Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of artificial chaperones in a novel type of Pickering emulsion for glycoprotein†
Tao Zhao ,
Wenjun Wen,
Junping Wang
,
Wenjun Wen,
Junping Wang * and
Shuo Wang
* and
Shuo Wang
Key Laboratory of Food Nutrition and Safety, Ministry of Education, Tianjin University of Science & Technology, 29 The Thirteenth Road, Tianjing Economy and Technology Development Area, Tianjin, 300457, PR China. E-mail: wangjp@tust.edu.cn; wangshuo@nankai.edu.cn; Fax: +86 22 60601332; Fax: +86 22 83534450; Tel: +86 22 6060145 Tel: +86 22 83534450
First published on 22nd November 2017
Abstract
Novel particles of poly(DVB-co-PBA) were synthesized by a one-step solvothermal route instead of distillation precipitation polymerization (DPP), which was simple to operate. The poly(DVB-co-PBA) showed excellent versatility in the preparation of functional materials. On the one hand, it could be applied to the preparation of a sugar fluorescent sensor due to the features of molecular recognition of cis-diol-containing compounds and fluorescence; on the other hand, it showed flexibility in the preparation of a Pickering emulsion. Further, the core–shell microspheres named W-type and W-II type were synthesized in emulsions of water-in-toluene and water-in-chloroform with stabilizers of poly(DVB-co-PBA), respectively. The core–shell microspheres named O-type were synthesized in the emulsion of oil-in-water with stabilizers poly(DVB-co-PBA)@L-Cys. The W-type polymer microspheres were used as thermally stable materials for the glycoprotein of horseradish peroxidase (HRP) to study the change of conformation and catalytic activity of HRP under heat stress through methods of UV-vis spectrophotometry, circular dichroism spectroscopy, ANS probe, chromogenic substrates, FITC fluorescence labelling, TEM and enzyme catalysis reaction. The results showed the W-type polymer microspheres with a core of hydrophilic/hydrophobic moieties reaching 2/1 could effectively maintain the natural conformation of HRP and longer catalytic activity under heat stress.
Introduction
Denaturation and aggregation of protein is the most acute problem in the utilization of protein.1–3 Protein is vulnerable to environmental impact in vitro. In the conditions of extreme pH4, heating,5 metal ions6 denaturants and organic solvents,7 the structure of protein changes and hydrophobic groups are exposed leading to irreversible denaturation. Therefore, protein protective materials that maintain the natural conformation and inhibit the aggregation of protein are of great significance.Many elegant works have been proposed for protein protective materials, such as surfactants,8 polymer microspheres,9 polymer nanoparticles,10 polymer micelles11 cyclodextrin12 and amphiphilic polysaccharides.13 Surfactants could form reverse micelles in the phases of ethanol and water; the interior of the micelles formed a water core which could create an environment for protein refolding.14 Although there were various types of surfactants, fewer surfactants were suitable for protein refolding and the surfactants were difficult to remove. Polymer microspheres and polymer nanomaterials have received increasing concerns in protein protection and refolding. For instance, Masahiko and co-workers15 have synthesized polymer nanoparticles (NPs) capable of facilitating resolubilization and refolding of an aggregated protein of positively charged lysozyme. The NPs could be prepared by copolymerizing optimized combinations and populations of functional monomers. Polymer micelles could be used as a substitute for surfactants to promote protein refolding. Huang and co-workers16 have synthesized the mixed-shell polymeric micelles (MSPMs) which were used as a suppressor of Alzheimer's disease. The balance of hydrophilic/hydrophobic moieties on the surface of MSPMs was important for their enhanced therapeutic effect. The amphiphilicity of cyclodextrin and polysaccharides was very important for the refolding of the protein, especially the balance between the hydrophilic and hydrophobic properties played a key role in inhibiting the aggregation of protein monomers.17 Pickering emulsion as a two-phase or multi-phase system, which has a great advantage in the preparation of the amphiphilic materials. However, there were few reports on the preparation of artificial chaperones in Pickering emulsions.
Pickering emulsion is an emulsion that stabilized by solid particles in place of surfactants.18 In the past studies, inorganic particles and organic particles of a single component were used as stabilizers for Pickering emulsions, such as silica,19 LAPONITE® clay,20 zinc oxide,21 titania22 and polystyrene latex particles.23 There were limited reports on the organic hybrid particles as stabilizers. Multi-component organic particles endow the Pickering emulsion more features for the preparation of versatile hybrid materials, especially in the preparation of amphiphilic materials for protein protection.
In this study, three types of Pickering emulsion were first prepared based on the stabilizers of poly(DVB-co-PBA) microspheres which were synthesized by the method of distillation precipitation polymerization (DPP).24 To demonstrate the flexibility of the multi-component organic particles microspheres of poly(DVB-co-PBA) in the preparation of hybrid materials, three hybrid polymer microspheres of core–shell structure were prepared in three types of emulsions by Pickering emulsion polymerization, respectively. The amphiphilic polymer microspheres of similar pine cone structure were used as thermal stabilizers to study the change of conformation and catalytic activity of horseradish peroxidase (HRP) under heat stress.
Experimental
Materials
Divinylbenzene (DVB, 80%, technical grade, mixture of isomers) was purchased from Sigma-Aldrich (Germany), 2,2′-azodiisobutyronitrile (AIBN), acrylamide (AM), N,N,N′,N′-tetramethylethylenediamine (TEMED), benzoylperoxide, N,N′-methylenebis(acrylamide) (MBAA), 4-vinylphenylboronic acid, N-isopropylacrylamide (NIPAAm), fluorescein isothiocyanate (FITC), guaiacol and 8-anilino-1-naphthalenesulfonic acid ammonium salt (ANS-NH4) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), horseradish peroxidase (HRP), L-cysteine and ammonium persulfate (APS) were purchased from Sigma-Aldrich (USA).Synthesis of poly(DVB-co-PBA) particles by DPP
The poly(DVB-co-PBA) particles were first fabricated by the DPP technique. In detail, the monomers, 4-vinyl phenylboronic acid (1.48 g, 10 mmol) and divinylbenzene (1.30 g, 10 mmol), and initiator AIBN (60 mg, 0.37 mmol) were dissolved in 100 mL of acetonitrile in around-bottom flask. The mixture was purged with pure N2 for 10 min. And then the Dean–Stark receiver was connected to the flask. After that the flash heated to 70 °C until the reaction solution appears cloudy in an oil bath. The polymerized reaction was proceeded under reflux with the gradually increase of the oil bath to 110 °C. The reaction was stopped when half of the reaction solvent was distilled through the Dean–Stark receiver within 2 h. The flask was removing from the oil bath. The resultant product was filtered with a sand core funnel of G5, and then the products were washed with methanol for three times to remove the residual oligomers and monomers, then vacuum drying.Synthesis of poly(DVB-co-PBA) particles by one-step solvothermal
The poly(DVB-co-PBA) particles were fabricated by the solvothermal method. In detail, the monomers, 4-vinyl phenylboronic acid (735 mg, 5 mmol) and DVB (710 μL, 5 mmol), and initiator AIBN (30 mg) were dissolved in 80 mL of acetonitrile in reaction kettle. The mixture was purged with pure N2 for 10 min. The polymerized reaction was carried out at 110 °C for 8 h. The resultant product was filtered with a sand core funnel of G5, and then the product was washed with methanol for three times to remove the residual oligomers and monomers, then vacuum drying.Synthesis of poly(DVB-co-PBA)@L-Cys particles
The poly(DVB-co-PBA) particles (100 mg), L-cysteine (50 mg) and AIBN (5 mg), were dispersed in the ethanol–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) mixture in a round-bottomed flask. After purged with pure N2 for 10 min, the mixture was heated to 70 °C for 24 h in an oil bath. When the flask was removed from the oil bath, the reaction was stopped. The product was washed repeatedly with ethanol–water mixture and the pure poly(DVB-co-PBA)@L-Cys particles were collected by centrifugation.
3, v/v) mixture in a round-bottomed flask. After purged with pure N2 for 10 min, the mixture was heated to 70 °C for 24 h in an oil bath. When the flask was removed from the oil bath, the reaction was stopped. The product was washed repeatedly with ethanol–water mixture and the pure poly(DVB-co-PBA)@L-Cys particles were collected by centrifugation.
Synthesis of W-type polymer microspheres
The poly(DVB-co-PBA) particles (200 mg) were dissolved in 15 mL toluene as an oil phase, and then ultrasound for 10 minutes. AM (150 mg), MBAA (10 mg) and NIPAAm (50 mg) were dissolved in 5 mL DDW as a water phase. After mixing the two phases, shaking vigorously for 5 min by hand. The initiator of APS (10 mg) and TEMED (20 μL) were added to emulsion, the polymerization reaction was carried out at 30 °C for 24 h. After polymerization, the solvent was removed by decantation. The resultant product was filtered with a sand core funnel of G5, and then the products were washed with methanol for three times to remove the residual oligomers and monomers, then vacuum drying.Synthesis of W-II type polymer microspheres
The poly(DVB-co-PBA) particles (50 mg) were dissolved in 15 mL chloroform as an oil phase, and then ultrasound for 10 minutes. AM (150 mg), MBAA (10 mg) and NIPAAm (50 mg) were dissolved in 10 mL DDW as a water phase. After mixing the two phases, shaking vigorously for 5 min by hand. The initiator of APS (10 mg) and TEMED (20 μL) were added to emulsion, the polymerization reaction was carried out at 30 °C for 24 h. The purification process was consistent with the above.Synthesis of O-type polymer microspheres
The poly(DVB-co-PBA) @L-Cys (20 mg) was dissolved in 3 mL distilled water (DDW) as a water phase, the initiator of benzoylperoxide (4.0 mg) was dissolved in 1.5 mL divinylbenzene as an oil phase. After mixing the two phases, shaking vigorously for 5 min by hand, polymerization was conducted for 14 hours at room temperature. After polymerization, the resultant product was filtered with a sand core funnel of G5, and then the products were washed with methanol for three times to remove the residual oligomers and monomers, then vacuum drying.Result and discussion
Synthesis and characterization of poly(DVB-co-PBA)
The poly(DVB-co-PBA) microspheres were first synthesized by the method of distillation precipitation polymerization (DPP).18 To simplify the synthesis process, one-step solvothermal was used to prepare the poly(DVB-co-PBA) microspheres instead of the method of DPP. The one-step solvothermal which used the begin time as clear criteria for the initial of the polymerization reaction was easier to operate compared to the method of DPP. The poly(DVB-co-PBA) microspheres with good morphology (Fig. S1†) were fabricated by one-step solvothermal.Fig. 1(A) was the FT-IR spectrum of the polymer, the peaks of 3226 and 1342 cm−1 were the stretching of the –OH of the boronic acid group, the peak of 3018 and 1608 cm−1 were the C–H bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the benzene ring, respectively. The feature of 2923 cm−1 was C–H of the methylene of straight chain (–CH2–), the feature of 2237 cm−1 was B–O bond, the feature of 1803 and 1922 cm−1 were C–H and C–C vibrations (R–CH
C of the benzene ring, respectively. The feature of 2923 cm−1 was C–H of the methylene of straight chain (–CH2–), the feature of 2237 cm−1 was B–O bond, the feature of 1803 and 1922 cm−1 were C–H and C–C vibrations (R–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) of straight chain, respectively. The results indicated that poly (DVB-co-PBA) has been successfully synthesized. Fig. 1(B) show the UV-visible absorption spectra and emission spectrum of poly(DVB-co-PBA). The polymer microspheres have the maximum UV absorption peak at 258 nm. The fluorescence emission spectra of the polymer microspheres were obtained by using 258 nm as the excitation wavelength. The maximum emission wavelength was found at 323.5 nm.
CH) of straight chain, respectively. The results indicated that poly (DVB-co-PBA) has been successfully synthesized. Fig. 1(B) show the UV-visible absorption spectra and emission spectrum of poly(DVB-co-PBA). The polymer microspheres have the maximum UV absorption peak at 258 nm. The fluorescence emission spectra of the polymer microspheres were obtained by using 258 nm as the excitation wavelength. The maximum emission wavelength was found at 323.5 nm.
 | ||
| Fig. 1 FT-IR spectrum (A), the UV-visible absorption spectra and emission spectrum (B) of poly(DVB-co-PBA) microsphere. | ||
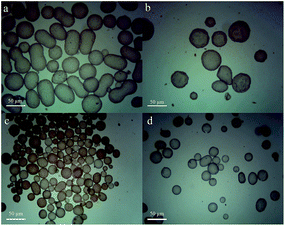
The results in Fig. 2a and b indicated that the morphology of the polymer microspheres was structured. The size of poly(DVB-co-PBA) microspheres ranged from 1 to 2 μm. The effective diameter was 1385.3 nm (Fig. S2†). The characterization aspects of carbohydrate affinity and TGA were detailed in ESI (Fig. S3 and S4†).
The poly(DVB-co-PBA) as stabilizer of Pickering emulsion
The synthetic poly(DVB-co-PBA) microspheres as stabilizer had some new features compared to the inorganic particles and organic particles of a single component. On one hand, due to the hydrophilicity and carbohydrate affinity of the boronic acid groups, the poly(DVB-co-PBA) microspheres were more hydrophilic than the polystyrene microspheres. The contact angle of water droplet was 52.7° (Fig. S5†). The poly(DVB-co-PBA) microspheres were easier to form Pickering emulsions without the addition of other chemical agents in conventional organic reagents, which could form two kinds of water-in-oil Pickering emulsions of water-in-toluene and water-in-chloroform. The phenylboronic acid as carbohydrate affinity agent, which endow the poly(DVB-co-PBA) microsphere the feature of molecular recognition of cis-diol-containing compounds.25,26 On the other hand, the synthetic poly(DVB-co-PBA) microspheres were susceptible to chemical modification. We used the poly(DVB-co-PBA) @L-Cys microspheres as the stabilizers for the preparation of a type of oil-in-water Pickering emulsion.Preparation of the core–shell microspheres in the water-in-toluene emulsion
In the system of water-in-toluene, acrylamide (AM), N-isopropylacrylamide (NIPAAm) and N,N′-methylenebis(acrylamide) were dissolved in distilled water (DDW) as the aqueous phase, the poly(DVB-co-PBA) microspheres were added to toluene as the oil phase, the water-in-toluene Pickering emulsion was prepared by mixing the two phases, and then the initiator was added to Pickering emulsion for polymerization. Finally, we obtained a type of amphiphilic particle with core–shell structure after polymerization. The surface was composed of hydrophobic particles of poly(DVB-co-PBA) microspheres and the core was composed of a hydrophilic hydrogel (Fig. 2c and d). This type of the core–shell microsphere was referred simply as W-type.Preparation of the core–shell microspheres in the water-in-chloroform emulsion
In the system of water-in-chloroform, we tried to use the corrosion characteristic of chloroform to reduce the size of the surface microspheres and improve its porosity. A novel type of amphiphilic core–shell structure microsphere which had a nanoscale surface was fabricated by Pickering emulsion polymerization (Fig. 2e and f). This type of the core–shell microspheres was referred simply as W-II type.Preparation of the core–shell microspheres in the oil-in-water emulsion
For further study, L-cysteine was grafted on the surface of the poly(DVB-co-PBA) microspheres by “click chemistry”.27 We used the poly(DVB-co-PBA)@L-Cys as stabilizers to prepare an oil-in-water Pickering emulsion. We used the oil-in-water Pickering emulsion to prepare a novel core–shell strong hydrophobic polymer microsphere that had hydrophobic core of poly(DVB) and was enveloped by the shell of poly(DVB-co-PBA)@L-Cys particles by Pickering emulsion polymerization (Fig. 2g and h). This type of the core–shell microspheres was referred simply as O-type.The synthetic poly(DVB-co-PBA) microspheres showed versatile for the preparation of Pickering emulsion. Three types of microspheres of core–shell structure with different morphology and properties were prepared by Pickering emulsion polymerization. The W-type microspheres with well-arranged structure undergo a conformational switching by water absorbing swelling (Fig. S7†). The W-II type which had a nanoscale surface could be stable in the water for a long time without damage, the reason may be the chloroform corrosiveness enhancing the binding between the core and the shell (Fig. S8†). The O-type microspheres that had many “gully” structures in the surface may be used as a good adsorbent.
What is more, the core in the microspheres of core–shell structure could be prepared by copolymerizing optimized combinations and populations of functional monomers, which endow the hybrid materials more features. The synthesis process of the three types of core–shell microspheres were detailed in ESI (Fig. S6 and S9†).
The protective function of W-type polymer microspheres for natural structure of HRP
We used the W-type microsphere as thermal stabilizers to study the change of conformation and catalytic activity of HRP under heat stress.The balance of hydrophilic/hydrophobic moieties is important for maintaining protein conformation. Acrylamide as a hydrophilic functional monomer and N-isopropylacrylamide as a hydrophobic functional monomer, the balance of hydrophilic/hydrophobic moieties of W-type materials was controlled by changing the proportions of acrylamide (AM) and N-isopropylacrylamide (NIPAAm) in the core of the W-type microspheres. Four types of the W-type microspheres of the different proportions of AM and NIPAAm (the molar ratio of AM/NIPAAm: all AM, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, all NIPAAm). The synthesis process is detailed in ESI (Table S1†). The materials of all AM and 4
1, all NIPAAm). The synthesis process is detailed in ESI (Table S1†). The materials of all AM and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used as the hydrophilic control groups, the materials of 2
1 were used as the hydrophilic control groups, the materials of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used as the neutral control groups, the materials of all NIPAAm and the O-type microspheres were used as the hydrophobic control groups.
1 were used as the neutral control groups, the materials of all NIPAAm and the O-type microspheres were used as the hydrophobic control groups.
The analysis of UV-vis spectrophotometer
The groups of adding W-type microspheres could maintain good UV-vis peak of horseradish peroxidase (HRP) compared with the blank group after the adsorption process of a long time (Fig. S11†). In consideration of this phenomenon, we assumed that W-type microspheres could maintain the conformation of the protein. To evaluate the properties of the W-type microspheres for maintaining protein conformations stability, HRP was used as a test protein and the W-type microspheres of different proportions of AM and NIPAMM were used as thermal stabilizers through the methods of circular dichroism spectrum (CD), ANS probe, chromogenic substrates, and FITC fluorescence label under heating conditions. Despite a lot of elegant works on the thermal stability materials for protein, there were few reports about the microspheres of core–shell structure for maintaining thermal stability of glycoprotein.The analysis of circular dichroism (CD)
To evaluate the capability of the W-type polymer microspheres for maintaining the natural conformation of protein, horseradish peroxidase (HRP) solution (1 mg mL−1, pH = 7.4, PBS buffer) with different types of materials were heated at 60 °C for 30 min, then the solution was filtered with 0.22 μm water filters and determined by using CD spectrophotometer.The contents of the secondary structure of the horseradish peroxidase in the group of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was similar to the group of untreated HRP (Table 1). For the strong hydrophilic groups (all AM, 4
1 was similar to the group of untreated HRP (Table 1). For the strong hydrophilic groups (all AM, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the strong hydrophobic groups (all NIPAAm), the capability of maintaining protein conformation under heat stress was weak. Especially when the hydrophobicity was too strong (O-type, poly(DVB-co-PBA)), the hydrophobic force could cause irreversible denaturation.
1) and the strong hydrophobic groups (all NIPAAm), the capability of maintaining protein conformation under heat stress was weak. Especially when the hydrophobicity was too strong (O-type, poly(DVB-co-PBA)), the hydrophobic force could cause irreversible denaturation.
| Result | Helix | Strand | Turns | Unordered |
|---|---|---|---|---|
| Untreated HRP | 0.349 | 0.112 | 0.233 | 0.319 |
| Denatured HRP | 0.209 | 0.247 | 0.195 | 0.382 |
| All AM | 0.209 | 0.242 | 0.191 | 0.370 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.130 | 0.252 | 0.180 | 0.400 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.347 | 0.119 | 0.247 | 0.330 |
| All NIPAAm | 0.805 | 0.006 | 0.066 | 0.160 |
| O-type | 0.800 | 0.006 | 0.071 | 0.169 |
| Poly(DVB-co-PBA) | 0.793 | 0.007 | 0.075 | 0.162 |
The results indicated that the W-type microspheres could maintain the secondary structure of glycoprotein in the heating conditions only when the hydrophilic/hydrophobic moieties reached a certain equilibrium. There were two reasons that may lead to this result. On one hand, the protein monomers through the hydrophobic interaction to form the natural structure, the materials that lack of hydrophobic force were not conducive for protein refolding. On the other hand, the hydrophilic environment provided a living environment for the protein, too strong hydrophobic effect would denature the protein and accelerate irreversible aggregation.
The analysis of ANS probe
To further verify the capability of the W-type microspheres for maintaining the conformation of HRP under heating conditions. ANS probe which could be combined into the exposed hydrophobic region of the protein in water was utilized to characterize part of unfolded intermediates of protein.The ANS probe has a maximum excitation wavelength at 350 nm and a maximum emission wavelength at 514 nm when present alone. However, the maximum emission wavelength becomes 460 nm when combined into the hydrophobic region of the protein, corresponding to a significant enhancement of the fluorescence intensity. The result in Fig. 3 showed that only the group of (c) and the unheated group of (HRP) did not occur a blue shift. The results indicated that the material in group of (c) had the ability to stable the conformation of protein, which could reduce the hydrophobic area of HRP under heat stress denaturation.
Table 2 showed the changes of the maximum fluorescence emission peak of ANS. The denatured HRP was much like the groups of ALL AM. The result indicated materials that composed of all hydrophilic monomers are less capable of maintaining the native structure of the glycoprotein. Meanwhile, the degree of blue shift of the strong hydrophobic groups (all NIPAAm, poly(DVB-co-PBA)) were the maximum. The results indicated too much hydrophobicity would accelerate the protein denaturation process these results were also consistent with the CD spectrophotometer.
| Result | Wavelength (nm) | Fluorescence intensity (a.u.) |
|---|---|---|
| ANS | 511 | 8458.9 |
| Untreated HRP | 511 | 7897.5 |
| Denatured HRP | 483 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996.6 996.6 |
| All AM | 499 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315.6 315.6 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
491 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308.4 308.4 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
506 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135.9 135.9 |
| All NIPAAm | 479 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385.1 385.1 |
| Poly(DVB-co-PBA) | 479 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647.0 647.0 |
The analysis of chromogenic substrates
To verify that the W-type microspheres could not only sustain the secondary structure of the HRP but also maintain a certain activity of the HRP under heat stress. The HRP-DAB chromogenic reagent was used to evaluate the capability of the material for maintaining the activity of HRP protein under heating conditions.The color effect of group (c) was significantly superior to other control groups (Fig. 4). The results indicated that heat-denatured HRP in group (c) maintained a certain activity. Due to the activity of HRP is closely related to its tertiary structure, the W-type polymer microspheres of group (c) may have the capability of maintaining a certain tertiary structure of HRP under heating conditions.
The analysis of FITC-HRP
For further study, the FITC labeled HRP were used to study the superficial mechanism of thermal protection for glycoprotein. The solution of FITC-HRP were added into the flat bottles with different types of materials and then heated at 60 °C for half an hour. After heating, the mixture was dropped to a slide and observed with a confocal fluorescence microscope.The fluorescence intensity of FITC-HRP were the highest in the group of (c) (Fig. 5c), the results indicated that the W-type polymer microspheres of group (c) had the best capability of maintaining a certain structure of HRP under heating conditions, which were consistent with the other experimental results. The fluorescence intensity of the core of the W-type polymer microspheres was significantly higher than the surface (Fig. 5d). The results indicated that the FITC-HRP entered the internal hydrogel through the channel of the surface during the heating process. The hydrogel core of W-type polymer microspheres was indispensable for thermal protection.
Based on the verified results, we proposed a hypothesis that the W-type polymer microspheres as thermal stabilizers were like a surfactant. The W-type polymer microspheres could form a structure like reverse micelles directly in the aqueous phase. The internal hydrogel as a “water pool” could provide a survival environment for protein, the denatured proteins that were trapped to the internal hydrogel without aggregations.28 The hydrophobic surface of poly(DVB-co-PBA) was comparable to a protective cover, which could effectively reduce the thermal impact on the protein.
The analysis of TEM
The state of heat-denatured HRP in presence of different types of materials were studied by TEM, the process was detailed in ESI.†The result in Fig. S12† showed that the group of unheated HRP (Fig. S12(a)†) did not occur aggregation. But for the heating denaturation HRP without adding materials, many black spots occurred (Fig. S12(b)†). The reason may be that heating denaturation led to aggregation of protein monomers, which resulted in an irreversible deposition. For the groups of c–f, which were added with different types of W-type polymer microspheres, the particle size of HRP was small and no serious aggregation occurred. This result indicated that the materials of W-type had the capability of maintaining a certain structure of HRP under heating conditions. For the hydrophobic groups of f and g, it was found that a small amount of protein was present in the aggregation state. It indicated that strong hydrophobic materials were not conducive to the conformational stability of the protein during the heating process.
The protection for catalytic activity of HRP under heat stress
In order to investigate the catalysis of HRP in presence of the W-type polymer microspheres which were used as thermal stabilizers under heat stress. The catalyzing reaction of H2O2 oxidizing guaiacol as a test reaction. The assay procedure was detailed in ESI.†The results in Fig. 6(a) showed the catalytic activity of the group which is in absence of thermal stabilizers (Fig. 6 (HRP)) entered a linear descend section at 25 minutes of continuous heating, but for the group which is in the presence of thermal stabilizers (Fig. 6 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) was 30 minutes. The results indicated that W-type material could improve the thermostable temperature of the HRP, and which could maintain longer catalytic activity at high temperatures. Fig. 6(b) showed the catalytic activity of HRP after heated at 60 °C for half an hour. The group c (2
1)) was 30 minutes. The results indicated that W-type material could improve the thermostable temperature of the HRP, and which could maintain longer catalytic activity at high temperatures. Fig. 6(b) showed the catalytic activity of HRP after heated at 60 °C for half an hour. The group c (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) had the best ability to stabilize catalytic activity under heating conditions. This result indicated that the W-type microspheres could maintain the catalytic activity of HRP in the heating conditions only when the hydrophilic/hydrophobic moieties reached a certain equilibrium, which was consistent with other experimental results.
1) had the best ability to stabilize catalytic activity under heating conditions. This result indicated that the W-type microspheres could maintain the catalytic activity of HRP in the heating conditions only when the hydrophilic/hydrophobic moieties reached a certain equilibrium, which was consistent with other experimental results.
Conclusions
In summary, one-step solvothermal instead of the method of DPP successfully to synthesize the poly(DVB-co-PBA) which was a kind of new multi-function organic particle being used as the stabilizer of Pickering emulsion. The synthetic poly(DVB-co-PBA) microspheres of multi-component exhibited versatile compared with the inorganic particles and organic particles of a single component in the preparation of hybrid materials by Pickering emulsion polymerization. The synthetic poly(DVB-co-PBA) with some new features of regular morphology, fluorescence, carbohydrate affinity and easier to form different types of Pickering emulsions. Three kinds of microspheres of core–shell structure were prepared in three types of emulsions, respectively. The W-type of amphiphilic polymer microsphere with a core of the hydrophilic/hydrophobic moieties reached to 2/1 could suppresses HRP thermal aggregation with high efficiency and longer catalytic activity of HRP under heat stress. Further studies on the deep mechanism of thermal protection for glycoprotein are now in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully appreciate the financial support by the National Key Research and Development Program of China (no. 2016YFD0401202). Special Fund for Grain-Scientific Research in the Public Interest (201513006-04). The National Key Technology R&D Program (2015BAD17B03).Notes and references
- K. Nishinari, Y. Fang, S. Guo and G. O. Phillips, Food Hydrocolloids, 2014, 39, 301–318 CrossRef CAS.
- K. N. Ryan and E. A. Foegeding, Food Hydrocolloids, 2015, 43, 265–274 CrossRef CAS.
- H. B. Wijayanti, N. Bansal and H. C. Deeth, Compr. Rev. Food Sci. Food Saf., 2014, 13, 1235–1251 CrossRef CAS.
- O. E. Mäkinen, E. Zannini, P. Koehler and E. K. Arendt, Food Chem., 2016, 196, 17–24 CrossRef PubMed.
- N. T. Wright and J. D. Humphrey, Annu. Rev. Biomed. Eng., 2002, 4, 109–128 CrossRef CAS PubMed.
- B. Song, Q. Sun, H. Li, B. Ge, J. S. Pan, A. T. S. Wee and F. Huang, Angew. Chem., Int. Ed., 2014, 126, 6476–6481 CrossRef.
- G. Stirnemann, S. G. Kang, R. Zhou and B. J. Berne, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3413–3418 CrossRef CAS PubMed.
- D. B. Wetlaufer and Y. Xie, Protein Sci., 1995, 4, 1535–1543 CrossRef CAS PubMed.
- H. Shimizu, K. Fujimoto and H. Kawaguchi, Biotechnol. Prog., 2000, 16, 248–253 CrossRef CAS PubMed.
- K. Akiyoshi, A. Yoshihiro Sasaki and J. Sunamoto, Bioconjugate Chem., 1999, 10, 321 CrossRef CAS PubMed.
- X. Liu, Y. Liu, Z. Zhang, F. Huang, Q. Tao, R. Ma, Y. An and L. Shi, Chem.–Eur. J., 2013, 19, 7437–7442 CrossRef CAS PubMed.
- Y. Sasaki and K. Akiyoshi, Curr. Pharm. Biotechnol., 2010, 11, 300–305 CAS.
- H. Takahashi, S. Sawada and K. Akiyoshi, ACS Nano, 2011, 5, 337–345 CrossRef CAS PubMed.
- M. Sakono, T. Maruyama, N. Kamiya and M. Goto, Biochem. Eng. J., 2004, 19, 217–220 CrossRef CAS.
- M. Nakamoto, T. Nonaka, K. J. Shea, Y. Miura and Y. Hoshino, J. Am. Chem. Soc., 2016, 138, 4282 CrossRef CAS PubMed.
- F. Huang, J. Wang, A. Qu, L. Shen, J. Liu, J. Liu, Z. Zhang, Y. An and L. Shi, Angew. Chem., Int. Ed., 2014, 53, 8985–8990 CrossRef CAS PubMed.
- S. Takeda, H. Takahashi, S. I. Sawada, Y. Sasaki and K. Akiyoshi, RSC Adv., 2013, 3, 25716–25718 RSC.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100, 503–546 CrossRef.
- J. Frelichowska, M. A. Bolzinger and Y. Chevalier, Colloids Surf., A, 2009, 343, 70–74 CrossRef CAS.
- N. P. Ashby and B. P. Binks, Phys. Chem. Chem. Phys., 2000, 2, 5640–5646 RSC.
- Y. He, Powder Technol., 2004, 147, 59–63 CrossRef CAS.
- X. Song, Y. Zhao, H. Wang and Q. Du, Langmuir, 2009, 25, 4443–4449 CrossRef CAS PubMed.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2001, 17, 4540–4547 CrossRef CAS.
- F. Bai, X. Yang and W. Huang, Macromolecules, 2004, 37, 3641–3649 CrossRef.
- D. Li, Y. Chen and Z. Liu, Chem. Soc. Rev., 2015, 44, 8097–8123 RSC.
- T. Zhao, J. Wang, J. He, Q. Deng and S. Wang, Biosens. Bioelectron., 2017, 91, 756–761 CrossRef CAS PubMed.
- L. L. Guo, Q. X. Li, X. Tang, K. G. Neoh and E. T. Kang, Macromolecules, 2010, 43, 5797–5803 CrossRef.
- N. Morimoto, T. Endo, Y. Iwasaki and K. Akiyoshi, Biomacromolecules, 2005, 6, 1829–1834 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra11558j |
| This journal is © The Royal Society of Chemistry 2017 |