 Open Access Article
Open Access ArticleSynergic effect of copper-based metal–organic frameworks for highly efficient C–H activation of amidines†
Fen Xu *,
Wei-Fen Kang,
Xiao-Ning Wang,
Hao-Dong Kou,
Zhen Jin and
Chun-Sen Liu
*,
Wei-Fen Kang,
Xiao-Ning Wang,
Hao-Dong Kou,
Zhen Jin and
Chun-Sen Liu *
*
Department of Material and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450002, P. R. China. E-mail: fenxu_zzuli@163.com
First published on 7th November 2017
Abstract
A Cu–MOF-catalyzed C–H functionalization of substituted amidines was developed for the facile and efficient synthesis of benzimidazoles. More importantly, this transformation is compatible with various substrates derived from aryl nitriles without ortho substituents. Under reaction conditions devoid of O2 atmosphere or specific groups, 537-MOF has been revealed as a key parameter for expanding the reaction scope and increasing the yield up to 96%. Importantly, this strategy also provides numerous opportunities for further utilization of MOFs as catalysts for challenging organic reactions.
Activation of C–H bonds has been identified as a straightforward and untraditional strategy for the decoration of unreactive hydrocarbon skeletons in terms of its broad application in synthesis of pharmacological active agents, natural products, and functional materials.1 Given the prevalence and relevance of heteroatoms in molecules of interest, C–Het bond formation via direct functionalization of C–H bonds has received considerable attention. In this context, numerous transition metal complexes, especially of Rh,2 Ir,3 Co,4 Ru,5 Pd6 and others,7 have exhibited outstanding catalytic efficiency towards C–H bond functionalization reactions.
Benzimidazoles have been deemed to be valuable intermediates and significant building blocks for the construction of natural products and for achieving the extension of molecule complexity.8 Conventional synthetic routes to this scaffold rely predominantly on condensation of carbonyl derivatives with 1,2-phenylenediamines.9 Nevertheless, this strategy was impeded because of the limited availability of 1,2-phenylene-diamines and the disadvantage of further derivations. Several other approaches toward the synthesis of benzimidazoles have also been reported, though the use of stoichiometric amounts of reagents and limited substrate scope were formidable obstacles.10 Chemists have attempted to fabricate benzimidazole derivates by replacement of a C–H bond from amidines,11 refraining from utilization of halogenated and pseudohalogenated substrates to abbreviate or avoid waste byproducts (Scheme 1).12 In general, high temperature, inert atmosphere or high loading of oxidants was required.13 Besides noble transition-metal catalysts, there have been reports on low-cost and low-toxic copper-catalyze C–H derivation of amidines to deliver benzimidazoles, whereas there remained several issues to be resolved. Representatively, Buchwald et al. developed an C–H functionalization/C–N bond formation reaction to afford benzimidazoles under an oxygen atmosphere.14 However, amidines derived from aryl nitriles without ortho substituents demonstrated frustrating conversion due to the decomposition of substrates, which mainly retard the catalytic cycle. In addition, 2-phenyl-1H-benzo[d]imidazole was obtained in only 44% yield under an air atmosphere. Thus, the development of effective and low-cost catalytic system under reaction conditions devoid of O2 atmosphere and specific groups was highly demanded.
Metal–organic frameworks (MOFs), assembled from metal cluster secondary building units (SBUs) and organic linkers, have emerged as a class of highly promising porous materials with the advantages of high porosity, tunable compositions, and decorative pore surface,15 as evidenced by wide potential for various applications in drug deliver16 and separation,17 gas storage,18 chemical sensing.19 Furthermore, using crystalline MOFs as heterogeneous catalyst provides opportunities for sustainable chemistry, thereby reducing waste and costs as well as boosting the overall efficiency of a catalyst.20 For example, Cohen et al.21 discovered that UiO-66-PdTCAT with site-isolated Pd can be applied to C–H derivation and high catalytic activity of UiO-66-PdTCAT can be attributed to the strong covalent metalthiocatecholato binding. Recently, a heterogeneous nanoscaled [Cu3(BTC)2] catalyst was furnished and applied to the aerobic oxidation of aromatic alcohols with excellent catalytic reactivity originated from the aromatic carboxylate ligand.22 Inspired by the above work, combined with the advantages of shape and size selectivity,23 and lower activity comparing with homogeneous catalysts to decrease the side reaction, we devote to develop a new effective MOFs catalyst to control the selectivity and catalytic activity in C–H functionalization of amidines. Herein, we described successful fabrication of a highly porous MOF, Cu2N2(CO2)4 (537-MOF), that shows remarkably high efficiency on catalytic synthesis of benzimidazoles via C–H functionalization under air atmosphere with broad substrates scope (Scheme 2).
 | ||
| Scheme 2 Synthesis of benzimidazoles from amidines via C–H functionalization reaction catalyzed by 537-MOF. | ||
Single-crystal X-ray diffraction analysis reveals that 537-MOF {[Cu2(TPPB)2](DMF)6} crystallizes in the triclinic space group ![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) 1. The Cu1 and Cu2 atoms are bridged by four bridging carboxylate groups from TPPB2− ligands, forming a {Cu2(O2C)4} secondary building unit (SBU) with a short Cu–Cu distance of 2.70 Å. As a result, each TPPB2− ligand binding to three 6-connected paddlewheel dimers leads to the assembly of a (3,6)-connected rtl network displaying 1D atactic channels along b axis with the pore diameter of 5.1 Å and the available void of 1579.9 Å3, corresponding to 43.5% of the crystal volume (3629.0 Å3) (Fig. 1).
1. The Cu1 and Cu2 atoms are bridged by four bridging carboxylate groups from TPPB2− ligands, forming a {Cu2(O2C)4} secondary building unit (SBU) with a short Cu–Cu distance of 2.70 Å. As a result, each TPPB2− ligand binding to three 6-connected paddlewheel dimers leads to the assembly of a (3,6)-connected rtl network displaying 1D atactic channels along b axis with the pore diameter of 5.1 Å and the available void of 1579.9 Å3, corresponding to 43.5% of the crystal volume (3629.0 Å3) (Fig. 1).
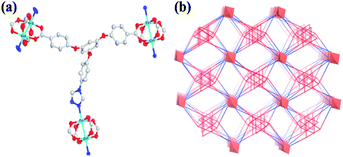 | ||
| Fig. 1 (a) Illustrations of the coordination mode of TPPB2− ligand. (b) View of (3,6)-connected rtl topology of 537-MOF. | ||
Our initial discovery of C–H functionalization of amidines in the presence of 537-MOF and the subsequent screening investigations for the optimization are detailed in Table 1. The initial discovery utilized a mixture of 537-MOF, 5 equiv. of HOAc and 4 Å MS (molecular sieve) as additive in (methylsulfinyl)methane (DMSO) at 100 °C under O2 atmosphere to deliver 2-phenyl-1H-benzo[d]imidazole 2a in 64% HPLC yield (entry 1). To our delight, treatment of the transformation under air atmosphere also gave satisfactory result (entry 2). The catalytic efficiency was decreased when 4 Å MS was absent (entry 3). Further optimization using typical HKUST-1 failed to give superior result (entry 4). We next turned our attention to enhancing the yield of 2a by extensively screening solvents. The yield of 2a was indeed improved when a mixed solvent (DMSO/DMF = 1/1) was used (entry 5). Of note, when benzoic acid was employed as an acidic additive, the targeted product was detected in 96% HPLC yield. The reason for this phenomena is probably due to the suppression of decomposition products under this condition (entry 8).
| Entry | Catalyst | Additive | Yieldb |
|---|---|---|---|
| a Reaction conditions: N-phenylbenzimidamide 1a (0.25 mmol), acid (5 equiv.), 537-MOF (10 mg), DMSO/DMF (2 mL, 1/1) at 100 °C under air atmosphere for 24 h.b HPLC yield.c O2.d DMSO as solvent.e Without 4 Å MS. | |||
| 1c,d | 537-MOF | HOAc | 64 |
| 2d | 537-MOF | HOAc | 59 |
| 3d,e | 537-MOF | HOAc | 54 |
| 4 | HKUST-1 | HOAc | 40 |
| 5 | 537-MOF | HOAc | 73 |
| 6 | 537-MOF | HCOOH | 21 |
| 7 | 537-MOF | MBSA | Trace |
| 8 | 537-MOF | Benzoic acid | 96 |
| 9 | 537-MOF | 2-Fluorobenzoic acid | 62 |
| 10 | 537-MOF | 2-Bromobenzoic acid | 13 |
| 11 | 537-MOF | 2,3,4-Trifluorobenzoic acid | 26 |
| 12 | 537-MOF | 2,3,4,5-Tetrafluorobenzoic acid | 11 |
With the optimized reaction conditions in hand, we investigated the scope of the various substituted amidines (Table 2). Functionalized amidines derived from aryl nitriles with electron-neutral, electron-withdrawing, and electron-donating substituents in ortho, para or meta positions, were smoothly transformed into corresponding benzimidazoles 2a–2p in moderate to excellent yields under air atmosphere. Among these transformations, decomposition of amidines was inhibited effectively with 537-MOF as the catalyst (entries 1–16). Regardless of their positions (ortho, para or meta), electron-donating substituents for the starting nitriles, exerted no apparently negative effect on the reaction efficiency and provided the expected products 2c–2e in high yields (entries 3–5). The amidine with ortho-MeO group was also found to be compatible with the reaction conditions, while 2g was obtained in 70% yield (entry 7). Using the disubstituted amidines 2h–2o, the corresponding products could be isolated in admirable yields (entries 8–15). 6-Bromo-2-(o-tolyl)-1H-benzo[d]imidazole 1j, benefiting from ortho substituent (–Me), demonstrated preferable reactivity than 1i, provided 2j in 94% yield (entries 10–11). Para-Substituted electron-withdrawing (p-F, Cl, Br, I) on the N-aryl ring of the substituted N-phenylbenzimidamide gave superior yields than the one with electron-donating group (p-MeO), which presumably ascribed to the partial decomposition of 2m–2o (entries 10–15). Notably, we were pleased to find N-(4-bromophenyl)-5-chloro-2-methylbenzimidamide 1p could deliver the desired benzimidazole derivative in an excellent yield of 87% (entry 16).
| Entry | R1 | R2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: N-phenylbenzimidamide 1a (0.25 mmol), benzoic acid (5 equiv.), 537-MOF (10 mg), DMSO/DMF (2 mL, 1/1) at 100 °C under air atmosphere for 24 h.b Isolated yield. | ||||
| 1 | H | C6H5 |  |
96 (2a) |
| 2 | H | 4-ClC6H4 |  |
84 (2b) |
| 3 | H | 4-MeC6H5 |  |
90 (2c) |
| 4 | H | 3-MeC6H4 | 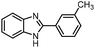 |
87 (2d) |
| 5 | H | 2-CH3C6H4 |  |
89 (2e) |
| 6 | H | 2-CF3C6H4 |  |
85 (2f) |
| 7 | H | 2-MeOC6H4 |  |
70 (2g) |
| 8 | H | 5-F-2-MeC6H5 | 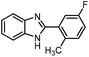 |
83 (2h) |
| 9 | 4-Cl | 3-Me |  |
85 (2i) |
| 10 | 4-Br | 2-MeC6H5 |  |
94 (2j) |
| 11 | 4-F | 2-CF3C6H5 | 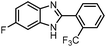 |
85 (2k) |
| 12 | 4-I | 2-MeC6H5 |  |
89 (2l) |
| 13 | 3-MeO | 4-ClC6H4 |  |
75 (2m) |
| 14 | 4-MeO | 4-CF3C6H5 |  |
70 (2n) |
| 15 | 3-F-4-MeO | C6H5 |  |
73 (2o) |
| 16 | Br | 2-Me,5-ClC6H5 | 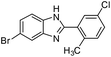 |
87 (2p) |
The catalyst was retrieved after reaction of 1a and characterized by powder X-ray diffraction (PXRD), which evidently confirmed that the catalyst maintained excellent crystallinity and was not damaged during the catalytic process (Fig. S4†). After three cycles, the high yields and high selectivity of the reactions were maintained (93% HPLC yield). Although it was failed to isolate the intermediate to illustrate the accurate mechanism, on the basis of the preliminary mechanistic studies for related processes,14 two possible paths of the C–H functionalization of amidines were proposed in Scheme 3. Path 1: coordination of 537-MOF24 and amidines is followed by electrophilic addition of copper center to N-phenyl ring to afford the metallacycle B.25 B is proposed to undergo reductive elimination of the metal and rearomatization to provide target product D. Alternatively, path 2: the amidines reacts with 537-MOF to generate copper nitrene C.26 Insertion of the nitrogen into a C–H bond of amidine, electrocyclic ring closure and subsequent [1,3]-shift of a hydrogen lead to the formation of D.
Taken together, the experiment shows that propeller type copper-catalyst has a positive catalytic activity, wherein {Cu2(O2C)4} secondary building unit (SBU) with a short Cu–Cu distance and four bridging carboxylate groups from TPPB2− ligands were crucial to the reaction. Compared with Cu(OAc)2, TPPB ligand was probably deemed to be the really key parameter for enhancement of coordination ability of 537-MOF as well as stabilizing intermediates B or C. Therefore, 537-MOF comprised of metal catalytic site and auxiliary ligand was utilized as a synergic catalyst, providing benzimidazoles in moderate to high yields from amidines without the requirement of ortho substituents for the staring nitriles under the atmosphere of air.
Conclusions
In summary, we have developed an efficient, practical and reliable route for 537-MOF-catalyzed C–H functionalization of amidines to the synthesis of diverse benzimidazoles. This new process features not only air condition instead of O2 atmosphere, but also extremely broad substrate scope and good functional-group compatibility, wherein challenging amidines derived from aryl nitriles without ortho-substituent afforded products in excellent yields. The synergic effect of 537-MOF was proposed to be the critical factor of high efficiency and selectivity. We anticipate that Cu–MOF will be utilized in other challenging reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the support from the National Natural Science Foundation of China (21471134, 21571158, and 21701148), Program for Science & Technology Innovative Research Team in University of Henan Province (15IRTSTHN002), Innovation Scientists and Technicians Troop Construction Projects of Henan Province (15-2101510003), Plan for Scientific Innovation Talent of Henan Province (154200510011), and Universities in Henan Province (17A150053).Notes and references
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (b) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS PubMed.
- F. Xie, S. Yu, Z. Qi and X. Li, Angew. Chem., Int. Ed., 2016, 55, 15351 CrossRef CAS PubMed.
- (a) W. Shu and C. Nevado, Angew. Chem., Int. Ed., 2017, 56, 1881 CrossRef CAS PubMed; (b) H. L. Li, Y. Kuninobu and M. Kanai, Angew. Chem., Int. Ed., 2017, 56, 1495 CrossRef CAS PubMed.
- (a) S. Wang, S.-Y. Chen and X.-Q. Yu, Chem. Commun., 2017, 53, 3165 RSC; (b) N. Thrimurtulu, A. Dey, D. Maiti and C. M. R. Volla, Angew. Chem., Int. Ed., 2016, 55, 12361 CrossRef CAS PubMed.
- (a) S. Takemoto, E. Shibata, M. Nakajima, Y. Yumoto, M. Shimamoto and H. Matsuzaka, J. Am. Chem. Soc., 2016, 138, 14836 CrossRef CAS PubMed; (b) Z. Ruan, S. K. Zhang, C. Zhu, P. N. Ruth, D. Stalke and L. Ackermann, Angew. Chem., Int. Ed., 2017, 56, 2045 CrossRef CAS PubMed.
- (a) H. Fu, P.-X. Shen, J. He, F. Zhang, S. Li, P. Wang, T. Liu and J.-Q. Yu, Angew. Chem., Int. Ed., 2017, 56, 1873 CrossRef CAS PubMed; (b) H. Shi, P. Wang, S. Suzuki, M. E. Farmer and J.-Q. Yu, J. Am. Chem. Soc., 2016, 138, 14876 CrossRef CAS PubMed; (c) S. Gao, H. Liu, Z. Wu, H. Yao and A. Lin, Green Chem., 2017, 19, 1861 RSC.
- D. C. Fabry, Y. A. Ho, R. Zapf, W. Tremel, M. Panthöfer, M. Rueping and T. H. Rehm, Green Chem., 2017, 19, 1911 RSC; P. Wang, S. Tang and A. Lei, Green Chem., 2017, 19, 2092 RSC.
- (a) S. Lin, W. Gao, Z. Tian, C. Yang, L. Lu, J.-L. Mergny, C.-H. Leung and D.-L. Ma, Chem. Sci., 2015, 6, 4284 RSC; (b) F. Wang, J. Hu, X. Cao, T. Yang, Y. Tao, L. Mei, X. Zhang and W. Huang, J. Mater. Chem. C, 2015, 3, 5533 RSC; (c) K.-C. Liu, S. M. Sakya, C. J. O Donnell, A. C. Flick and J. Li, Bioorg. Med. Chem., 2011, 21, 1136 CrossRef PubMed; (d) H. Xu, D.-H. Yu, L.-L. Liu, P.-F. Yan, L.-W. Jia, G.-M. Li and Z.-Y. Yue, J. Phys. Chem. B, 2010, 114, 141 CrossRef CAS PubMed; (e) M. Boiani and M. Gonzalez, Mini-Rev. Med. Chem., 2005, 5, 409 CrossRef CAS PubMed; (f) T. Fekner, J. Gallucci and M. K. Chan, J. Am. Chem. Soc., 2004, 126, 223 CrossRef CAS PubMed; (g) S. R. LaPlante, A. Jakalian, N. Aubry, Y. Bousquet, J. M. Ferland, J. Gillard, S. Lefebvre, M. Poirier, Y. S. Tsantrizos, G. Kukolj and P. L. Beaulieu, Angew. Chem., Int. Ed., 2004, 43, 4306 CrossRef CAS PubMed.
- (a) D. Yang, D. Fokas, J. Li, L. Yu and C. M. Baldino, Synthesis, 2005, 47 Search PubMed; (b) C. Zhu and Y. Wei, ChemSusChem, 2011, 4, 9513 CrossRef PubMed.
- (a) S.-K. Xiang, W. Tan, D.-X. Zhang, X.-L. Tian, C. Feng, B.-Q. Wang, K.-Q. Zhao, P. Hu and H. Yang, Org. Biomol. Chem., 2013, 11, 7271 RSC; (b) J. Huang, Y. He, Y. Wang and Q. Zhu, Chem.–Eur. J., 2012, 18, 13964 CrossRef CAS PubMed.
- Q. Xiao, W.-H. Wang, G. Liu, F.-K. Meng, J.-H. Chen, Z. Yang and Z.-J. Shi, Chem.–Eur. J., 2009, 15, 7292 CrossRef CAS PubMed.
- (a) J. S. Peng, M. Ye, C. J. Zong, F. Y. Hu, L. T. Feng, X. Y. Wang, Y. F. Wang and C. X. Chen, J. Org. Chem., 2011, 76, 716 CrossRef CAS PubMed; (b) N. Zheng, K. W. Anderson, X. Huang, H. N. Nguyen and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 7509 CrossRef CAS PubMed.
- M. Sun, C. Chen and W. Bao, RSC Adv., 2014, 4, 47373 RSC.
- G. Brasche and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 1932 CrossRef CAS PubMed.
- (a) O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 15507 CrossRef PubMed; (b) H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5403 RSC; (c) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed.
- (a) X. Gao, X. Hai, H. Baigude, W. Guan and Z. Liu, Sci. Rep., 2016, 6, 37705 CrossRef CAS PubMed; (b) H. Zheng, Y. Zhang, L. Liu, W. Wan, P. Guo, A. M. Nystrom and X. Zou, J. Am. Chem. Soc., 2016, 138, 962 CrossRef CAS PubMed; (c) C. He, K. Lu, D. Liu and W. Lin, J. Am. Chem. Soc., 2014, 136, 5181 CrossRef CAS PubMed.
- (a) L. H. Wee, M. Meledina, S. Turner, G. V. Tendeloo, K. Zhang, L. M. Rodriguez-Albelo, A. Masala, S. Bordiga, J. Jiang, J. Navarro, C. E. Kirschhock and J. A. Martens, J. Am. Chem. Soc., 2017, 139, 819 CrossRef CAS PubMed; (b) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (c) J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- (a) M. Witman, S. Ling, A. Gladysiak, K. C. Stylianou, B. Smit, B. Slater and M. Haranczyk, J. Phys. Chem. C, 2017, 121, 1171 CrossRef CAS PubMed; (b) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. OKeeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed.
- (a) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC; (b) K. C. Stylianoum, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119 CrossRef PubMed.
- (a) M. T. Zhao, K. Yuan, Y. Wang, G. D. Li, J. Guo, L. Gu, W. P. Hu, H. J. Zhao and Z. Y. Tang, Nature, 2016, 539, 76 CrossRef CAS PubMed; (b) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed; (c) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (d) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC.
- H. Fei and S. M. Cohen, J. Am. Chem. Soc., 2015, 137, 2191 CrossRef CAS PubMed.
- Y. Qi, Y. Luan, J. Yu, X. Peng and G. Wang, Chem.–Eur. J., 2015, 21, 1589 CrossRef CAS PubMed.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502 CrossRef CAS PubMed.
- (a) G.-C. Kuang, P. M. Guha, W. S. Brotherton, J. T. Simmons, L. A. Stankee, B. T. Nguyen, R. J. Clark and L. Zhu, J. Am. Chem. Soc., 2011, 133, 13984 CrossRef CAS PubMed; (b) M. Ahlquist and V. V. Fokin, Organometallics, 2007, 26, 4389 CrossRef CAS.
- K. Yamada, T. Kubo, H. Tokuyama and T. Fukuyama, Synlett, 2002, 231 CrossRef CAS.
- (a) K. Fauché, L. Nauton, L. Jouffret, F. Cisnetti and A. Gautier, Chem. Commun., 2017, 53, 2402 RSC; (b) K. Hou, D. A. Hrovat and X. Bao, Chem. Commun., 2015, 51, 15414 RSC; (c) P. Brandt, M. J. SNdergren, P. G. Andersson and P.-O. Norrby, J. Am. Chem. Soc., 2000, 122, 8013 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystal data and structural figures, gas adsorption, and characterization data. CCDC 1547691. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra10682c |
| This journal is © The Royal Society of Chemistry 2017 |




