Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of Gd2Zr2O7 defect-fluorite oxide nanoparticles via a homogeneous precipitation-solvothermal method
Zhe Tangab,
Zhangyi Huangac,
Jianqi Qi *ac,
Xiaofeng Guo
*ac,
Xiaofeng Guo de,
Wei Hanab,
Mao Zhouab,
Shuting Penga and
Tiecheng Lu*abc
de,
Wei Hanab,
Mao Zhouab,
Shuting Penga and
Tiecheng Lu*abc
aCollege of Physical Science and Technology, Sichuan University, Chengdu 610064, P. R. China. E-mail: qijianqi@scu.edu.cn; lutiecheng@scu.edu.cn
bKey Laboratory of High Energy Density Physics of Ministry of Education, Sichuan University, Chengdu 610064, Sichuan, P. R. China
cKey Laboratory of Radiation Physics and Technology of Ministry of Education, Sichuan University, Chengdu 610064, Sichuan, P. R. China
dDepartment of Chemistry, Washington State University, Pullman, Washington 99163, USA
eThe Alexandra Navrotsky Institute for Experimental Thermodynamics, Washington State University, Pullman, Washington 99163, USA
First published on 1st December 2017
Abstract
Defect-fluorite structured Gd2Zr2O7 nanoparticles were successfully synthesized via a homogeneous precipitation-solvothermal method using urea as a precipitant. The obtained nanoparticles were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) analysis and transmission electron microscopy (TEM). Compared to the traditional solvothermal method, this homogeneous precipitation-solvothermal method has the advantage of producing nanoparticles with small grain sizes, a narrow size-distribution, high surface areas and little agglomeration. Particularly, the mean crystallite size of Gd2Zr2O7 obtained by this method is 20–30 nm, providing a great opportunity of using these nanoparticles as starting nano-sized building blocks for low temperature preparation of homogeneous and dense ceramics.
Introduction
Gd2Zr2O7 is one of the most important rare-earth-stabilized zirconia (RESZ) materials due to its potential application as a ceramic waste matrix for immobilizing actinides and fission products.1,2 This crystalline system has a general formula A2B2O7, with one eight-coordinated A site (16c) and one six-coordinated B site (16d) that are capable of incorporating a large amount of radionuclides (e.g. Pu, U, Np, Hf, Th, Am and Cm).3 Previous studies show that Gd2Zr2O7 has a high thermal stability, high chemical resistance and durability,4–7 which suggests that it can serve as a stable, robust and long-lived waste host. In addition, due to the good mechanical properties (strength and hardness), thermodynamic stability and leaching durability, Gd2Zr2O7 also finds versatile applications in many fields, such as thermal barrier coatings, solid electrolytes in solid oxide fuel cells, transparent ceramics, photoactive materials, etc.8–11Controls of sizes and morphologies of grains can greatly affect the physical and chemicals properties of material in related to its applications. Due to the significant advantages in small grain sizes, nanostructured materials have been reported to display enhanced or discrepant properties comparing to coarse-grained counterparts.12–14 Previous studies on RESZ show that compared to the conventionally synthesized microcrystalline structures, nanosized ceramics show enhanced radiation resistance, decreased thermal conductivity and increased conductivity.15–19 So far, there are reports on using wet chemistry methods, the sol–gel processing,5 the gel combustion processing,20 the co-precipitation method,21 the hydrothermal method22 and others,6 to prepare Gd2Zr2O7 nanoparticles with different chemical homogeneity and phase assemblage. However, those former methods cannot produce nanoparticles with well-controlled grain sizes due to the agglomeration as a result of pH variation.
Thus, in this paper, we introduced a facile homogeneous-solvothermal precipitation method to synthesize well-crystallized and dispersed Gd2Zr2O7 nanoparticles with grain sizes of 20–30 nm. We demonstrated that this method has a better control of the grain size distribution through pH variation by adjusting the molar ratio of urea. In addition, it provides a more efficient way to synthesis well-dispersed nanoparticles than other wet chemistry routes.
Experimental section
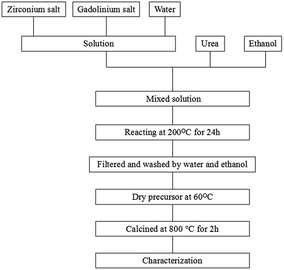
In the synthesis experiment, Gd(NO3)3·6H2O (>99.99%, Ruike RE, China), ZrOCl2·8H2O (>99.99%, Aladdin) were mixed with a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and dissolved in deionized water. Ethanol and different amounts of urea were added to the mixed solution while it was constantly stirring. After a fully mixing, the solution was heated at 200 °C for 24 h in a Teflon beaker and a precipitate was yielded. The precipitate was then filtered and washed with deionized water and ethanol in turns for several times. The reactants diluted by ethanol were dried at 60 °C. Finally, the dry precursors were calcined at 800 °C for 2 h. The synthesis scheme is shown in Fig. 1. We compared our synthetic samples by the homogeneous precipitation-solvothermal method with those obtained by the co-precipitation method. In the co-precipitation method, the dilute ammonium hydroxide was used as the precipitant, which was mixed with the solution containing Gd3+ and Zr4+. After stirring the solution for 60 min and aging for 24 h, the formed gel-like precipitate was obtained from the solution through the centrifugal separation. Then the precipitate was washed with deionized water and ethanol for several times and dried at 60 °C. Gd2Zr2O7 powders were finally synthesized after a calcination at 800 °C for 2 h.
1 and dissolved in deionized water. Ethanol and different amounts of urea were added to the mixed solution while it was constantly stirring. After a fully mixing, the solution was heated at 200 °C for 24 h in a Teflon beaker and a precipitate was yielded. The precipitate was then filtered and washed with deionized water and ethanol in turns for several times. The reactants diluted by ethanol were dried at 60 °C. Finally, the dry precursors were calcined at 800 °C for 2 h. The synthesis scheme is shown in Fig. 1. We compared our synthetic samples by the homogeneous precipitation-solvothermal method with those obtained by the co-precipitation method. In the co-precipitation method, the dilute ammonium hydroxide was used as the precipitant, which was mixed with the solution containing Gd3+ and Zr4+. After stirring the solution for 60 min and aging for 24 h, the formed gel-like precipitate was obtained from the solution through the centrifugal separation. Then the precipitate was washed with deionized water and ethanol for several times and dried at 60 °C. Gd2Zr2O7 powders were finally synthesized after a calcination at 800 °C for 2 h.
The synthetic products were characterized by X-ray diffraction (XRD), Scanning electron microscope (SEM), transmission electron microscopy (TEM), Infrared spectroscopy (IR), and Brunauer–Emmett–Teller (BET) surface area measurements. The quantitative phase analysis was derived from the refinement of the XRD patterns using MAUD Rietveld program.
Results and discussion
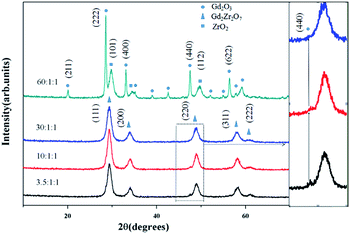
The XRD patterns (Fig. 2) of Gd2Zr2O7 synthesized by the homogeneous-solvothermal precipitation method suggest a good crystallinity. Particularly, when the molar ratio of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+
Gd3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr4+ is 30
Zr4+ is 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the phase is completely crystallized in the defect-fluorite structure, evidenced by the strong and sharp peaks resulting from the (111), (200), (220), (311) and (222) reflections. By using the Scherrer equation implemented by JADE 6, we performed a pattern profile fitting that determined the average crystallite size to be 7.0 ± 0.3 nm which is similar to that reported by Popov et al. (∼10 nm).23,24 Because of our powders were well crystallized in small sizes, we can control the particle size by applying different calcination temperatures to the preliminary particles. Furthermore, the XRD patterns suggest that the molar ratio of urea
1, the phase is completely crystallized in the defect-fluorite structure, evidenced by the strong and sharp peaks resulting from the (111), (200), (220), (311) and (222) reflections. By using the Scherrer equation implemented by JADE 6, we performed a pattern profile fitting that determined the average crystallite size to be 7.0 ± 0.3 nm which is similar to that reported by Popov et al. (∼10 nm).23,24 Because of our powders were well crystallized in small sizes, we can control the particle size by applying different calcination temperatures to the preliminary particles. Furthermore, the XRD patterns suggest that the molar ratio of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+
Gd3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr4+ has an influence on the formation of the final products (Fig. 2). At a lower concentrated level of urea, we observed a weak peak attributed to the (440) reflection of Gd2O3, which was not shown in the product when the urea molar ratio is 30. Though at a very high urea concentration (molar ratio of 60), the XRD pattern shows no formation of Gd2Zr2O7 but Gd2O3 and ZrO2 instead (Fig. 3).
Zr4+ has an influence on the formation of the final products (Fig. 2). At a lower concentrated level of urea, we observed a weak peak attributed to the (440) reflection of Gd2O3, which was not shown in the product when the urea molar ratio is 30. Though at a very high urea concentration (molar ratio of 60), the XRD pattern shows no formation of Gd2Zr2O7 but Gd2O3 and ZrO2 instead (Fig. 3).
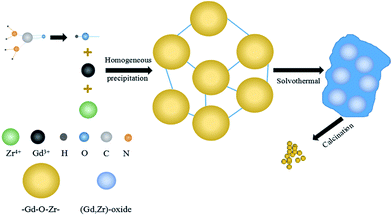 | ||
| Fig. 3 Schematic diagram illustrating the formation and structural evolution of Gd2Zr2O7 nanocrystalline powders. | ||
The relationship between the urea concentration and the final product can be explained by the pH variation. When the temperature of mixed solution increases, the hydrolysis reaction takes place and provides hydroxide ions described in eqn (1) and (2),
| CO(NH2)2 + H2O = CO2 + 2NH3 | (1) |
| NH3 + H2O = NH34+ + OH− | (2) |
The hydrolysis reaction in the solution can constantly provide hydroxide ions with which Gd3+ and Zr4+ are combined, so that the mixing of Gd and Zr happens in a molecular level which is the basis for synthesizing homogeneous Gd2Zr2O7 nanocrystals. Thus, the concentration of hydroxide ions is critical as it can determine the phases formed in the final products. If the concentration of urea is low, the concentration of hydroxide ions is also low and not enough for OH− to be reacted with Gd3+ and Zr4+. In addition, though theoretically urea could provide enough hydroxide ions for reactions with Gd3+ and Zr4+, the hydrolysis reactions described in eqn (1) and (2) are in dynamic equilibrium in a hermetic reactor, which could result in an insufficient concentration of hydroxide ions. For instance, no precipitate is formed when the molar ratio of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+
Gd3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr4+ is lower than 2
Zr4+ is lower than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As the concentration of urea increased, the content of hydroxide ions is also increased as seen from eqn (1) and (2). After the molar ratio of urea reaches 3.5, the solution contains enough hydroxide ions to be combined with Gd3+ and Zr4+ (Fig. 2) that yielded phases consist of a defect-fluorite phase Gd2Zr2O7 as the main phase with a tiny amount of Gd2O3 as a secondary phase. We found out that the amount of the impurity (Gd2O3) decreases as the mole ratio of urea increases up to certain values; and at a proper mole rate (30
1. As the concentration of urea increased, the content of hydroxide ions is also increased as seen from eqn (1) and (2). After the molar ratio of urea reaches 3.5, the solution contains enough hydroxide ions to be combined with Gd3+ and Zr4+ (Fig. 2) that yielded phases consist of a defect-fluorite phase Gd2Zr2O7 as the main phase with a tiny amount of Gd2O3 as a secondary phase. We found out that the amount of the impurity (Gd2O3) decreases as the mole ratio of urea increases up to certain values; and at a proper mole rate (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we produced pure Gd2Zr2O7 in defect-fluorite structure (Fig. 2). However, on the other hand, if the concentration of urea is too high, such as 60
1), we produced pure Gd2Zr2O7 in defect-fluorite structure (Fig. 2). However, on the other hand, if the concentration of urea is too high, such as 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the hydroxide ions would segregate Gd3+ and Zr4+, and react with them separately to form Gd(OH)3 and Zr(OH)4 instead of (Gd, Zr) hydroxide that eventually leads to products as Gd2O3 and ZrO2 (Fig. 2).
1, the hydroxide ions would segregate Gd3+ and Zr4+, and react with them separately to form Gd(OH)3 and Zr(OH)4 instead of (Gd, Zr) hydroxide that eventually leads to products as Gd2O3 and ZrO2 (Fig. 2).
The hydrolysis reactions described by eqn (1) and (2) are known as homogeneous precipitations that produce a homogeneous precipitation of (Gd, Zr) hydroxide as described in eqn (3).22 Then the next step occurred under heating is the solvothermal process, during which the added ethanol can effectively break up the aggregates in the (Gd, Zr)-oxides at 200 °C, and the alkoxyl group can substitute hydroxyl group as described in eqn (4),
| ZrOCl2 + Gd(NO3)3 + OH− → (Gd, Zr)-hydroxide | (3) |
| (Gd, Zr)-hydroxide + ROH → Gd2Zr2O7 nanocrystal + mH2O | (4) |
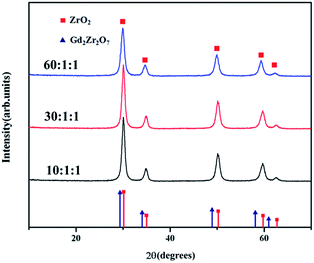
In this homogeneous precipitation-solvothermal method, the solvothermal process is the key step for synthesizing pure defect-fluorite phase Gd2Zr2O7 nanocrystals. As a comparison, we performed a co-precipitation synthesis without the solvothermal process. The mixed solution resulted from the homogeneous precipitation was heated in a thermostatted oil bath that maintained at 120 °C for 2 h with a constant stirring. Then the suspension was cooled and the white precipitate was washed multiple times with deionized water and ethanol. Then the precursors were dried in vacuum at 60 °C and then calcined at 800 °C for 2 h. The XRD patterns of the products are given in Fig. 4. It's clear that without a solvothermal process, the synthesized powder is pure ZrO2. Numbers of studies show both A2B2O7 oxides and rare earth oxides can be synthesized by homogeneous precipitation method using urea as precipitant.25,26 Nevertheless, due to the low-temperature calcination, using only homogeneous precipitation method could lead to A2B2O7 oxides mixed with BO2 crystalline phases and perhaps poor crystallized AO2 or A-containing polymers, partly due to the closed formation enthalpies of these phases at 800 °C. Similar phenomena were observed in other complex oxide systems, such as garnet.27,28 During the solvothermal process, Gd2Zr2O7 was formed because of more OH− was produced by the hydrolysis of urea with the aids of high temperature and high pressure. Compared with Gd3+, Zr4+ can be precipitated at lower pH value. With the absence of solvothermal process, only Zr4+, rather than Gd3+, was precipitated because of the relatively low pH value provided by the insufficient hydrolysis of urea, giving rise to the formation of the precursor of ZrO2. Thus, our homogeneous precipitation-solvothermal method was proved to be a new feasible route for synthesizing pure phase Gd2Zr2O7 as a base for various functional nanomaterials.
IR absorption spectra are used to determine the site preferences of Gd and Zr in Gd2Zr2O7 as it is sensitivity to the change of the local structure resulting from distortion of polyhedra and element substitutions. The IR spectra of samples prepared by the homogeneous precipitation-solvothermal method with different concentrations of urea are presented in Fig. 5. According to former studies, the infrared spectra of A2B2O7 compounds contain seven IR-active optic modes.29,30 Fig. 5(a) shows the IR absorption spectra of powders with different concentrations of urea in the wave range of 400 cm−1 to 4000 cm−1. There are several characteristic absorption bands at about 436 cm−1, 540 cm−1, 1380–1630 cm−1, and 3500 cm−1. It has been known that the appearances of the band centered at approximately the 1380–1630 cm−1 and 3500 cm−1 bands are evidence of the existence of water molecules attached to the powders. In Fig. 5(b), the spectrum of the sample with the mole ratio of urea as 60 shows peaks in the range of 400 cm−1 to 600 cm−1, from which the peak at 540 cm−1 is attributed from Zr–O stretching modes, and that at 436 cm−1 is caused by the O–Gd–O bending modes. These are another evidence besides XRD that show the presence of Gd2O3 and ZrO2 when the urea is high concentrated during the homogeneous precipitation-solvothermal synthesis.
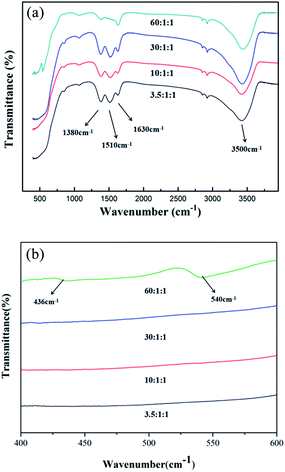 | ||
| Fig. 5 IR absorption spectra of calcined powders with different concentrations of urea (a) wavenumber between 400 cm−1 and 4000 cm−1; (b) wavenumber between 400 cm−1 and 600 cm−1. | ||
In order to understand the micro-morphologies and micro-structures of synthetic products made by the homogeneous precipitation-solvothermal method as compared to co-precipitation method, we performed SEM on Gd2Zr2O7 powders synthesized by these two methods (Fig. 6). Fig. 6(b) shows large particles of pure defect-fluorite nanocrystalline phase Gd2Zr2O7 powders prepared by the homogeneous precipitation-solvothermal method with urea mole ratio as 30. The morphology suggests well-dispersed and homogeneous features. While in Fig. 6(a), particles prepared by the co-precipitation method show elevated agglomerations that grains are interconnected with each other to form larger micro-structures that have irregular morphologies and porous networks. Compared to co-precipitation method, our new method produces Gd2Zr2O7 powders with a better dispersity and a low level of aggregation. Furthermore, the particle sizes of samples synthesized by our method estimated by Nano Measurer 1.2 are in a relatively-narrow particle-size distribution within the range of 20–30 nm with a small mean particle size as 28.7 ± 0.8 nm (shown in Fig. 7).
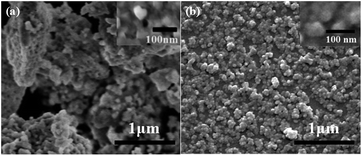 | ||
| Fig. 6 SEM of powders synthesized by (a) the co-precipitation method; (b) the homogeneous precipitation-solvothermal method. | ||
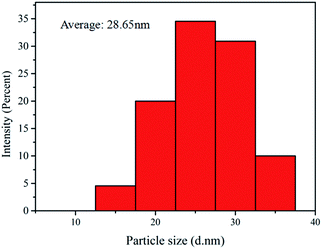 | ||
| Fig. 7 The particle size distribution of Gd2Zr2O7 nanocrystal powders synthesized by the homogeneous precipitation-solvothermal method. | ||
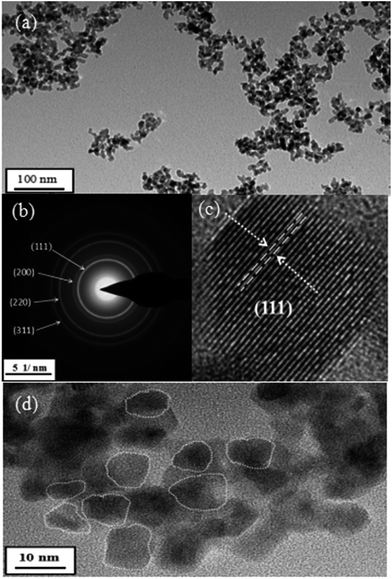
TEM micrographs of our synthetic samples suggest the nature of nanocrystalline as evidenced in Fig. 8. It is concluded by the morphological image (Fig. 8(a)) that Gd2Zr2O7 powders are well-dispersed nanoparticles without tight aggregations. The crystal structure is confirmed by selected area diffraction (SAED) to be defect-fluorite shown in Fig. 8(b). The crystallite size determined by HRTEM (Fig. 8(c)) is ∼7 nm in agreement with the one by Scherrer formula; and the clear and regular crystal lattice distance indicates that Gd2Zr2O7 phases are highly crystalline. Well-dispersed Gd2Zr2O7 nanoparticles shown in the Fig. 8(d) HRTEM image have sphere-like or ellipsoid shapes. As mentioned above, this homogeneous precipitation-solvothermal method can be used to synthesize Gd2Zr2O7 in smaller particle sizes and with a good dispersity at a relatively low temperature about 200 °C.
The BET surface area and total pore volume of the calcined powder were studied by the gas absorption analysis. The N2 adsorption/desorption isotherms of the powders which produced by the mole ratio of urea, Gd3+ and Zr4+ is 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown in Fig. 9. The Gd2Zr2O7 powders prepared via homogeneous precipitation-solvothermal processing displays a type H3 hysteresis loop (according to the IUPAC classification scheme), indicating smaller particle sizes and a good dispersity. The specific surface area of the pure defect-fluorite phase Gd2Zr2O7 powder is 76.9 m2 g−1, and the total pore volume is about 0.63 m2 g−1, and with an average pore radius of 26.04 nm, which further proves our conclusion compared with the SEM result. Thus, we can conclude that via the homogeneous precipitation-solvothermal method, Gd2Zr2O7 nanoparticles are well dispersed and the particle sizes are small.
1 are shown in Fig. 9. The Gd2Zr2O7 powders prepared via homogeneous precipitation-solvothermal processing displays a type H3 hysteresis loop (according to the IUPAC classification scheme), indicating smaller particle sizes and a good dispersity. The specific surface area of the pure defect-fluorite phase Gd2Zr2O7 powder is 76.9 m2 g−1, and the total pore volume is about 0.63 m2 g−1, and with an average pore radius of 26.04 nm, which further proves our conclusion compared with the SEM result. Thus, we can conclude that via the homogeneous precipitation-solvothermal method, Gd2Zr2O7 nanoparticles are well dispersed and the particle sizes are small.
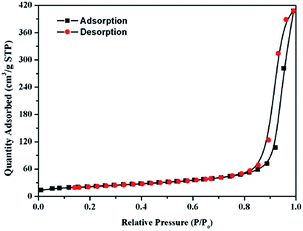 | ||
| Fig. 9 Nitrogen adsorption–desorption isotherms of Gd2Zr2O7 nanocrystalline powders prepared by the homogeneous precipitation-solvothermal method. | ||
Conclusions
In summary, well-crystalized and well-dispersed defect-fluorite phase Gd2Zr2O7 nanocrystalline powders have been successfully synthesized by the homogeneous precipitation-solvothermal method. The reactions of precipitation and crystallization both occur at a relatively low temperature so that the high-temperature calcination can be eliminated. We discovered that under the appropriate mole ratio of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+
Gd3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr4+ as 30
Zr4+ as 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the formed Gd2Zr2O7 nanocrystalline powder is most sphere-like (with some oval shape) and has a narrow particle distribution with an average diameter of 20–30 nm. This research provides a new facile and efficient route to prepare Gd2Zr2O7 nanocrystals at a lower temperature with shortened reaction time, which can also be extended to the synthesis of Gd2Zr2O7-based functional nanomaterials and other fluorite-oxide nanocrystals (like La2Zr2O7).
1, the formed Gd2Zr2O7 nanocrystalline powder is most sphere-like (with some oval shape) and has a narrow particle distribution with an average diameter of 20–30 nm. This research provides a new facile and efficient route to prepare Gd2Zr2O7 nanocrystals at a lower temperature with shortened reaction time, which can also be extended to the synthesis of Gd2Zr2O7-based functional nanomaterials and other fluorite-oxide nanocrystals (like La2Zr2O7).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant No. 11505122 and Grant No. 91326103, the ITER Program (No. 2014GB125002).References
- A. Gomez-Perez, J. Prado-Gonjal, D. Munoz-Gil, A. Andrada-Chacon, J. Sanchez-Benitez, E. Moran, M. T. Azcondo, U. Amador and R. Schmidt, RSC Adv., 2015, 5, 85229–85241 RSC.
- M. K. Patel, V. Vijayakumar, S. Kailas, D. K. Avasthi, J. C. Pivin and A. K. Tyagi, J. Nucl. Mater., 2008, 380, 93–98 CrossRef CAS.
- L. Wang, X. Y. Shu, X. R. Lu, Y. L. Wu, Y. Ding and S. Zhang, Mater. Lett., 2017, 196, 403–405 CrossRef CAS.
- M. Lang, F. X. Zhang, J. M. Zhang, J. W. Wang, B. Schuster, C. Trautmann, R. Neumann, U. Becker and R. C. Ewing, Nat. Mater., 2009, 8, 793–797 CrossRef CAS PubMed.
- J. Shamblin, M. Feygenson, J. Neuefeind, C. L. Tracy, F. X. Zhang, S. Finkeldei, D. Bosbach, H. D. Zhou, R. C. Ewing and M. Lang, Nat. Mater., 2016, 15, 507 CrossRef CAS PubMed.
- X. R. Lu, Y. Ding, X. Y. Shu, X. L. Mao and X. L. Wang, RSC Adv., 2015, 5, 64247–64253 RSC.
- A. Navrotsky, T. Shvareva and X. Guo, in Uranium – Cradle to Grave, ed. P. C. Burns and G. E. Sigmon, Mineralogical Association of Canada, Winnipeg, MB, Cananda, 2013, vol. Short Course, ch. 5, pp. 147–164 Search PubMed.
- Z. G. Liu, J. H. Ouyang, Y. Zhou and X. L. Xia, Mater. Lett., 2008, 62, 4455–4457 CrossRef CAS.
- Z. G. Liu, J. H. Ouyang, Y. Zhou and X. L. Xia, J. Alloys Compd., 2010, 490, 277–281 CrossRef CAS.
- Z. J. Wang, G. H. Zhou, X. P. Qin, Y. Yang, G. J. Zhang, Y. Menke and S. W. Wang, J. Alloys Compd., 2014, 585, 497–502 CrossRef CAS.
- M. S. Rabasovic, D. Sevic, J. Krizan, M. Terzic, J. Mozina, B. P. Marinkovic, S. Savic-Sevic, M. Mitric, M. D. Rabasovic and N. Romcevic, J. Alloys Compd., 2015, 622, 292–295 CrossRef CAS.
- M. G. Bellino, D. G. Lamas and N. E. W. de Reca, Adv. Funct. Mater., 2006, 16, 107–113 CrossRef CAS.
- G. Xu, Y. W. Zhang, C. S. Liao and C. H. Yan, Phys. Chem. Chem. Phys., 2004, 6, 5410–5418 RSC.
- C. W. Nan, A. Tschope, S. Holten, H. Kliem and R. Birringer, J. Appl. Phys., 1999, 85, 7735–7740 CrossRef CAS.
- S. K. Gupta, P. S. Ghosh, C. Reghukumar, N. Pathak and R. M. Kadam, RSC Adv., 2016, 6, 44908–44920 RSC.
- J. M. Zhang, J. Lian, A. F. Fuentes, F. X. Zhang, M. Lang, F. Y. Lu and R. C. Ewing, Appl. Phys. Lett., 2009, 94, 243110 CrossRef.
- S. Dey, J. W. Drazin, Y. Q. Wang, J. A. Valdez, T. G. Holesinger, B. P. Uberuaga and R. H. R. Castro, Sci. Rep., 2015, 5, 7746 CrossRef CAS PubMed.
- G. Soyez, J. A. Eastman, L. J. Thompson, G. R. Bai, P. M. Baldo, A. W. McCormick, R. J. DiMelfi, A. A. Elmustafa, M. F. Tambwe and D. S. Stone, Appl. Phys. Lett., 2000, 77, 1155–1157 CrossRef CAS.
- Y. W. Zhang, S. Jin, Y. Yang, G. B. Li, S. J. Tian, J. T. Jia, C. S. Liao and C. H. Yan, Appl. Phys. Lett., 2000, 77, 3409–3411 CrossRef CAS.
- B. P. Mandal, A. Banerji, V. Sathe, S. K. Deb and A. K. Tyagi, J. Solid State Chem., 2007, 180, 2643–2648 CrossRef CAS.
- Z. Y. Huang, J. Q. Qi, L. Zhou, Z. Feng, X. H. Yu, Y. C. Gong, M. Yang, Q. W. Shi, N. Wei and T. C. Lu, J. Appl. Phys., 2015, 118, 214901 CrossRef PubMed.
- Y. W. Zhang, G. Xu, Z. G. Yan, Y. Yang, C. S. Liao and C. H. Yan, J. Mater. Chem., 2002, 12, 970–977 RSC.
- V. V. Popov, V. F. Petrunin, S. A. Korovin, A. P. Menushenkov, O. V. Kashurnikova, R. V. Chernikov, A. A. Yaroslavtsev and Y. V. Zubavichus, Russ. J. Inorg. Chem., 2011, 56, 1538–1544 CrossRef CAS.
- V. V. Popov, Y. V. Zubavichus, A. P. Menushenkov, A. A. Yaroslavtsev, E. S. Kulik, V. F. Petrunin, S. A. Korovin and N. N. Trofimova, Russ. J. Inorg. Chem., 2014, 59, 279–285 CrossRef CAS.
- H. S. Xue, W. N. Zhang, X. Y. Li, X. C. You, J. S. Rao and F. S. Pan, Electron. Mater. Lett., 2017, 13, 255–259 CrossRef CAS.
- R. Gunawidjaja, T. Myint and H. Eilers, J. Phys. Chem. C, 2013, 117, 14427–14434 CAS.
- X. Guo, A. H. Tavakoli, S. Sutton, R. K. Kukkadapu, L. Qi, A. Lanzirotti, M. Newville, M. Asta and A. Navrotsky, Chem. Mater., 2014, 26, 1133–1143 CrossRef CAS.
- X. Guo, A. Navrotsky, R. K. Kukkadapu, M. H. Engelhard, A. Lanzirotti, M. Newville, E. S. Ilton, S. Sutton and H. Xu, Geochim. Cosmochim. Acta, 2016, 189, 269–281 CrossRef CAS.
- L. Zhou, Z. Y. Huang, J. Q. Qi, Z. Feng, D. X. Wu, W. Zhang, X. H. Yu, Y. B. Guan, X. T. Chen, L. D. Xie, K. Sun and T. C. Lu, Metall. Mater. Trans. A, 2016, 47, 623–630 CrossRef CAS.
- M. A. Subramanian, G. Aravamudan and G. V. S. Rao, Prog. Solid State Chem., 1983, 15, 55–143 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |