and the Growth of Knowledge, Cambridge, UK: Cambridge University Press.
- Lybeck L., Marton F., Stromdahl H. and Tullberg A., (1988), The phenomenography of the ‘mole concept’ in chemistry, in Ramsden P. (ed.), Improving Learning: New Perspectives, London: Kogan Page, pp. 81–108.
- Marton F., (1981), Phenomenography-describing conceptions of the world around us, Instruct. Sci., 10, 177–200.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69, 191–196.
- Ozden M., (2009), Prospective science teachers' conceptions of the solution chemistry, J. Balt. Sci. Educ., 8(2), 69–78.
- Pınarbasi T. and Canpolat N., (2003), Students' understanding of solution chemistry concepts, J. Chem. Educ., 80, 1328–1332.
- Pinarbasi T., Sozbilir M. and Canpolat N., (2009), Prospective chemistry teachers' misconceptions about colligative properties: Boiling point elevation and freezing point depression, Chem. Educ. Res. Pract., 10, 273–280.
- Robson C., (1998), Real word researc h, Oxford, UK: Blackwell Publishers Ltd.
- Sadler T. D., (2004), Informal reasoning regarding socioscientific issues: A critical review of research, J. Res. Sci. Teach., 41(5), 513–536.
- Solomon J. and Aikenhead G. (ed.), (1994), STS education: international perspectives on reform, New York: Teachers College Press.
- Titrek O. and Cobern W. W., (2011), Valuing science: A Turkish–American comparison, Int. J. Sci. Educ., 33(3), 401–421.
- Tosun C. and Taskesenligil Y., (2013), The effect of problem-based learning on undergraduate students' learning about solutions and their physical properties and scientific processing skills, Chem. Educ. Res. Pract., 14, 36–50.
- Ültay N. and Çalik M., (2012), A thematic review of studies into the effectiveness of context-based chemistry curricula, J. Sci. Educ. Technol., 26(6), 686–701.
- Wegner W. and Yaggi M., (2001), Environmental impacts of road salt and alternatives in the New York City watershed, Stormwater, 2, 24–31.
- Wong A. F. L. and Fraser B. J., (1996), Environment-attitude associations in the chemistry laboratory classroom, Res. Sci. Technol. Educ., 14(1), 91–102.
- Wood L. C., Ebenezer J. and Boone R., (2013), Effects of an intellectually caring model on urban African American alternative high school students' conceptual change and achievement in chemistry, Chem. Educ. Res. Pract., 14, 390–407.
- Zeidler D. L., (1984), Moral issues and social policy in science education: Closing the literacy gap, Sci. Educ., 68, 411–419.
| This journal is © The Royal Society of Chemistry 2017 |