Open Access Article
Open Access ArticleSynthesis of polyethylene/exfoliated MoS2 nanocomposites by in situ exfoliation polymerization using Ziegler–Natta catalyst intercalated MoS2†
Hao Zhanga,
He-Xin Zhang *bc and
Keun-Byoung Yoon*c
*bc and
Keun-Byoung Yoon*c
aCollege of Food Science and Engineering, National Engineering Lab. for Wheat and Corn Deep Processing, Jilin Agricultural University, Changchun, China
bSchool of Chemistry and Chemical Engineering, Anhui University of Technology, China. E-mail: polyhx@ciac.ac.cn
cDepartment of Polymer Science and Engineering, Kyungpook National University, Korea. E-mail: kbyoon@knu.ac.kr
First published on 9th November 2017
Abstract
A new synthetic route for polyethylene (PE)/exfoliated MoS2 (EMoS2) nanocomposites using novel Ziegler–Natta catalyst intercalated MoS2 was designed. The catalyst was synthesized by the intercalation of MoS2 with a Grignard reagent, followed by anchoring of TiCl4 into the MoS2 galleries. The intercalated MoS2 was exfoliated to form PE/EMoS2 nanocomposites during in situ ethylene polymerization. The resultant PE/EMoS2 nanocomposites had a layered morphology, and they were well dispersed in the PE matrix. In addition, the thermal stability and mechanical properties of PE were significantly enhanced with the introduction of EMoS2. Thus, this work provides a facile approach to the production of PE/EMoS2 nanocomposites.
Introduction
Polyolefins are the most widely used materials, because of their excellent combination of chemical and physical properties, along with low production cost, superior processability and good recyclability. However, for advanced applications, it is necessary to improve the performance of polyolefins, such as stiffness and rigidity. Over the past decades, the study of polyolefin nanocomposites has attracted considerable attention, because of their high potential as materials with improved properties, such as mechanical and thermal stability, flame resistance, and thermal and electrical conductivities.Two-dimensional (2D) nanomaterials, including graphite, graphene, and inorganic graphene analogs, have been drawing tremendous attention for many potential applications due to the fascinating properties associated with their ultrathin layer structures.1–4 Among the layered nanomaterials, molybdenum disulfide (MoS2) is one of the most attractive; it has a structure similar to that of graphite.5–9 It has been reported that a monolayer of MoS2 has extraordinarily high breaking strength (∼23 GPa) and Young's modulus (∼300 GPa), which are higher than those of chemically reduced graphene.10,11 The aforementioned fascinating properties make MoS2 an attractive substitute in the fabrication of high-performance organic–inorganic polymer nanocomposites. For example, Hu et al. reported a solution blending method to prepare EMoS2-based nanocomposites of polymethyl methacrylate, polystyrene, and polyvinyl alcohol.12–14 The resultant polymer/EMoS2 nanocomposites exhibited enhanced mechanical properties, thermal stability, and fire resistance properties. The uniform dispersion of EMoS2 in the polymer matrix can be achieved by solution mixing, which is the ideal strategy, wherein these polymers are dissolved in common organic solvents. However, the solution mixing process is difficult and uneconomical in the case of polyolefin, since polyolefin are soluble in limited solvents, such as xylene and trichlorobenzene, at high temperature. In our previous reports,15,16 the PE/EMoS2 nanocomposites were prepared through an in situ polymerization of ethylene using EMoS2 containing Ziegler–Natta catalyst. The resulted nanocomposites exhibited enhanced physical properties than neat PE. However, the catalyst preparation process are very complicate, including use excess amount of n-butyl lithium to lithiation of MoS2, the longtime lithiation reaction, violent exfoliation reaction, waste too much water to remove lithium salt and longtime vacuum freeze-dried process, and so on.
Therefore, in this research, we reported a Ziegler–Natta catalyst intercalated MoS2. The catalyst preparation process is very simple, including intercalation of a Grignard reagent into MoS2 galleries and anchoring of TiCl4. During the ethylene polymerization, the intercalated MoS2 layers are in situly exfoliated and dispersed in the polymer matrix (no need additional exfoliation process), producing PE/EMoS2 nanocomposites directly.
Experimental
Materials
Molybdenum disulfide (MoS2, ∼6 μm), n-butylmagnesium chloride (BuMgCl, 2.0 M in THF) triethylaluminum (TEA, 1.0 M in hexane), and titanium tetrachloride (TiCl4, >99%) were purchased from Sigma-Aldrich and used as received. Polymer-grade ethylene was provided by Korea Petrochemical Ind. Co. Ltd., Korea. n-Hexane was distilled from sodium/benzophenone under N2 prior to use.Preparation of Ziegler–Natta catalyst intercalated MoS2
1 g of MoS2 was first placed in the autoclave and 20 mL of BuMgCl was added. The autoclave was heated at 150 °C for 12 h under argon atmosphere. After that, the autoclave was cooled to room temperature and the product was filtered, and washed with anhydrous n-hexane. The resulting powder was suspended in n-hexane (200 mL). Then, TiCl4 (10 mL) was added dropwise to the suspension of MoS2–MgCl at 0 °C, after which the temperature was increased to 80 °C (2 °C min−1) and the suspension stirred for 4 h. The mixture was filtered to remove the unreacted TiCl4 and then washed several times with hot n-hexane. The obtained powdery catalyst was dried under vacuum at 60 °C for 3 h. The contents of MoS2, Mg and Ti in the resultant catalyst as determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis were: MoS2 – 44.7 wt%, Mg – 6.6 wt% and Ti – 7.4 wt%. The catalyst was also synthesized without the addition of MoS2 for comparison. For this, a similar procedure was followed to prepare the catalyst without including MoS2. The contents of Mg and Ti in the resultant catalyst determined by ICP analysis were: Mg – 2.3 wt%, Ti – 14.1 wt%.Ethylene polymerization
Ethylene polymerization was performed in a three-neck glass reactor (300 mL). The reactor was thrice back-filled with N2 and charged with 100 mL distilled n-hexane. The reaction solution was stirred at 40 °C under 1 bar of ethylene for 5 min, followed by addition of the TEA cocatalyst. Subsequently, the catalyst was added into the reactor, and polymerization was started under a continuous feed of ethylene (1 bar). After 0.5 h polymerization, 10 mL HCl–methanol solution (10%) was added to the suspension to terminate to polymerization. The mixture was poured into large quantity of methanol (500 mL) to precipitate the polymer. The product was collected by filtration, and washed with methanol. Then, the product was dried under vacuum at 60 °C until a constant weight was achieved.Characterization
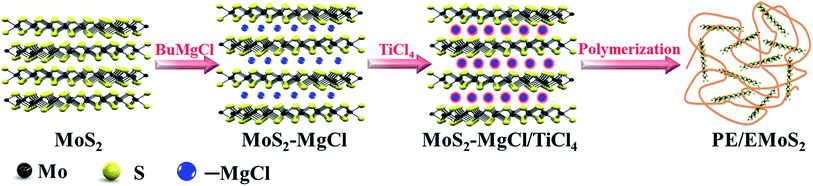
The contents of Mg and Ti in the catalyst were determined using inductively coupled plasma-atomic emission spectroscopy (PerkinElmer, Optima 7300DV). Scanning electron microscopy (SEM) images were recorded using a JEOL JSM-6380LV microscope. The morphologies of the support and the catalyst were studied by an optical microscope (ANA-006, Leitz, Germany) equipped with a charge coupled device-based camera for recording the images. The X-ray diffraction patterns were obtained on a Philips X-Pert PRO MRD diffractometer using Cu-Kα radiation.The melting temperature (Tm) of the obtained polymer was determined using differential scanning calorimetry (DSC; DSC131evo, Setaram) at a heating rate of 10 °C min−1. The sample was heated to 200 °C and held in the molten state for 3 min to eliminate the influence of the thermal history. The polymer melt was cooled to 30 °C at the rate of 10 °C min−1. The melting point was determined in the second scan. The analysis of the decomposition temperature was conducted under N2 atmosphere using a thermogravimetric analyzer (TGA; Setaram Labsys evo) from 30 to 800 °C with a programmed heating rate of 10 °C min−1. The tensile mechanical properties of the polymers were measured using a universal testing machine (Instron M4465). The sample sizes for the tensile drawing experiments were 5.0 × 75.0 × 1.0 mm3. The sample gauge length was 25.0 mm, and the crosshead speed was 50.0 mm min−1. The preparation process of Ziegler–Natta catalyst intercalated MoS2 (MoS2–MgCl/TiCl4 catalyst) and PE/EMoS2 nanocomposites are illustrated in Scheme 1. In the first step, the Grignard reagent (BuMgCl) was intercalated into the MoS2 galleries and obtained MoS2–MgCl support, and then treatment with excess TiCl4 to generate Mg/Ti catalyst species between MoS2 layers. During the ethylene polymerization process, the layered MoS2 will in situly exfoliated by the polymerization force arising from the propagation of PE chain.
Results and discussion
The preparation process of Ziegler–Natta catalyst intercalated MoS2 (MoS2–MgCl/TiCl4 catalyst) and PE/EMoS2 nanocomposites are illustrated in Scheme 1. In the first step, the Grignard reagent (BuMgCl) was intercalated into the MoS2 galleries and obtained MoS2–MgCl support, and then treatment with excess TiCl4 to generate Mg/Ti catalyst species between MoS2 layers. During the ethylene polymerization process, the layered MoS2 will in situly exfoliated by the polymerization force arising from the propagation of PE chain.The morphologies of the pristine MoS2 sample and the MoS2–MgCl/TiCl4 catalyst were studied by SEM analysis. It could be clearly seen from Fig. 1(a), the pristine MoS2 sample contains a large number of tightly stacked MoS2 layers and the diameters are in the range of several micrometers. As shown in Fig. 1(b), after reacted with Grignard reagent and TiCl4, the sheet structure remained and the tightly stacked MoS2 layers cannot be observed on the edge of MoS2. This could be corresponding to the intercalation of Ziegler–Natta catalyst into the MoS2 galleries that blocked the edge of MoS2. The MoS2–MgCl/TiCl4 catalyst was also characterized by SEM-EDS (Fig. S1†). It was found that the catalyst not only covered the surface of MoS2, but also the galleries of MoS2. Concerning the catalyst in the absence of MoS2 (BuMgCl/TiCl4), the morphology were exhibited as irregular particles.
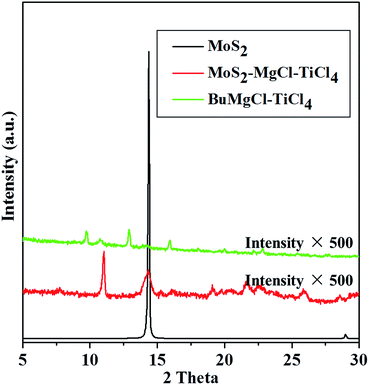
XRD analyses were conducted for the pristine MoS2, MoS2–MgCl–TiCl4, and BuMgCl-TiCl4 catalyst to confirm the successful intercalation of the catalyst into the gallery of MoS2. As shown in Fig. 2, an intense reflection at 2θ = 14.3° (the corresponding interlayer distance was 0.62 nm) was observed for pristine MoS2, which was attributed to the (002) plane of MoS2. After the treatment with the Grignard reagent and TiCl4, a new peak at 2θ = 11.2°, which correspond to the layer distances of 0.8 nm, respectively were observed. This clearly indicated that the expansion of the interlayer space is due to the successful intercalation of the Ziegler–Natta catalyst into the MoS2 galleries. Although the reflection at 2θ = 14.3° was observed after the intercalation, the peak became very broad and the intensity was drastically reduced compared to that of the pristine MoS2. This phenomenon also proves that MoS2 was successful intercalated by Ziegler–Natta catalyst.
After activation with the TEA cocatalyst, the ethylene polymerization behaviors of the catalysts in the absence and the presence of MoS2 were evaluated. As shown in Table 1, the catalyst activity of BuMgCl–TiCl4 catalyst were much lower than those with the MoS2–MgCl–TiCl4 catalyst. When the equivalent weight of catalyst was added (entry 1 vs. 4), the catalyst activity of MoS2–MgCl–TiCl4 is 3.6 times the catalyst in the absence of MoS2. This phenomenon may be due to the fact that MoS2 act as a template to provide a large specific surface area for the Ziegler–Natta catalyst. The PE/EMoS2 nanocomposites with EMoS2 content of 0.4–3.1 wt% were obtained in this research by changing the feed weight of the catalyst and [Al]/[Ti] ratio.
| Entry | Cat. | Cat. (mg) | [Al]/[Ti] | Activity (kg molTi−1 h−1) | ODA-MoS2 (wt%) | Tm (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|---|---|---|---|---|
| a Polymerization conditions: 100 mL n-hexane, TEA co-catalyst, 0.5 h, 1 atm, 40 °C. | ||||||||
| 1 | BuMgCl/TiCl4 | 100 | 50 | 17.3 | — | 133.2 | 116.2 | 52.5 |
| 2 | MoS2–MgCl–TiCl4 | 25 | 200 | 129.7 | 0.4 | 136.0 | 118.0 | 60.9 |
| 3 | 50 | 100 | 85.6 | 0.7 | 135.8 | 118.3 | 62.6 | |
| 4 | 100 | 50 | 62.3 | 0.9 | 135.4 | 118.3 | 68.9 | |
| 5 | 200 | 25 | 29.8 | 2.0 | 135.5 | 118.6 | 71.9 | |
| 6 | 300 | 17 | 19.5 | 3.1 | 135.2 | 119.1 | 78.5 | |
As shown in Fig. 3, PE obtained using the BuMgCl–TiCl4 catalyst comprised irregularly shaped white particles, while the PE/EMoS2 nanocomposites showed layered shapes (1–3 mm) with homogeneous gray color. This could be ascribed to the morphology of the resultant PE and the PE/EMoS2 nanocomposites directly mirror the morphology of the catalyst.
In order to investigate the dispersion of EMoS2, the resultant PE and PE/EMoS2 nanocomposites were hot-pressed into films. The polymer films were characterized by optical microscope in the transparent mode; the micrographs are presented in Fig. 4. It was found that the EMoS2 fillers were well dispersed in the PE matrix.
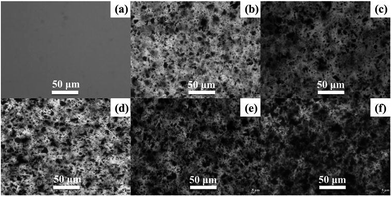 | ||
| Fig. 4 Optical micrographs of (a) PE and PE/EMoS2 nanocomposites with EMoS2 contents of (b) 0.4, (c) 0.7, (d) 0.9, (e) 2.0 and (f) 3.1 wt%. | ||
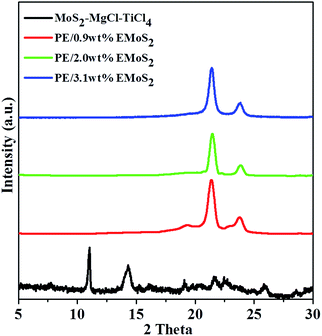
The dispersion of EMoS2 in the PE/EMoS2 nanocomposites was further confirmed by XRD analyses and the data are presented in Fig. 5. The XRD data of PE/EMoS2 nanocomposites showed the presence of two diffraction peaks at 21.4° and 23.8°, which correspond to the (110) and (200) planes of PE (Fig. 5). The peak due to intercalation (11.2°) disappeared completely for PE/EMoS2 nanocomposites. The disappearance of these peaks is ascribed to the complete exfoliation of the intercalated EMoS2 by the chain propagation force of propylene polymerization. No conspicuous diffraction peaks were observed in addition to the ones of crystalline PE, indicating that no obvious stacking of EMoS2 sheets occurs in the PE/EMoS2 nanocomposites and that the stacked MoS2 sheets of the catalyst are completely exfoliated.
The effects of the EMoS2 fillers on the melting temperature, degree of crystallinity of PE was characterized using DSC; the typical DSC curves are summarized in Fig. S2.† As shown in Table 1, the Tm of PE prepared by MoS2-free catalyst was 133.2 °C. Upon the introduction of the EMoS2 fillers, the values of Tm and Xc of PE gradually increased with the EMoS2 amount. The increase in Tm may be due to the interaction between the EMoS2 and the PE matrix, which restricts the motion of the PE chain.17 As compared to the neat PE sample, the Tc also increased with the introduction of MoS2 fillers, which demonstrates that the EMoS2 fillers can act as nucleating agents to induce PE crystallization.
The thermal degradation of PE and the PE/EMoS2 nanocomposites with different weight fractions of EMoS2 fillers were investigated by TGA under N2 atmosphere. The results are given in Table 2, and the TGA curves are shown in Fig. S3.† In comparison to the case of neat PE, the thermal degradation temperatures are linearly shifted to the higher-temperature region with the introduction of the EMoS2 fillers, indicating significant improvement in the thermal oxidation stability of PE. This enhancement of polymer thermal stability upon incorporation of the EMoS2 filler has already been reported by both ourselves and other groups.11–16 With the incorporation of EMoS2, the value of Tdmax of the PE/EMoS2 nanocomposites increased to ∼486.5 °C, which is 17.3 °C higher than that of the pure PE. The significant enhancement in the thermal stability of PE after the incorporation of EMoS2 could be ascribed to the good dispersion of EMoS2 in the PE matrix, which may act as an insulator between the heat source and the polymer surface where combustion occurs. In addition, the char yield of virgin PE is 0.9 wt% at 600 °C, and those of all the PE/EMoS2 nanocomposites are higher than that of the virgin PE. It is clear that the EMoS2 can catalyze the char formation of PE during the process of thermal degradation. These results are not unusual because the transition metal, molybdenum, can catalyze the char formation of polymers and sulfur can improve the flame retardancy of the polymers.14,18 Considering the above results, it is believable that the introduction of inorganic components into organic polymers, such as PE, can improve their thermal stabilities on the basis of the fact that EMoS2 fillers have good thermal stability due to the heat insulation effect of the EMoS2 layers and to the mass transport barrier to the volatile products generated during decomposition.
| EMoS2 content (wt%) | Td5% (°C) | Tdmax (°C) | Char yield (wt%) |
|---|---|---|---|
| — | 399.8 | 469.2 | 0.9 |
| 0.9 | 430.9 | 485.3 | 4.8 |
| 2.0 | 430.1 | 482.5 | 5.9 |
| 3.1 | 431.8 | 486.5 | 7.8 |
The mechanical properties of PE and the PE/EMoS2 nanocomposites with various EMoS2 loadings were investigated by tensile tests and the results are presented in Table 3. The values of the tensile strength, the modulus, and the elongation at break for the resultant PE nanocomposites are significantly enhanced even at very low EMoS2 nanofiller loadings. With increasing the EMoS2 loading, the tensile strength and modulus drastically improved. The elongation at break value was also increased with the introduction of relatively lower amount of EMoS2. The maximum increase in tensile strength, modulus, and elongation at break value is 54%, 119% and 60%, respectively. Hu et al.19 reported that the largest increase in tensile strength and modulus was 16.8 and 37.5% for PE/MoS2 nanocomposites that prepared from solution mixing method, which is much lower than our results. These results indicate that the PE/EMoS2 nanocomposites obtained by in situ polymerization with the MoS2–MgCl–TiCl4 catalyst, exhibit remarkable combination of stiffness and toughness.
| EMoS2 content (wt%) | Tensile strength (MPa) | Modulus (MPa) | Elongation at break (%) | |
|---|---|---|---|---|
| Neat PE | — | 25.6 ± 1 | 480 ± 20 | 1000 ± 80 |
| PE/EMoS2 nanocomposites | 0.9 | 32.3 ± 2 | 720 ± 30 | 1600 ± 90 |
| 2.0 | 35.4 ± 2 | 780 ± 30 | 1500 ± 90 | |
| 3.1 | 39.5 ± 2 | 1050 ± 40 | 1000 ± 80 |
Conclusions
A new concept for ethylene polymerization using MoS2–MgCl-supported Ti-based Ziegler–Natta catalyst was successfully established by the intercalation of Ziegler–Natta catalyst into MoS2 galleries. After the in situ polymerization of ethylene, PE/EMoS2 nanocomposites with well-dispersed EMoS2 nanofillers were fabricated. The resultant PE/EMoS2 nanocomposites displayed enhanced thermal stability as compared to PE obtained from the MoS2-free catalyst system. The mechanical properties of PE were enhanced significantly even with a very small amount of the EMoS2 nanofiller. Thus, this work provides a facile approach for the production of high-performance PE with good thermal stability and excellent stiffness–toughness balance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U1462124) and National Research Foundation of Korea (NRF) grant (NRF-2015R1D1A1A0161012). The authors would also like to acknowledge the financial support from Key Scientific Research Project of Education Department (No. 2016-183) and Young Elite Scientist Sponsorship Program of Jilin Province.Notes and references
- H. Kim and C. W. Macosko, Macromolecules, 2008, 41, 3317 CrossRef CAS.
- Y. Huang, Y. Qin, Y. Zhou, H. Niu, Z. Z. Yu and J. Y. Dong, Chem. Mater., 2010, 22, 4096 CrossRef CAS.
- H. X. Zhang, M. G. Bae, J. H. Park, E. B. Ko, D. H. Lee, X. Q. Zhang and K. B. Yoon, RSC Adv., 2016, 6, 20734 RSC.
- H. X. Zhang, Y. M. Hu, D. H. Lee, K. B. Yoon and X. Q. Zhang, RSC Adv., 2016, 6, 26553 RSC.
- E. Benavente, M. A. S. Ana, F. Mendizábal and G. González, Coord. Chem. Rev., 2002, 224, 87 CrossRef CAS.
- P. Joensen, R. Frindt and S. R. Morrison, Mater. Res. Bull., 1986, 21, 457 CrossRef CAS.
- H. Li, J. Wu, Z. Yin and H. Zhang, Acc. Chem. Res., 2014, 47, 1067 CrossRef CAS PubMed.
- X. Fan, P. Xu, D. Zhou, Y. Sun, Y. C. Li, M. A. T. Nguyen, M. Terrones and T. E. Mallouk, Nano Lett., 2015, 15, 5956 CrossRef CAS PubMed.
- X. Li and H. Zhu, J. Materiomics, 2015, 1, 33 CrossRef.
- Z. Tang, Q. Wei and B. Guo, Chem. Commun., 2014, 50, 3934 RSC.
- K. Zhou, J. Liu, W. Zeng, Y. Hu and Z. Gui, Compos. Sci. Technol., 2015, 107, 120 CrossRef CAS.
- K. Zhou, W. Yang, G. Tang, B. Wang, S. Jiang, Y. Hu and Z. Gui, RSC Adv., 2013, 3, 25030 RSC.
- K. Zhou, J. Liu, Q. Zhang, Y. Shi, S. Jiang, Y. Hu and Z. Gui, Mater. Lett., 2014, 126, 159 CrossRef CAS.
- K. Zhou, S. Jiang, C. Bao, L. Song, B. Wang, G. Tang, Y. Hu and Z. Gui, RSC Adv., 2012, 2, 11695 RSC.
- H. X. Zhang, E. B. Ko, J. H. Park, Y. K. Moon, X. Q. Zhang and K. B. Yoon, Compos. Sci. Technol., 2016, 137, 9 CrossRef CAS.
- H. X. Zhang, E. B. Ko, J. H. Park, Y. K. Moon, X. Q. Zhang and K. B. Yoon, Composites, Part A, 2017, 93, 82 CrossRef CAS.
- L. Cui and S. I. Woo, Polym. Bull., 2008, 61, 453 CrossRef CAS.
- T. Tang, X. C. Chen, X. Y. Meng, H. Chen and Y. P. Ding, Angew. Chem., Int. Ed., 2005, 44, 1517 CrossRef CAS PubMed.
- X. Feng, P. Wen, Y. Cheng, L. Liu, Q. Tai, Y. Hu and K. M. Liew, Composites, Part A, 2016, 81, 61 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10853b |
| This journal is © The Royal Society of Chemistry 2017 |