Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Superparamagnetic nanoparticles as a recyclable catalyst: a new access to phenol esters via cross dehydrogenative coupling reactions†
Chung K. Nguyen,
Hoang H. Vu,
Ha V. Dang,
Ngon N. Nguyen,
Nhan T. H. Le and
Nam T. S. Phan *
*
Faculty of Chemical Engineering, HCMC University of Technology, VNU-HCM, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam. E-mail: ptsnam@hcmut.edu.vn; Fax: +84 8 38637504; Tel: +84 8 38647256 ext. 5681
First published on 8th December 2017
Abstract
CuFe2O4 superparamagnetic nanoparticles were utilized as a recyclable heterogeneous catalyst for the direct synthesis of chemical structures containing both phenol ester and benzothiazole moieties via cross dehydrogenative coupling reactions. Several substrates were reactive towards the transformation in the presence of the nano catalyst, including benzaldehyde, benzyl alcohol, dibenzyl ether, 2-oxo-2-phenylacetaldehyde, and 2-iodo-1-phenylethanone. This nano catalyst displayed higher catalytic efficiency than many homogeneous and heterogeneous catalysts. The nano CuFe2O4 was easily isolated by using a magnet, and reutilize numerous times without a remarkable deterioration in catalytic performance. To our best knowledge, these reactions were not previously reported in the literature.
1. Introduction
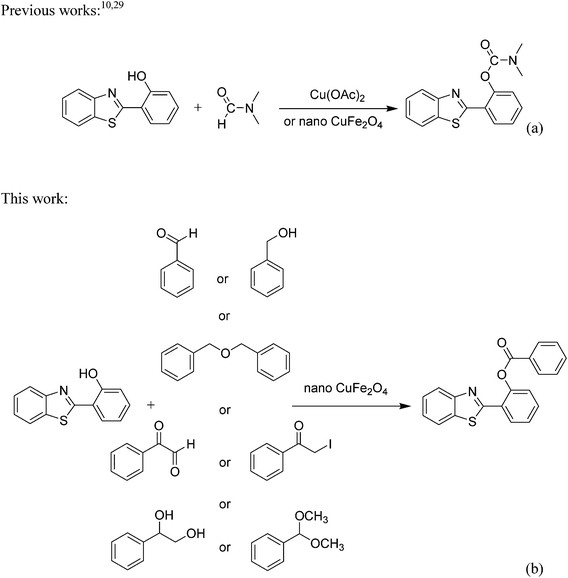
Esters and their derivatives are prevalent structures existing in diverse collections of biologically active molecules, with a broad range of applications in many fields such as pharmaceutical chemicals, fragrance chemicals, agricultural chemicals, and functional materials.1–3 Phenol esters are conventionally generated from phenols and carboxylic acid derivatives utilizing a considerable quantity of bases, showing numerous drawbacks, and therefore, synthetic pathways to these skeletons must be improved.4,5 Benzothiazole derivatives are precious heterocyclic scaffolds present in numerous natural products or synthetic chemicals, offering valuable biological activities.6,7 Organic structures containing both phenol ester and benzothiazole moieties would benefit from both functional parts in terms of pharmaceutical and biological activities. Overcoming drawbacks of traditional synthetic approaches where two functional groups must be pre-installed in the reactants, coupling reactions via direct C–H bond activation should provide more efficacious synthetic routes for these valuable skeletons.8,9 Previously, Ali and et al. developed the first illustration of a synthetic method to produce structures containing both carbamates and benzothiazoles via the Cu(OAC)2-catalyzed coupling reaction between dialkylformamides and phenols having benzothiazole directing substituents (Scheme 1a).10 Zheng et al. also synthesized phenol esters possessing cyano, azo, and pyridine moieties by using the Cu(OAC)2-catalyzed transformation between aldehydes and 2-substituted phenols.11 Certainly, the field still remains to be explored, in which recyclable catalysts should be investigated for these transformations.Catalysis has an essential role in the chemical industry, since it contributes cost-effective, environmentally benign, and selective routes for what are contrastingly expensive, eco-hazardous, or even unapproachable.12–14 As inspired by green chemistry principles, utilizing heterogeneous catalysts is favored owing to the possibility of separation and reusability.15–17 “Nanocatalysis” has appeared as an speedily expanding area throughout the last few years, in which nanoparticles have been considered as alternative candidates, combining benefits from both homogeneous and heterogeneous catalysts.18–20 Nevertheless, because of their nanometer-scaled sizes, the isolation from liquid phase and the reutilizing of the nanoparticles is undoubtedly challenging, and more efforts are needed to solve this issue.21,22 Superparamagnetic nanoparticles as catalysts would link the merit of superior reactivity and good dispersion in reaction media with straightforward isolation procedure by using magnets.23,24 Certainly, both functionalized and unfunctionalized superparamagnetic nanoparticles have been utilized as catalysts for numerous organic reactions.25–28 We recently performed the coupling reaction between dialkylformamides and phenols having benzothiazole directing substituents utilizing nano CuFe2O4 catalyst (Scheme 1a).29 In this work, we would like to describe the direct synthesis of chemical structures containing both phenol ester and benzothiazole moieties via cross dehydrogenative coupling reactions, in the presence of CuFe2O4 superparamagnetic nanoparticles as recyclable catalyst (Scheme 1b). To our best knowledge, these reactions were not previously reported in the literature.
2. Experimental
CuFe2O4 superparamagnetic nanoparticles were obtained from Sigma-Aldrich. The nanoparticles was subsequently characterized by employing different analysis methods (see Fig. S1–S4 in ESI†). In an illustrative catalytic run, a solution of 2-(benzo[d]thiazol-2-yl)phenol (0.0568 g, 0.25 mmol) and benzaldehyde (0.0795 g, 0.75 mmol) in p-xylene (0.5 mL) was introduced into a pressurized vial as reactor. The catalyst was then added to the solution, and the reactor was shaken to disperse the solid catalyst into the liquid phase. The catalyst quantity was utilized based on the copper/2-(benzo[d]thiazol-2-yl)phenol mole ratio. After that, tert-butyl hydroperoxide (tBuOOH, 70% wt. in water; 0.145 mL, 1.0 mmol) as an oxidant was added. The vial was magnetically stirred under argon at 80 °C for 24 h. The mixture was then cooled down to ambient temperature, and diphenyl ether (0.0425 g, 0.25 mmol) as internal standard was introduced. Samples were withdrawn, and quenched with water (1 mL). Organic constituents were extracted into ethyl acetate (2 mL), dried with anhydrous Na2SO4 to remove any water residue, and analyzed by GC regarding diphenyl ether. The major product, 2-(benzo[d]thiazol-2-yl)phenyl benzoate, was purified by column chromatography. 1H NMR, 13C NMR, and GC-MS analyses were also conducted to verify product structure. For the catalyst reutilizing experiment, the superparamagnetic nanoparticles were collected by decantation using a permanent magnet, washed carefully with p-xylene and methanol, heated at 150 °C under vacuum on a Schlenk line for 6 h, and then reused as catalyst for new experiment.3. Results and discussion
The CuFe2O4 superparamagnetic nanoparticles were explored as a heterogeneous catalyst for the cross dehydrogenative coupling reaction between 2-(benzo[d]thiazol-2-yl)phenol and benzaldehyde to produce 2-(benzo[d]thiazol-2-yl)phenyl benzoate as the major product (Scheme 1b). Initially, the influence of solvent on the formation of the hybrid benzothiazole–phenol ester was investigated, having conducted the reaction in various solvents, including DMSO, dichlorobenzene, tert-butanol, DMA, dioxane, and p-xylene (entries 1–6, Table 1). Indeed, liquid-phase organic transformations utilizing metal catalysts are generally affected by the reaction solvents. Zheng et al. previously synthesized phenol esters possessing cyano, azo, and pyridine moieties, and demonstrated that solvent expressed a noticeable influence on the transformation, in which DMSO emerged as the best solvent.11 The reaction was performed at 100 °C for 24 h, with 2 equivalents of benzaldehyde, at 3 mol% catalyst, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant. Compared to other solvents, p-xylene should be the solvent of choice, and the reaction conducted in this solvent generated 2-(benzo[d]thiazol-2-yl)phenyl benzoate in 66% yield after 24 h (entry 6, Table 1). Next, we tried to improve the yield of the desired product by changing the reaction temperature (entries 7–12). The reaction conducted at room temperature afforded only 11% yield after 24 h (entry 7, Table 1). As anticipated, boosting the temperature caused a noticeable enhancement in the generation of the expected product. Best yield was achieved when the reaction temperature was extended to 80 °C (entry 10, Table 1). It was noticed that increasing the temperature to over 80 °C did not favor the transformation (entries 11 and 12, Table 1). Indeed, Zheng et al. previously performed the Cu(OAc)2-catalyzed oxidative esterification of ortho-formyl phenols with aldehydes, and pointed out that the coupling reaction should be conducted at 80 °C.30| Entry | Solvent | Temperature (°C) | Catalyst concentration (mol%) | Oxidant | Oxidant amount (equiv.) | Yieldb |
|---|---|---|---|---|---|---|
| a Reaction conditions: 2-(benzo[d]thiazol-2-yl)phenol (0.25 mmol); benzaldehyde (0.75 mmol); solvent (0.5 mL); 24 h. TBHP: tert-butyl hydroperoxide in water; TBHP (decane): tert-butyl hydroperoxide in decane; DTBP: di-tert-butyl peroxide; TBPB: tert-butyl peroxybenzoate; CHP: cumyl hydroperoxide; H2O2: hydrogen peroxide; DMSO: dimethyl sulfoxide; DMA: N,N-dimethylacetamide; DCB: 1,2-dichlorobenzene.b GC yield. | ||||||
| 1 | DMSO | 100 | 3 | TBHP | 4 | 54 |
| 2 | DMA | 100 | 3 | TBHP | 4 | 29 |
| 3 | t-BuOH | 100 | 3 | TBHP | 4 | 50 |
| 4 | Dioxane | 100 | 3 | TBHP | 4 | 50 |
| 5 | DCB | 100 | 3 | TBHP | 4 | 48 |
| 6 | p-Xylene | 100 | 3 | TBHP | 4 | 66 |
| 7 | p-Xylene | RT | 3 | TBHP | 4 | 11 |
| 8 | p-Xylene | 50 | 3 | TBHP | 4 | 61 |
| 9 | p-Xylene | 60 | 3 | TBHP | 4 | 69 |
| 10 | p-Xylene | 80 | 3 | TBHP | 4 | 72 |
| 11 | p-Xylene | 100 | 3 | TBHP | 4 | 66 |
| 12 | p-Xylene | 120 | 3 | TBHP | 4 | 49 |
| 13 | p-Xylene | 80 | 0 | TBHP | 4 | 3 |
| 14 | p-Xylene | 80 | 1 | TBHP | 4 | 36 |
| 15 | p-Xylene | 80 | 3 | TBHP | 4 | 72 |
| 16 | p-Xylene | 80 | 5 | TBHP | 4 | 89 |
| 17 | p-Xylene | 80 | 7 | TBHP | 4 | 89 |
| 18 | p-Xylene | 80 | 10 | TBHP | 4 | 89 |
| 19 | p-Xylene | 80 | 5 | TBHP | 4 | 89 |
| 20 | p-Xylene | 80 | 5 | TBHP(decane) | 4 | 65 |
| 21 | p-Xylene | 80 | 5 | CHP | 4 | 40 |
| 22 | p-Xylene | 80 | 5 | TBPB | 4 | 16 |
| 23 | p-Xylene | 80 | 5 | DTBP | 4 | 3 |
| 24 | p-Xylene | 80 | 5 | H2O2 | 4 | 2 |
| 25 | p-Xylene | 80 | 5 | TBHP | 0 | 0 |
| 26 | p-Xylene | 80 | 5 | TBHP | 2 | 56 |
| 27 | p-Xylene | 80 | 5 | TBHP | 3 | 68 |
| 28 | p-Xylene | 80 | 5 | TBHP | 4 | 89 |
| 29 | p-Xylene | 80 | 5 | TBHP | 5 | 72 |
Next, the required catalyst amount was investigated for the cross dehydrogenative coupling transformation (entries 13–18, Table 1). It should be emphasized that the reaction could not progress in the absence of the catalyst, and only 3% yield of the major product was detected after 24 h (entry 13, Table 1). This observation verified that the catalyst should be compulsory for the transformation. Low yield of the expected product was recorded for the reaction utilizing 1 mol% catalyst (entry 14, Table 1). This value could be upgraded to 89% for the reaction conducted with 5 mol% catalyst (entry 16, Table 1). Extending the catalyst quantity to 7 mol% or 10 mol% was not needed, as the yield of 2-(benzo[d]thiazol-2-yl)phenyl benzoate was not augmented markedly (entries 17 and 18, Table 1). It should be noted that 10 mol% catalyst was previously employed for similar transformations,11,30 while the cross dehydrogenative coupling with dialkylformamides reacquired 5 mol% catalyst.10 Concerning other reactions via C–H bond activation, an oxidant should be compulsory for the reaction. We therefore studied the influence of oxidant on the transformation (entries 19–24, Table 1). Compared to other oxidants, tert-butyl hydroperoxide in water expressed better performance, producing 2-(benzo[d]thiazol-2-yl)phenyl benzoate in 89% yield (entry 19, Table 1). Indeed, tert-butyl hydroperoxide was also the oxidant of choice in previous works.10,11,30 Having these results, we also explored the influence of oxidant concentration on the transformation (entries 25–29, Table 1). The reaction could not proceed without an oxidant, and no trace quantity of the expected product was detected after 24 h (entry 25, Table 1). Best result was recorded when 4 equivalents of the oxidant was present in the reaction mixture (entry 28, Table 1). However, extending the quantity of the oxidant to more than 4 equivalents did not favor the generation of 2-(benzo[d]thiazol-2-yl)phenyl benzoate (entry 29, Table 1).
Since the cross dehydrogenative coupling reaction utilizing the superparamagnetic nanoparticle catalyst was conducted in solvent, an important issue is the potentiality that a number of active sites might go into solution phase, and they would be responsible for the formation of the expected product. To verify if leaching of active sites was a serious problem for the reaction, a control experiment was implemented to assess the contribution of homogeneous catalysis. If more 2-(benzo[d]thiazol-2-yl)phenyl benzoate was produced after the catalyst was isolated, this might imply that the active species were in solution phase rather than on the solid superparamagnetic nanoparticle catalyst. The reaction was performed in p-xylene at 80 °C for 24 h, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant, at 5 mol% catalyst, with 3 equivalents of benzaldehyde. The liquid phase was removed from the solid catalyst after 4 h reaction time by magnetic decantation, and subsequently added to a new and clean reactor. The mixture was consequently stirred at 80 °C for an additional 20 h to verify if more 2-(benzo[d]thiazol-2-yl)phenyl benzoate was generated under this condition. Certainly, almost no additional product was recorded after the catalyst was isolated. These data verified that the cross dehydrogenative coupling transformation required the presence of the superparamagnetic nanoparticle catalyst, and almost no product was produced via homogeneous catalysis (Fig. 1).
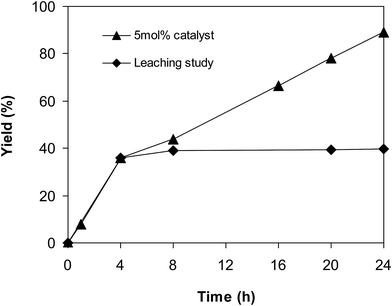 | ||
| Fig. 1 Leaching studies verified that 2-(benzo[d]thiazol-2-yl)phenyl benzoate was not generated after the catalyst was isolated. | ||
To underline the benefit of utilizing the CuFe2O4 superparamagnetic nanoparticles as catalyst, numerous homogeneous and heterogeneous catalysts were consequently employed for the transformation (Table 2). The reaction was conducted in p-xylene at 80 °C for 24 h, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant, at 5 mol% catalyst, with 3 equivalents of benzaldehyde. Copper salts were noticed to be more active than iron salts. The reaction using CuI catalyst afforded 67% yield (entry 1, Table 2), while 61% and 77% yields were observed for the case of CuBr (entry 2, Table 2) and CuCl (entry 3, Table 2) catalysts, respectively. Cu(OAc)2 displayed higher activity, producing 2-(benzo[d]thiazol-2-yl)phenyl benzoate in 78% yield (entry 5, Table 2). Low yields of the expected product was detected for the reaction utilizing FeCl2 (entry 6, Table 2), FeCl3 (entry 7, Table 2), and Fe(OAc)2 (entry 8, Table 2). Moving to heterogeneous catalysts, the difference in activity between the CuFe2O4 superparamagnetic nanoparticles and other catalysts was significant. Nano Fe2O3 (entry 9, Table 2), nano Fe3O4 (entry 10, Table 2), and nano NiFe2O4 (entry 11, Table 2) were almost unactive for the cross dehydrogenative coupling reaction, while 39% yield was observed for the case of nano CoFe2O4 catalyst (entry 12, Table 2). Nano CuO (entry 13, Table 2) and nano Cu2O (entry 14, Table 2) were more active, affording 68% and 61% yields, respectively. Compared to these homogeneous and heterogeneous catalysts, the nano CuFe2O4 catalyst (entry 15, Table 2) displayed the best catalytic efficiency, with 89% yield being recorded.
| Entry | Homogeneous catalyst | Heterogeneous catalyst | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 2-(benzo[d]thiazol-2-yl)phenol (0.25 mmol); benzaldehyde (0.75 mmol); p-xylene (0.5 mL); tert-butyl hydroperoxide in water (1 mmol); CuFe2O4 (0.0125 mmol); 80 °C; 24 h.b GC yield. All catalysts were purchased from Sigma-Aldrich. | |||
| 1 | CuI | 67 | |
| 2 | CuBr | 61 | |
| 3 | CuCl | 77 | |
| 4 | CuBr2 | 72 | |
| 5 | Cu(OAc)2 | 78 | |
| 6 | FeCl2 | 26 | |
| 7 | FeCl3 | 11 | |
| 8 | Fe(OAc)2 | 34 | |
| 9 | Nano Fe2O3 | 5 | |
| 10 | Nano Fe3O4 | 7 | |
| 11 | Nano NiFe2O4 | 4 | |
| 12 | Nano CoFe2O4 | 39 | |
| 13 | Nano CuO | 68 | |
| 14 | Nano Cu2O | 61 | |
| 15 | Nano CuFe2O4 | 89 | |
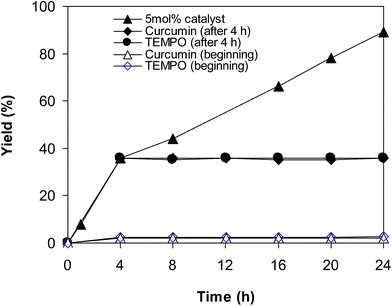
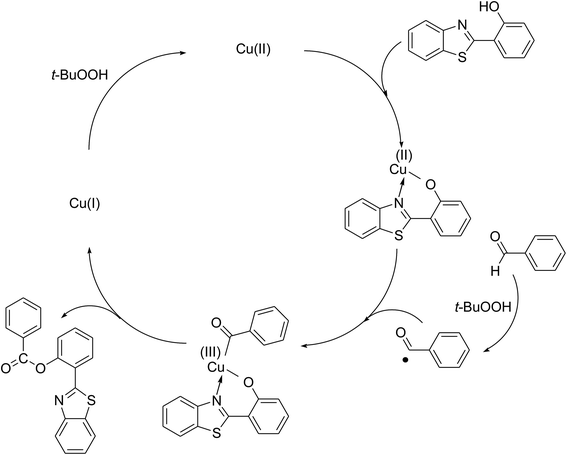
To achieve more information for the possible pathway of the cross dehydrogenative coupling reaction between 2-(benzo[d]thiazol-2-yl)phenol and benzaldehyde to produce 2-(benzo[d]thiazol-2-yl)phenyl benzoate, additional mechanistic studies were then performed. The reaction was conducted in p-xylene at 80 °C for 24 h, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant, at 5 mol% catalyst, with 3 equivalents of benzaldehyde. It was noted that 36% yield was recorded for the first 4 hour reaction time. After that, (2,2,6,6-tetramethylpiperidin-1-yl)oxy (TEMPO) or curcumin as a radical trapping reagent was added to the reactor, and the mixture was consequently stirred at 80 °C for an additional 20 h. Under these conditions, 36% yield was detected (Fig. 2). In another experiment series, TEMPO or curcumin was added to the reaction mixture at the beginning of the reaction. It was noticed that less than 3% yield of 2-(benzo[d]thiazol-2-yl)phenyl benzoate was detected after 24 h. These data suggested that the antioxidant would decompose the radicals produced in the catalytic cycle, thus terminating the transformation. Based on these observations and the literature,10,11 a possible reaction pathway was suggested (Scheme 2). Chelation of 2-(benzo[d]thiazol-2-yl)phenol to the copper sites on the catalyst afforded a cyclometalated intermediate. Next, oxidative addition of acyl radical formed a copper(III) species, which consequently underwent the reductive elimination step to produced the desired product and copper(I) sites. Finally, the oxidation of copper(I) species by tert-butyl hydroperoxide regenerated the catalyst.
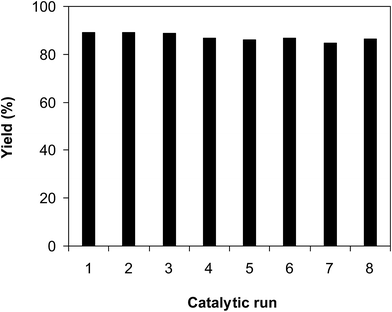
As noted earlier, the CuFe2O4 superparamagnetic nanoparticles were more active towards the cross dehydrogenative coupling reaction between 2-(benzo[d]thiazol-2-yl)phenol and benzaldehyde to produce 2-(benzo[d]thiazol-2-yl)phenyl benzoate than numerous homogeneous and heterogeneous catalysts. To additionally highlight the meaning of employing this catalyst, the simplicity of catalyst recycling was then explored over 8 successive runs. The reaction was conducted in p-xylene at 80 °C for 24 h, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant, at 5 mol% catalyst, with 3 equivalents of benzaldehyde. After each run, the nanoparticles were collected by decantation using a permanent magnet, washed carefully with p-xylene and methanol, heated at 150 °C under vacuum on a Schlenk line for 6 h. The recovered superparamagnetic nanoparticles were consequently reutilized for new catalytic experiment under similar conditions to the first run. It was noticed that the CuFe2O4 nanoparticles could be reutilized many times for the cross dehydrogenative coupling reaction, and its performance as catalyst was maintained throughout the experiment sequences. Certainly, 2-(benzo[d]thiazol-2-yl)phenyl benzoate was produced with 86% yield in the 8th catalytic run (Fig. 3). The fact that the CuFe2O4 nanoparticles could be reutilized many times was accordingly of significance, as compared with organic transformations using conventional homogeneous catalysts.
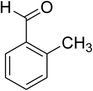
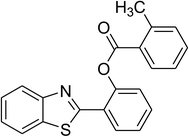
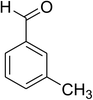
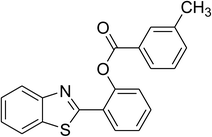
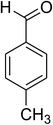
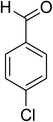
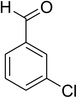
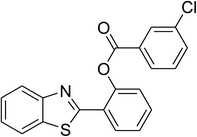
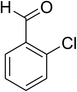
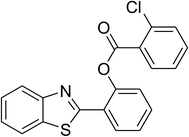
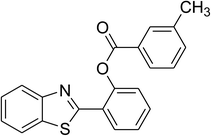
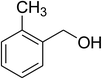
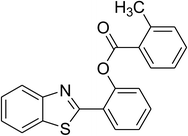
The scope of this protocol was subsequently expanded to the cross dehydrogenative coupling reaction between 2-(benzo[d]thiazol-2-yl)phenol and several benzaldehydes utilizing the CuFe2O4 nanoparticles (Table 3). The reaction was conducted in p-xylene at 80 °C for 24 h, in the presence of 4 equivalents of aqueous tert-butyl hydroperoxide as oxidant, at 5 mol% catalyst, with 3 equivalents of benzaldehyde. The end of the experiment, expected products were purified on silica gel using column chromatography. Under these conditions, 2-(benzo[d]thiazol-2-yl)phenyl benzoate was obtained in 83% isolated yield (entry 1, Table 3). Benzaldehydes containing substituent was also reactive in this transformation. 2-(Benzo[d]thiazol-2-yl)phenyl 2-methylbenzoate (entry 2, Table 3) was produced in 82% yield for the case of 2-methylbenzaldehyde. Similarly, 2-(benzo[d]thiazol-2-yl)phenyl 3-methylbenzoate (entry 3, Table 3) and 2-(benzo[d]thiazol-2-yl)phenyl 4-methylbenzoate (entry 4, Table 3) were achieved in 88% and 84%, respectively, for the case of 3-methylbenzaldehyde and 4-methylbenzaldehyde. Moving to 4-chlorobenzaldehyde, the reaction generated 2-(benzo[d]thiazol-2-yl)phenyl 4-chlorobenzoate (entry 5, Table 3) in 70% yield, while 2-(benzo[d]thiazol-2-yl)phenyl 3-chlorobenzoate (entry 6, Table 3) and 2-(benzo[d]thiazol-2-yl)phenyl 2-chlorobenzoate (entry 7, Table 3) were formed in 74% and 71% yields for the case of 3-chlorobenzaldehyde and 2-chlorobenzaldehyde, respectively.
| Entry | Reactant 1 | Reactant 2 | Products | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-(benzo[d]thiazol-2-yl)phenol (0.25 mmol); benzaldehydes (0.75 mmol); p-xylene (0.5 mL); tert-butyl hydroperoxide in water (1 mmol); CuFe2O4 (0.0125 mmol); 80 °C; 24 h.b Isolated yield. | ||||
| 1 | 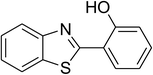 |
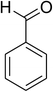 |
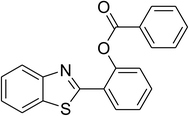 |
83 |
| 2 | 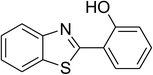 |
 |
 |
82 |
| 3 | 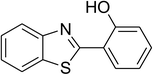 |
 |
 |
88 |
| 4 | 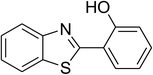 |
 |
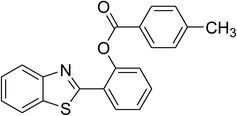 |
84 |
| 5 | 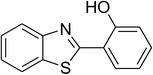 |
 |
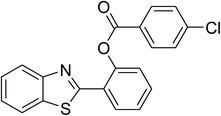 |
70 |
| 6 | 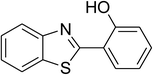 |
 |
 |
74 |
| 7 | 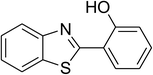 |
 |
 |
71 |
| 8 | 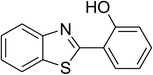 |
 |
 |
83 |
| 9 | 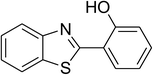 |
 |
 |
73 |
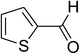
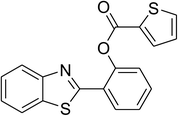
| 10 | 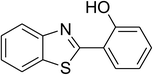 |
 |
 |
56 |
| 11 | 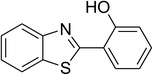 |
 |
 |
59 |
| 12 | 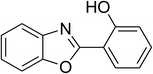 |
 |
 |
55 |
| 13 | 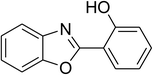 |
 |
 |
75 |
| 14 | 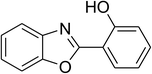 |
 |
 |
59 |
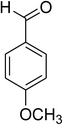
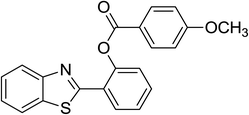
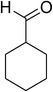
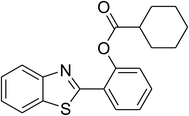
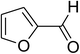
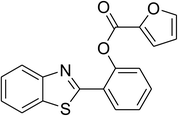
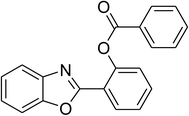
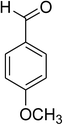
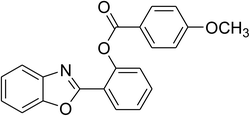
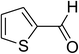
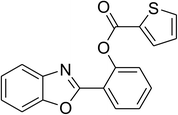
Similarly, the reaction between 2-(benzo[d]thiazol-2-yl)phenol and 4-methoxybenzaldehyde afforded 2-(benzo[d]thiazol-2-yl)phenyl 4-methoxybenzoate (entry 8, Table 3) in 83% yield. Interestingly, cyclohexanecarbaldehyde was reactive towards the cross dehydrogenative coupling reaction. Under these conditions, 2-(benzo[d]thiazol-2-yl)phenyl cyclohexanecarboxylate (entry 9, Table 3) was produced in 73% yield. Heteroaromatic aldehydes were found to be less reactive towards the reaction utilizing the CuFe2O4 superparamagnetic nanoparticles as catalyst. Indeed, the reaction between 2-(benzo[d]thiazol-2-yl)phenol and thiophene-2-carbaldehyde proceeded with more difficulty, though 56% yield of 2-(benzo[d]thiazol-2-yl)phenyl thiophene-2-carboxylate (entry 10, Table 3) was obtained. Similarly, 2-(benzo[d]thiazol-2-yl)phenyl furan-2-carboxylate (entry 11, Table 3) was achieved for the case of furan-2-carbaldehyde. As shown in the proposed mechanism (Scheme 2), the benzothiazole moiety worked as a directing group for the transformation. We then decided to explore the cross dehydrogenative reaction of phenols containing benzoxazole moiety. It was noticed that 2-(benzo[d]oxazol-2-yl)phenol was also reactive towards the reaction, confirming the importance of the benzoxazole moiety. Under similar reaction conditions, the coupling of 2-(benzo[d]oxazol-2-yl)phenol with benzaldehyde afforded 2-(benzo[d]oxazol-2-yl)phenyl benzoate (entry 12, Table 3) in 55% yield. Moving to 4-methoxybenzaldehyde, the transformation generated 2-(benzo[d]oxazol-2-yl)phenyl 4-methoxybenzoate (entry 13, Table 3) in 75% yield. Similarly, 2-(benzo[d]oxazol-2-yl)phenyl thiophene-2-carboxylate (entry 14, Table 3) was produced in 59% yield for the case of thiophene-2-carbaldehyde.
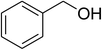
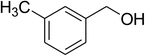
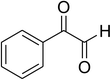
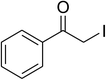
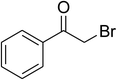
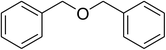
Inspired by these observations, we then expanded the research scope to other substrates. As mentioned in Scheme 2, the acyl radical was produced in the catalytic cycle. Therefore, benzyl alcohol was tested in place of benzaldehyde. It was noticed that benzyl alcohol was less reactive towards the cross dehydrogenative coupling reaction with 2-(benzo[d]thiazol-2-yl)phenol than benzaldehyde. Nevertheless, performing the experiment at 100 °C in the presence of 10 mol% catalyst, reasonable yields was obtained (Table 4). Moving to benzyl alcohol, 60% yield of 2-(benzo[d]thiazol-2-yl)phenyl benzoate (entry 1, Table 4) was achieved. Similarly, 2-(benzo[d]thiazol-2-yl)phenyl 2-methylbenzoate (entry 2, Table 4), 2-(benzo[d]thiazol-2-yl)phenyl 3-methylbenzoate (entry 3, Table 4), 2-(benzo[d]thiazol-2-yl)phenyl 4-methylbenzoate (Table 4, entry 4), and 2-(benzo[d]thiazol-2-yl)phenyl 4-chlorobenzoate (entry 5, Table 4) were obtained in 65%, 63%, 75%, and 66% yields, respectively. We found that dibenzyl ether could be used in place of benzaldehyde or benzyl alcohol. Certainly, the reaction using dibenzyl ether afforded 2-(benzo[d]thiazol-2-yl)phenyl benzoate (entry 6, Table 4) in 60% yield. Interestingly, 2-oxo-2-phenylacetaldehyde was also reactive in this reaction, producing 2-(benzo[d]thiazol-2-yl)phenyl benzoate (entry 7, Table 4) in 73% yield. Moreover, it was found that 2-iodo-1-phenylethanone could be used in place of benzaldehyde, and 2-(benzo[d]thiazol-2-yl)phenyl benzoate (entry 8, Table 4) was achieved in 75% yield. 2-Bromo-1-phenylethanone (entry 9, Table 4) was less reactive than 2-iodo-1-phenylethanone, though the desired product was achieved in 69% yield. Interestingly, 1-phenylethane-1,2-diol (entry 10, Table 4) and (dimethoxymethyl)benzene (entry 11, Table 4) could also be alternative substrates, affording 2-(benzo[d]thiazol-2-yl)phenyl benzoate in 80% and 68% yields, respectively. As expected, 2-(benzo[d]oxazol-2-yl)phenol was also reactive toward these reactions (entries 12–14, Table 4).
| Entry | Reactant 1 | Reactant 2 | Products | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-(benzo[d]thiazol-2-yl)phenol (0.25 mmol); second substrate (0.75 mmol); p-xylene (0.5 mL); tert-butyl hydroperoxide in water (1 mmol); CuFe2O4 (0.025 mmol); 100 °C; 24 h.b Isolated yield. | ||||
| 1 | 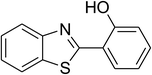 |
 |
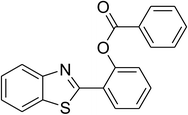 |
60 |
| 2 |  |
 |
 |
65 |
| 3 | 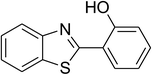 |
 |
 |
63 |
| 4 | 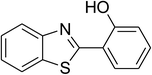 |
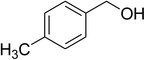 |
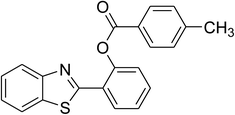 |
75 |
| 5 | 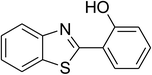 |
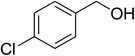 |
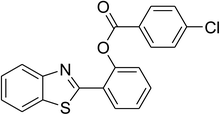 |
66 |
| 6 | 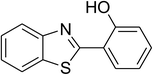 |
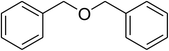 |
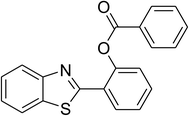 |
60 |
| 7 | 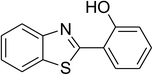 |
 |
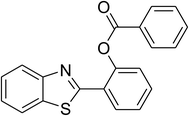 |
73 |
| 8 | 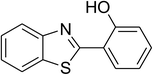 |
 |
 |
75 |
| 9 | 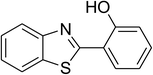 |
 |
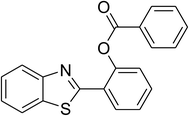 |
69 |
| 10 | 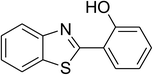 |
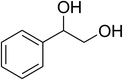 |
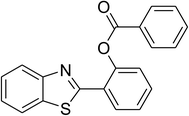 |
80 |
| 11 | 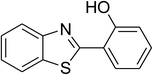 |
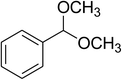 |
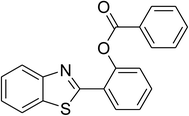 |
68 |
| 12 | 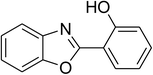 |
 |
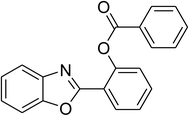 |
51 |
| 13 | 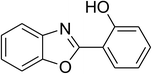 |
 |
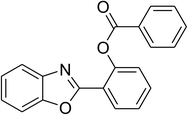 |
52 |
| 14 | 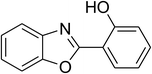 |
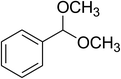 |
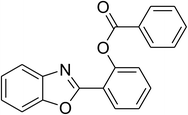 |
60 |
4. Conclusions
CuFe2O4 superparamagnetic nanoparticles exhibited high catalytic efficiency in the direct synthesis of chemical structures containing both phenol ester and benzothiazole moieties via cross dehydrogenative coupling reactions. Several substrates were reactive towards the transformation in the presence of the nano catalyst, including benzaldehyde, benzyl alcohol, dibenzyl ether, 2-oxo-2-phenylacetaldehyde, and 2-iodo-1-phenylethanone. To our best knowledge, these reactions were not previously reported in the literature. The CuFe2O4 superparamagnetic nanoparticles were more active than nano NiFe2O4, nano CoFe2O4, and nano Fe2O3. Moreover, the nano CuFe2O4 catalyst also offered higher catalytic efficiency than numerous conventional homogeneous catalysts. At the end of each catalytic reaction, the nano CuFe2O4 was isolated from the reaction mixture by utilizing a magnet. It was possible to reutilize the recovered catalyst numerous times for the cross dehydrogenative coupling reaction without a remarkable deterioration in catalytic performance. The fact that chemical structures containing both phenol ester and benzothiazole moieties were achieved using a recyclable nano catalyst was accordingly of significance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Viet Nam National University-Ho Chi Minh City (VNU-HCM) for financial support.References
- M. Neurock, Z. Tao, A. Chemburkar, D. D. Hibbitts and E. Iglesia, Faraday Discuss., 2017, 197, 59–86 RSC.
- M. Zhou, P. Liu, K. Wang, J. Xu and J. Jiang, Catal. Sci. Technol., 2016, 6, 7783–7792 CAS.
- E. M. Gayakwad, V. V. Patil and G. S. Shankarling, New J. Chem., 2017, 41, 2695–2701 RSC.
- G. Majji, S. K. Rout, S. Rajamanickam, S. Guin and B. K. Patel, Org. Biomol. Chem., 2016, 14, 8178–8211 CAS.
- S. Gaspa, A. Porcheddu and L. D. Luca, Org. Lett., 2015, 17, 3666–3669 CrossRef CAS PubMed.
- J. Gong, L. Huang, Q. Deng, K. Jie, Y. Wang, S. Guo and H. Cai, Org. Chem. Front., 2017, 4, 1781–1784 RSC.
- F. Penteado, M. M. Vieira, G. Perin, D. Alves, R. G. Jacob, C. Santi and E. J. Lenardão, Green Chem., 2016, 18, 6675–6680 RSC.
- W. Fan, C. Sun, F. Huang, J. Liu, X. Zhao, X. Sheng and D. Chen, Dalton Trans., 2017, 46, 5288–5296 RSC.
- V. Gauchot, D. R. Sutherland and A.-L. Lee, Chem. Sci., 2017, 8, 2885–2889 RSC.
- W. Ali, S. K. Rout, S. Guin, A. Modi, A. Banerjee and B. K. Patel, Adv. Synth. Catal., 2015, 357, 515–522 CrossRef CAS.
- Y. Zheng, W.-B. Song and L.-J. Xuan, Org. Biomol. Chem., 2015, 13, 10834–10843 CAS.
- F. Gao, D. Mei, Y. Wang, J. Szanyi and C. H. F. Peden, J. Am. Chem. Soc., 2017, 139, 4935–4942 CrossRef CAS PubMed.
- L. Zhu, X.-Q. Liu, H.-L. Jiang and L.-B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- K. P. Bryliakov, Chem. Rev., 2017, 117, 11406–11459 CrossRef CAS PubMed.
- A. Chatterjee and V. R. Jensen, ACS Catal., 2017, 7, 2543–2547 CrossRef CAS.
- P. Veerakumar, P. Thanasekaran, K.-L. Lu, S.-B. Liu and S. Rajagopal, ACS Sustainable Chem. Eng., 2017, 5, 6357–6376 CrossRef CAS.
- Y. Wang, T. Len, Y. Huang, A. D. Taboada, A. N. Boa, C. Ceballos, F. Delbecq, G. Mackenzie and C. Len, ACS Sustainable Chem. Eng., 2017, 5, 392–398 CrossRef CAS.
- T. Chen, S. Chen, P. Song, Y. Zhang, H. Su, W. Xu and J. Zeng, ACS Catal., 2017, 7, 2967–2972 CrossRef CAS.
- H. Duan, Y. Zeng, X. Yao, P. Xing, J. Liu and Y. Zhao, Chem. Mater., 2017, 29, 3671–3677 CrossRef CAS.
- S. Cao, F. F. Tao, Y. Tang, Y. Li and J. Yu, Chem. Soc. Rev., 2016, 45, 4747–4765 RSC.
- J. A. Delgado, O. Benkirane, C. Claver, D. Curulla-Ferré and C. Godard, Dalton Trans., 2017, 46, 12381–12403 RSC.
- C. Parmeggiani, C. Matassini and F. Cardona, Green Chem., 2017, 19, 2030–2050 RSC.
- R. K. Sharma, S. Dutta, S. Sharma, R. Zboril, R. S. Varma and M. B. Gawande, Green Chem., 2016, 18, 3189–3209 RSC.
- F. M. Moghaddam, G. Tavakoli, F. Latifi and B. Saeednia, Catal. Commun., 2016, 75, 37–41 CrossRef CAS.
- Y. Liu, L. Li, S. Liu, C. Xie and S. Yu, J. Mol. Catal. A: Chem., 2016, 424, 269–275 CrossRef CAS.
- Y. Liu, L. Li, C. Xie, S. Yu and S. Liu, Chem. Eng. J., 2016, 303, 31–36 CrossRef CAS.
- M. J. Nasab and A. R. Kiasat, RSC Adv., 2016, 6, 41871–41877 RSC.
- J. Zhang, T. Yao, H. Zhang, X. Zhang and J. Wu, Nanoscale, 2016, 8, 18693–18702 RSC.
- C. K. Nguyen, N. N. Nguyen, K. N. Tran, V. D. Nguyen, T. T. Nguyen, D. T. Le and N. T. S. Phan, Tetrahedron Lett., 2017, 58, 3370–3373 CrossRef CAS.
- Y. Zheng, W.-B. Song and L.-J. Xuan, Tetrahedron Lett., 2015, 56, 4569–4573 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra11706j |
| This journal is © The Royal Society of Chemistry 2017 |