pH effects in the acetaldehyde–ammonia reaction
Emanuele
Moioli
 *ab,
Leo
Schmid
b,
Peter
Wasserscheid
a and
Hannsjörg
Freund
a
*ab,
Leo
Schmid
b,
Peter
Wasserscheid
a and
Hannsjörg
Freund
a
aLehrstuhl für Chemische Reaktionstechnik, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstrasse 3, DE-91058 Erlangen, Germany. E-mail: emanuele.moioli@fau.de
bSpecialty Ingredients Research & Technology, Lonza Ltd., Lonzastrasse, CH-3930 Visp, Switzerland
First published on 28th February 2017
Abstract
The pH dependency of the reaction of acetaldehyde and ammonia to form the acetaldehyde-ammonia trimer has been studied in detail. The acetaldehyde-ammonia trimer is a molecule of interest in organic synthesis, since it can be used as a substrate in many reactions involving acetaldehyde or ammonia. This trimer is well known in the literature but no references are present so far to describe its formation from ammonia sources other than ammonium hydroxide. The focus of this study is on describing the course of reaction after addition of acetaldehyde to solutions of ammonia and various acids. Products have been analysed by means of 1H-NMR and IR spectroscopy and the complete range of pH values has been covered. Depending on the pH, two reaction regimes can be distinguished. At high pH, only the trimer is formed. In contrast, at low pH, only low quantities of the trimer are produced and the nature of the applied acid has a distinct effect on the reaction outcome. Inorganic acids result in low trimer concentration and high quantity of unreacted ammonia. Polymer formation dominates with simple carboxylic acids. Complex organic acids, such as e.g. maleic or nicotinic acid, lead to comparable quantities of the trimer and acetaldehyde. Based on our results, we propose some adjustments to the traditional reaction scheme developed for acetaldehyde-ammonia trimer formation at high pH.
Introduction
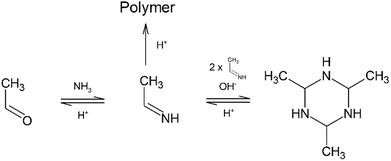
Mixing of acetaldehyde, CH3CHO, and ammonia, NH3, at room temperature and atmospheric pressure results in the formation of a cyclic structure usually referred to as the acetaldehyde-ammonia trimer (2,4,6-trimethyl-1,3,5-hexahydrotriazine).1–3 The reaction proceeds spontaneously and is highly exothermic. The trimer is in dry form a white solid that is only partially water-soluble.4 The molecule is unstable at room temperature, thus the product has to be maintained in organic solution. The acetaldehyde-ammonia trimer can be used to produce amines by hydrogenation5,6 or as a building block in organic synthesis.7 Other reported applications include its use as a scavenger for sulfhydryl compounds (thanks to its analogy to amines4) or as a precursor for ammonia production.8The mechanism of the formation of 2,4,6-trimethyl-1,3,5-hexahydrotriazine (C6H15N3) has been widely studied, and possible steps of RNH2 addition to a carbonyl group were postulated.9–11 Ogata et al. studied in 1964 the first step of the reaction, the formation of 1-aminoethanol (CH3CH(OH)NH2), by means of spectrophotometry.12 In this publication, equilibrium and kinetic data for the reaction at pH values ranging from 9 to 12 were reported. These authors came to the conclusion that the rate of the direct reaction to form 1-aminoethanol increases only slightly with increasing pH of the solution. Furthermore, it was found that the reverse reaction is subject to acid catalysis. In 1973, Hull et al. proposed a complete scheme for the formation of the acetaldehyde-ammonia trimer.13 By means of NMR analysis, the reaction of acetaldehyde and ammonia was extensively investigated at pH values higher than 7. The intermediate species were only tentatively identified as a direct determination via NMR analysis is not possible. The following scheme was proposed13 (see Fig. 1): the imine (C) represents the monomer for the formation of the trimer. The proposed values for the kinetic parameters are in good agreement with the earlier published calculations by Ogata et al.12 Recently, Vinogradoff et al. gave additional insight into the mechanism of acetaldehyde-ammonia trimer formation, analysing its formation at low temperature in the presence of formic acid.14 In this study, the intermediates proposed by Hull et al. were identified at low temperature using IR spectroscopy. Thanks to the theoretical prediction of vibrational frequencies, the assignment of all bands to the respective species was possible and all necessary steps to produce the acetaldehyde-ammonia trimer were elucidated. In particular, under cryogenic conditions, the dehydration of 1-aminoethanol into ethanimine is possible in the presence of formic acid. Ethanimine is stabilized at low temperature and can react to form the trimer only at temperatures higher than 230 K. A theoretical study based on molecular modelling by Chen et al. has also investigated the reaction of formaldehyde, acetone and acetaldehyde with ammonia at low temperature.15
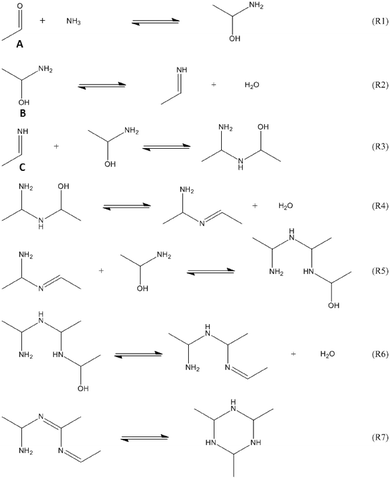 | ||
| Fig. 1 Proposed acetaldehyde–ammonia formation scheme (from ref. 13). | ||
Referring to experimental data for 1-aminoethanol formation,16 the production of this intermediate is influenced by the presence of excess ammonia in the solvent. Ammonia is more effective than water in transferring a proton from this intermediate formed by combination of an ammonia molecule and one molecule of acetaldehyde. This proton transfer decreases the energetic barrier for the reaction. Even though the approach from ref. 16 is not yet completely capable of describing the experimental results, it allows improving the understanding of the influence of various reaction parameters on the reaction. The results of these previous studies lead to a generally accepted reaction mechanism for the formation of the acetaldehyde-ammonia trimer under high pH conditions.
In contrast, no detailed report has been given to the best of our knowledge on the reaction of acetaldehyde and ammonia at lower pH so far. Therefore, the scope of this paper is to describe variations in the course of the reaction when mixing acetaldehyde with solutions of ammonia containing increasing quantities of various acids. We aim to check whether the formation of the acetaldehyde-ammonia trimer is also possible under conditions quite different from those discussed so far in the literature. In particular, we are interested in shedding light on the influence of different acids on the reaction outcome. We anticipate that a better understanding of the effect of pH on this reaction can open new ways of applying acetaldehyde and ammonia in the chemical industry, e.g., the design of new process schemes that are not limited to highly basic conditions.
Experimental
In our study, ammonia was applied as a 25–30% vol./vol. solution from Sigma Aldrich. For comparison purposes, ammonia solution in methanol was also prepared by condensing ammonia at −20 °C from a gas cylinder to form a 25–30% vol./vol. solution in a sealed reactor. Acetaldehyde was applied in its liquid form with 99% purity as purchased from Sigma Aldrich. Acetic acid, sulfuric acid, and formic acid were applied as commercially supplied (96–99%, Sigma Aldrich). Hydrochloric acid was used in the form of a 33% solution in water (Sigma Aldrich). Oxalic acid, maleic acid, and nicotinic acid (Sigma Aldrich) are solids and were applied in the form of their respective saturated solutions in water. The applied ammonium salts were also commercial samples from Sigma Aldrich. The reference acetaldehyde-ammonia trimer was purchased in its hydrated form with 96% purity from Sigma Aldrich.All reactions were carried out in a flask that was placed in an ice bath to avoid temperature increases due to the reaction exothermicity. For the reactions with salts, solutions of 10 M were prepared. Acetaldehyde was slowly added in a stoichiometric amount to the nominal ammonia content. Solutions of ammonia and acid at different pH values were prepared as follows: 50 mL of the commercial solution of ammonia were dosed into the flask. Subsequently, the desired acid was added dropwise while checking the pH using an electronic pH sensor. When the desired pH was reached, it was double checked using a universal indicator. All obtained solutions were cooled in the ice bath prior to dropwise addition of acetaldehyde in a stoichiometric amount to the nominal ammonia quantity. Acetaldehyde was always kept in the ice bath to prevent evaporation.
The obtained samples were analysed by means of 1H-NMR spectroscopy (Bruker, 400 MHz). All samples were prepared for NMR analysis in d6-DMSO and benzyl benzoate was applied as the internal standard for quantitative evaluation. Benzyl benzoate does not interact with the acetaldehyde-ammonia trimer and its characteristic peaks are well distinguishable from the product. Samples were analysed immediately after preparation to avoid product decomposition. In addition, control experiments were also carried out comparing commercial trimer samples with the ones obtained in our experiments using IR spectroscopy.
Results and discussion
Thermodynamics
No experimental thermodynamic data are available in the literature regarding acetaldehyde-ammonia trimer formation. In order to obtain a preliminary estimation of thermodynamic properties, theoretical predictions following theoretical prediction methodologies from the groups of Joback17 and Gani18,19 were carried out. The respective results are presented in Tables 1 and 2.| Component | Method | ΔH°f [kJ mol−1] | ΔG°f [kJ mol−1] |
|---|---|---|---|
| Acetaldehyde-ammonia trimer | Joback | −40.1 | 271.8 |
| Gani | −56.2 | 216.8 |
| Method | ΔH°R [kJ mol−1] | ΔG°R [kJ mol−1] |
|---|---|---|
| Joback | −123.6 | −9.6 |
| Gani | −157.5 | −53.9 |
The acetaldehyde-ammonia trimer is characterised by the presence of nitrogen and carbon in a 6-member ring together with three terminal methyl groups. The two prediction methods agree on the fact that the value of ΔH°f is negative and the value of ΔG°f is positive. The Joback method results in higher values for both quantities. This in turn has a direct influence on the calculation of reaction enthalpy and Gibbs free energy: the results from the Gani method suggest a stronger exothermic reaction with significantly higher thermodynamic driving force compared to the results obtained from the method of Joback. The origin of the difference between the two methods is the presence of a second order group contribution in the Gani method (absent in the Joback method) referring to ring effects that decrease the predicted enthalpy and Gibbs free energy. For the cyclic acetaldehyde-ammonia trimer, this feature should make the Gani method more suitable for the prediction of thermodynamic properties.
Note that the calculated results using the Gani method are in good agreement with our qualitative experimental observations. Acetaldehyde addition to ammonia results in a strongly exothermic reaction. A point of special interest is the calculation of the Gibbs free energy. The method of Joback predicts a ΔG°R close to 0. The value predicted by the Gani method is instead much more strongly negative. The equilibrium constant is predicted to be 2.8 × 109, which is very close to the experimental value given in ref. 13. For this reason, we consider in this study the values predicted using the Gani method since this method results in an obviously more appropriate representation of the system under investigation. The results of thermodynamic calculations for decreasing pH values show a decrease in the equilibrium constant value, due to the reaction of ammonia and acids. This results in a decrease in ΔG°R and ΔH°R, as experimentally observed by a decreasing heat release during reaction at lower pH. Nevertheless, even at low pH, the formation of the acetaldehyde-ammonia trimer is thermodynamically clearly favoured.
Initial screening of acetaldehyde and ammonia reactivity under non-basic conditions
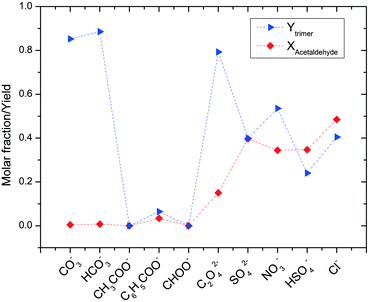 | ||
| Fig. 2 Molar fractions of acetaldehyde and acetaldehyde ammonia trimer in various ammonium salts (CH3CHOin = 5 M). | ||
| Anion | CO32− | HCO3− | Ac− | C6H6COO− | CHOO− | C2O42− | SO42− | NO3− | HSO4− | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 9 | 8 | 7 | 6.5 | 6 | 5.5 | 5 | 5 | 5 | 5 |
The polymer formed was characterised by MS analysis. The results of this analysis show the presence of species with molecular weight higher than that of the acetaldehyde-ammonia trimer. The analysis of the distribution of molecular weights suggests that these species are produced from aldol condensation of acetaldehyde in the presence of ammonia. This side reaction shows an irreversible character, in contrast to the reversible behaviour of the acetaldehyde–ammonia reaction system.
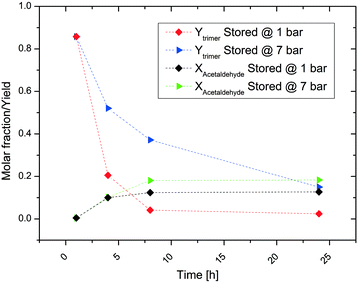
The results reported here were obtained few minutes after the preparation of the solutions. Repeated measurements over several hours verified the stability of the mixture. With regard to trimer yield, the results are quite dispersed and do not show any clear trend. In contrast, the influence of the pH value of the salt solution on acetaldehyde conversion is much clearer. The quantity of unreacted acetaldehyde is considerable for salts of strong inorganic acids (about 40–50%), while full acetaldehyde conversion is reached with the salts of carboxylic acids and with carbonates.
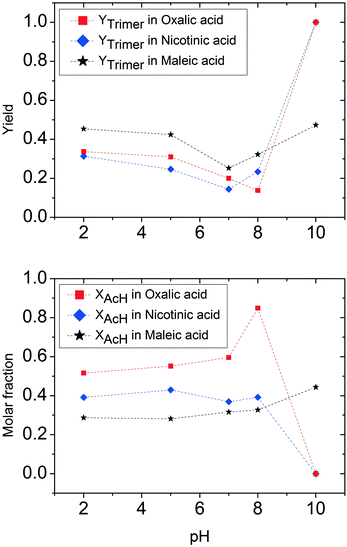
Interestingly, the results in Fig. 2 can be classified into three different groups of acids showing very similar results: salts of inorganic acids, salts of organic acids and carbonates. The use of ammonium salts of inorganic acids results in low production of undesired species, but also a large quantity of unreacted acetaldehyde (i.e., low acetaldehyde conversion) is observed. The sum of these two factors limits trimer formation to a yield of about 50%. The NMR spectra indicate the formation of acetaldehyde in its hydrate form under these conditions. Under low pH conditions, acetaldehyde is more likely to react with water producing acetaldehyde hydrate rather than to react with the ammonium ion to form the amino-alcohol which is the initial step for trimer formation. In this acidic environment, the acetaldehyde hydrate shows only a limited reactivity towards aldol condensation. This is the reason for the observed low polymer formation. Oxalic acid is at the border of this group as it allows much higher production of the trimer linked to a significantly larger acetaldehyde conversion.
The results for the second group, composed of salts of carboxylic acids, are completely different. When applying these salts, no signals of acetaldehyde and the trimer are found in the NMR spectra. The solutions are clear when prepared but they become dark within a few minutes. It was therefore impossible to reveal whether the obvious production of tar was the result of trimer decomposition or of direct aldol condensation of acetaldehyde. To shed more light on the relevant reaction processes, the reaction with ammonium acetate was repeated, adding a buffer to the solution. In this way, the production of tar was greatly slowed down and it was possible to analyse the actual reaction products. Under these modified conditions, NMR signals for the acetaldehyde-ammonia trimer could also be detected. Therefore, we expect that the reaction mechanism still involves initial trimer formation followed by acid-catalysed trimer decomposition.
The third group of ammonium salts applied comprises ammonium carbonate and bicarbonate salts. The reaction outcome in this group is characterised by total conversion of acetaldehyde and high selectivity to acetaldehyde ammonia trimer. This is in good agreement with the common assumption in the literature12 that the trimer is more easily formed under basic conditions. It is still surprising that such high trimer yields can be obtained using an ammonium salt. Another special aspect related to the use of carbonate salts is the incipient CO2 formation during acetaldehyde addition. This is most probably due to the decomposition of carbonic acid that goes hand in hand with the trimer formation reaction. CO2 is released by the reaction of acetaldehyde and carbonates (e.g. 3CH3CHO + 3NH4HCO3 ↔ trimer + H2O + CO2), and its presence in the solution should influence the stability of the produced trimer.
To sum up the effects of ammonium carbonate addition, it is fair to state that its addition leads to a pH range of 9–10 which is favourable for trimer formation. However, this does not prevent trimer decomposition over time.
Quantitative pH range screening
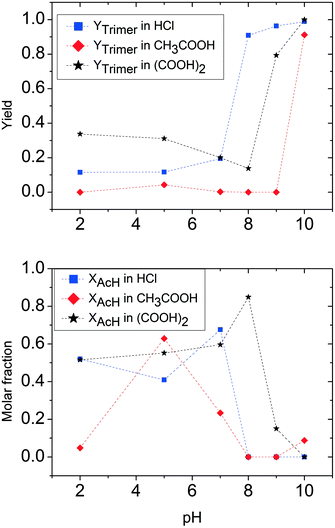 | ||
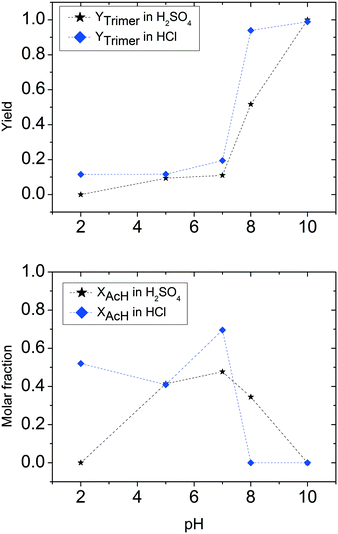
| Fig. 4 Comparison of hydrochloric acid, acetic acid and oxalic acid: trimer and acetaldehyde molar fraction (CH3CHOin = 5 M). | ||
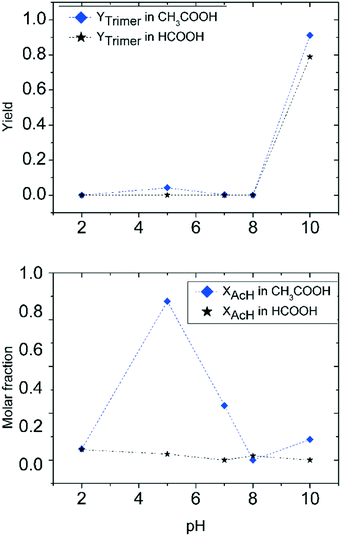
The influence of pH on the reaction in the presence of acetic acid is quite different. At high pH (low quantities of acetic acid added), the acetaldehyde ammonia trimer is the main product, even though it does not reach the full yield observed with hydrochloric acid. A drop in trimer molar yield due to protonation of ammonia is observable at relatively high pH. The range of pH with the largest polymer formation is particularly large for acetic acid (between pH 7 and 9). In the same pH range, unreacted acetaldehyde is not observed, in full agreement with the previous observations for the ammonium acetate case. A further decrease of pH following the addition of more acetic acid to ammonia solution does allow the formation of the acetaldehyde ammonia trimer. At pH 4, a maximum in acetaldehyde concentration is found. This means that the quantity of acetic acid present in this solution favours trimer decomposition, but also the aldehyde form compared to the imine form.
This phenomenon is not present at lower pH, where no acetaldehyde is recovered. The role of acetic acid in catalysing the various steps of acetaldehyde ammonia trimer formation is complex and strongly varies with the relative amounts of acetic acid and ammonia present.
Trimer preparation tests also give different results when the acid used to vary the pH is oxalic acid. Once again, at pH 10, the trimer is formed quantitatively. The decrease of pH lowers the trimer formation slower than in the cases of hydrochloric and acetic acid. In fact, at pH 9, an 80% trimer molar fraction is still observed, while at pH 8, the solution contains 20% trimer. It is important to underline in this case that the reaction system at certain pH values obtained by using oxalic acid is not completely comparable with the two previous cases because of the solubility limit of oxalic acid in water. For this reason, solutions prepared with oxalic acid are more diluted, thus a higher quantity of acid is necessary to achieve the same pH in the solution. A particular trend for solutions containing oxalic acid is found in trimer concentrations at low pH. Compared to the experiment at pH 8, the yield of the trimer tends to increase and stabilizes at pH lower than 5 at a value of around 30%. The trend for acetaldehyde is quite similar to that previously observed for hydrochloric acid. A maximum for acetaldehyde is found at pH 8, corresponding to a minimum in trimer. At lower pH, the molar fraction of acetaldehyde decreases and stabilizes at a value of around 50%. It is important to note that in the case of oxalic acid also at low pH values, less than 20% of the acetaldehyde fed is lost in polymerization products.
For all the three acids considered in this section, it is important to distinguish the pure effects of pH and the effects of acid concentration. Owing to the differing strength of the applied acids, the concentration of acid needed to adjust a certain pH is different. At high and neutral pH, the most important effect of the added acid is due to the protonation of the reactant. At low pH, the effect of concentration is more relevant, as high amounts of acid are required to lower the pH. In this case, all reactants are fully protonated and further side reactions are influenced by the nature and quantity of free acid present in the solution.
To summarize this first quantitative analysis, we have observed that at high pH all acids under investigation allow almost quantitative trimer production. This is an indication that a small quantity of acid does not affect the capability of ammonia to react with acetaldehyde to form the respective imine, and consequently the acetaldehyde ammonia trimer. The increase of acid concentration in solution results in the decrease of trimer yield. The differences found among the three different acids used are mainly the result of the different quantities of acid necessary to achieve the same pH in the solution. The most important differences appear when the threshold of complete ammonia transformation into the ammonium salt is reached. As already observed in the previous section, the use of ammonium salts gives results that depend on the nature of the salt used.
For pH values lower than the value identified for the corresponding ammonium salt, acidity has a catalytic effect on the trimer decomposition reaction. This is also confirmed by dissolution tests of commercial acetaldehyde ammonia trimer in 0.1 M hydrochloric acid. The unstable imine groups formed by trimer decomposition undergo a further reaction depending on the nature of the applied acid. A schematic and simplified representation of the reaction is depicted in Fig. 5. The difference between the different acids either causes a complete reverse reaction to acetaldehyde (or acetaldehyde does not react) or favours aldol condensation to polymerization products. For acetic acid, a catalyst for both named reactions, it is impossible to stabilize the trimer in the presence of free acid. The final product of the reaction network is determined by the quantity of free acetic acid present: at low and intermediate pH (regions <4 and 6–9), only polymer is formed. In the pH range between 4 and 6, acetaldehyde is found in the reaction mixture.
The case for the other two considered acids is less complex: there is no special effect of pH under acidic conditions on the course of the reaction. The relative quantity of acetaldehyde and trimer is practically constant for pH < 7.
Summary and conclusions
A detailed study of the reaction of acetaldehyde and ammonia under various pH conditions using different acids was carried out in order to understand the effect of acid addition on trimer formation. The acid additions under investigation included inorganic and organic acids. At high pH, the acetaldehyde ammonia trimer is the main product which is obtained in yields close to 100%. When lowering the pH value by acid addition, the trimer formation decreases. Minimum trimer formation is observed at the pH value that corresponds to that of the corresponding ammonium salt solution. This pH value is characteristic of the applied acid additive. At low pH, the reaction outcome differs significantly for the different types of acids applied. Inorganic acid additions at low pH lead to inhibition of the reaction with a large quantity of unreacted acetaldehyde and a small quantity of the trimer found in the reaction mixture. Carboxylic acid additions at low pH values lead to aldol condensation that converts acetaldehyde into polymer.Our results show the importance of pH control in the acetaldehyde–ammonia reaction. The least favourable pH-range for trimer formation is around neutral pH as all ammonium salts effectively catalyse polymer formation. High trimer formation is observed at high pH (with only small quantities of the respective acids added) or at low pH with inorganic acids. An exception is maleic acid addition at high pH that results in incomplete conversion and significant amounts of unreacted acetaldehyde.
Acknowledgements
This project has received funding from the European Union's Seventh Framework Program for research, technological development and demonstration under grant agreement no. 607114.References
- A. Novak, Spectrochim. Acta, 1960, 16, 1001–1009 CrossRef.
- E. W. Lund, Acta Chem. Scand., 1951, 5, 678 CrossRef CAS.
- E. W. Lund, Acta Chem. Scand., 1957, 12, 1768–1776 CrossRef.
- M. Callaway and G. T. Rivers, US Pat., 5958352, 1999 Search PubMed.
- G. Gobron and R. Proust, French Pat., 1548582, 1968 Search PubMed.
- G. Gobron and R. Proust, US Pat., 3594420, 1971 Search PubMed.
- W. J. Burke and R. J. Reynolds, J. Am. Chem. Soc., 1954, 76, 1291–1293 CrossRef CAS.
- J. MacLachlan, P. Morken, W. Schmiegel and K. Takahashi, US Pat., 6281296, 2001 Search PubMed.
- M. Sprung, Chem. Rev., 1940, 26(3), 297–338 CrossRef CAS.
- A. Nielsen, R. Atkins, D. Moore, R. Scott, D. Mallory and J. M. LaBerge, J. Org. Chem., 1973, 38, 3288–3295 CrossRef CAS.
- A. Nielsen, D. Moore, R. Atkins, D. Mallory, J. DiPol and J. M. LaBerge, J. Org. Chem., 1976, 41, 3221–3229 CrossRef CAS.
- Y. Ogata and A. Kawasaki, Tetrahedron, 1964, 20, 855–860 CrossRef CAS.
- W. Hull, B. Sykes and B. Babior, J. Org. Chem., 1973, 38, 2931–2939 CrossRef CAS.
- V. Vinogradoff, F. Duvernay, M. Farabet, G. Danger, P. Theulé, F. Borget, J. C. Guillemin and T. Chiavassa, J. Phys. Chem. A, 2012, 116, 2225–2233 CrossRef CAS PubMed.
- L. Chen and D. E. Woon, J. Phys. Chem. A, 2011, 115, 5166–5183 CrossRef CAS PubMed.
- F. Duvernay, V. Dufauret, G. Danger, P. Theulé, F. Borget and T. Chiavassa, Astron. Astrophys., 2010, 523, A79 CrossRef.
- K. G. Joback and R. C. Reid, Chem. Eng. Commun., 1987, 57, 233–243 CrossRef CAS.
- L. Costantinou and R. Gani, AIChE J., 1994, 40, 1697–1710 CrossRef.
- J. Marrero and R. Gani, Fluid Phase Equilib., 2001, 183–184, 183–208 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |