Open Access Article
Open Access ArticleStabilization of arsenic in waste slag using FeCl2 or FeCl3 stabilizer
Yilong Lin,
Bin Wu,
Ping Ning ,
Guangfei Qu
,
Guangfei Qu *,
Junyan Li,
Xueqian Wang and
Ruosong Xie
*,
Junyan Li,
Xueqian Wang and
Ruosong Xie
Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, China. E-mail: qgflab@sina.com
First published on 4th December 2017
Abstract
With the aim of stabilizing arsenic pollution in mine tailing, FeCl2 and FeCl3 were chosen as stabilizers. The changes in pH, speciation, and leaching concentration of arsenic were analyzed. The stabilization mechanism of the FeCl2 and FeCl3 stabilizers towards the removal of arsenic has been discussed based on FTIR spectroscopy and XRD results; the results show that both the FeCl2 and FeCl3 stabilizers can reduce the pH of arsenic waste slag, but pose the risk of acidification, especially for FeCl3. Both stabilizers could reduce the content of acid-soluble arsenic. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was 1.0 and an FeCl2 mixed solution at pH = 7 and FeCl3 mixed solution at pH = 4 and pH = 7 were used, the acid-soluble arsenic was decreased by 96.22%, 93.42%, and 96.22%, respectively. The arsenic leaching concentration <2.5 mg L−1, which meets the minimum requirements for the entrance of safe landfill sites, demonstrates that the FeCl2 and FeCl3 stabilizers have good stabilizing effects. The acid-soluble arsenic or reducible arsenic can be converted into residual arsenic by the FeCl2 stabilizer. Furthermore, acid-soluble arsenic was converted into residual arsenic and oxidized arsenic by the FeCl3 stabilizer.
As molar ratio was 1.0 and an FeCl2 mixed solution at pH = 7 and FeCl3 mixed solution at pH = 4 and pH = 7 were used, the acid-soluble arsenic was decreased by 96.22%, 93.42%, and 96.22%, respectively. The arsenic leaching concentration <2.5 mg L−1, which meets the minimum requirements for the entrance of safe landfill sites, demonstrates that the FeCl2 and FeCl3 stabilizers have good stabilizing effects. The acid-soluble arsenic or reducible arsenic can be converted into residual arsenic by the FeCl2 stabilizer. Furthermore, acid-soluble arsenic was converted into residual arsenic and oxidized arsenic by the FeCl3 stabilizer.
1 Introduction
In natural soil, the arsenic content is generally about 1–20 mg kg−1, which does not affect the natural growth of creatures. However, due to human activities, such as mining, smelting, and application of arsenic-containing pesticides and fertilizers, the soil can be polluted to different degrees in some areas,1,2 especially in the Yunnan province, which is known as a non-ferrous metals kingdom, that is rich in non-ferrous metal mineral resources. Due to the mining and smelting process of non-ferrous metals, the open stockpiling of tailings and metallurgical slag has caused a series of severe arsenic pollution issues. Heavy metals are persistent, covert, and non-biodegradable, and their pollution and hazards can exist in the environment for a long time. Arsenic (As) is a highly toxic and carcinogenic chemical element that causes serious environmental and health problems all over the world.3–5The biological toxicity and environmental behavior of heavy metals not only have a relationship with the total amount of heavy metals, but they also depend on their morphology. The speciation distribution of heavy metals can predict and explain their chemical activity, bioavailability, toxicity, and effects on the ecological system.6,7 The main forms of arsenic in soil are water-soluble arsenic, adsorptive arsenic, and arsenic compounds.8 Adsorptive arsenic and arsenic compounds, which are difficult to dissolve, are closely combined with soil and not easily released or degraded by microorganisms.9 Soluble arsenic is the active arsenic present in soil whose bioavailability is relatively high, and it can be absorbed by plants. This type of arsenic contamination is very serious and deserves significant attention. At present, the remediation of heavy metal pollution can be divided into in situ remediation and ex situ remediation. In situ remediation technologies include in situ physical technology, in situ chemical technology, and in situ bioremediation technology.10,11 Ex situ remediation technologies include washing, ultrasonic-assisted extraction, and stabilization; moreover, studies have shown that goethite, FeSO4, Fe2(SO4)3, and lime are effective towards the immobilization of arsenic.12–14 The valence state and morphology of arsenic in the environment are not static, and the arsenic species change over time. Thus, it is feasible to change the forms of arsenic waste slag by adding a stabilizing agent, which turns soluble arsenic into insoluble arsenic. There is a strong affinity between iron and arsenic, and they can produce an insoluble precipitate; therefore, iron has a good ability to stabilize arsenic.15,16 There are many studies on the use of ferric salts, iron oxide or zero-valent iron for the removal of arsenic in water, but only a few studies have been reported on their use towards arsenic waste slag pollution treatment and remediation.
Considering the good performance of ferric salts towards the removal of arsenic and the urgent need for efficient stabilization operating using a fast and low-cost approach, we have utilized FeCl2 and FeCl3 as stabilizers. Upon adding the FeCl2 and FeCl3 solution, the changes in pH, arsenic form, and the amount of arsenic leaching concentration were analyzed, and the stabilizing effects of the FeCl2 and FeCl3 stabilizers on arsenic were studied. The aim of this study was to find a stabilizer, which has a good repair effect on arsenic waste slag, and provide a scientific basis and theoretical support for the remediation of arsenic waste slag. Using the BCR method, arsenic speciation analysis in the waste slag of the mining area was performed, and the morphological changes before and after stabilization were detected. A preliminary study on the effect of the stabilizer on the migration of arsenic in the mining area was conducted to provide a scientific basis for the treatment of the historical legacy of the mining area and even other similar arsenic pollution waste slags.
2 Materials and methods
2.1 Raw materials
The raw material of waste rock and tailings were obtained from the Chuxiong Province of Yunnan (an abandoned arsenic mine). The samples were stored in sample bags, and the inside air was discharged. The samples were taken to the laboratory and then dried naturally; after all macroscopic and separable contaminants were eliminated, the samples were crushed into powder using a grinder. The powder was screened and divided into different pore size samples and then preserved. The physical and chemical properties of the samples are shown in Table 1, Fig. 1, and Table 2.| Elementary composition/% | O | Si | Ca | Al | Mg | S | As | C | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 1# Storage place | 47.5 | 14.9 | 8.1 | 6.6 | 5.9 | 1.4 | 4.1 | 3.6 | 3.4 |
| Sample | pH | Arsenic content/g kg−1 | Arsenic content/% | Arsenic oxides/% | Orpiment & realgar/% | Arsenopyrite/% | As leaching concentration/mg L−1 |
|---|---|---|---|---|---|---|---|
| 1# Tailings | 6.5 | 40.6 | 4.1 | 1.9 | 1.8 | 0.2 | 64.3 |
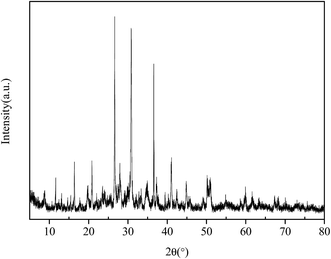
As observed from Table 1, the main heavy metal pollutant of slag is arsenic, as ascertained by the XRF analysis. The chemical composition is complex. Among the heavy metal elements present, arsenic is the major constituent. The following study was focused on the morphological analysis of arsenic utilizing XRD to analyze the compounds present in the powder of the arsenic residue.
The samples were analyzed using X-ray diffraction from the 1# storage place, which is presented in Fig. 1. The arsenic-containing compounds were divided into three forms mainly composed of arsenic oxides, orpiment, and realgar. By calculating the crystallinity of the compound, the relative content percentage can be obtained roughly.
As shown in Table 2, the content of arsenic in the sample 1# was 40.6 g kg−1, and the leaching concentration of arsenic was 64.3 g kg−1. The iron-containing materials FeCl2·4H2O and FeCl3·6H2O (analytically pure chemical reagents) were used in the experiments.
2.2 Experimental method
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratios was added to the beaker, and the solid–liquid ratio was controlled at 3
As molar ratios was added to the beaker, and the solid–liquid ratio was controlled at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pH was rapidly adjusted to the determined value using a 40% H2SO4 solution or 20% NaOH solution. Additional deionized water was to ensure the solid–liquid ratio was controlled at 3
1. The pH was rapidly adjusted to the determined value using a 40% H2SO4 solution or 20% NaOH solution. Additional deionized water was to ensure the solid–liquid ratio was controlled at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After the previous preparation was completed, each arsenic-containing mixed solution sample was mixed using a stirrer at 200 rpm for a certain period of time to ensure the stabilizers and samples were mixed thoroughly and reacted completely. The fully reacted samples (denoted as an FeCl2 mixed solution or FeCl3 mixed solution) were placed in an oven, then dried at 60 °C, stored, and used for characterization.
1. After the previous preparation was completed, each arsenic-containing mixed solution sample was mixed using a stirrer at 200 rpm for a certain period of time to ensure the stabilizers and samples were mixed thoroughly and reacted completely. The fully reacted samples (denoted as an FeCl2 mixed solution or FeCl3 mixed solution) were placed in an oven, then dried at 60 °C, stored, and used for characterization.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio of 0.1, 0.25, 0.5, 1.0, 2.0, 3.0, and 4.0. The reaction was performed for 24 h, and the pH was kept constant; after the reaction was complete, the stabilizer and arsenic waste slag mixed solution were dried for 24 h, ground, and sieved prior to use. Then, 0.50 g of the reaction product was used to extract the 4 arsenic species step-by-step in accordance with the BCR sequential extraction method.
As molar ratio of 0.1, 0.25, 0.5, 1.0, 2.0, 3.0, and 4.0. The reaction was performed for 24 h, and the pH was kept constant; after the reaction was complete, the stabilizer and arsenic waste slag mixed solution were dried for 24 h, ground, and sieved prior to use. Then, 0.50 g of the reaction product was used to extract the 4 arsenic species step-by-step in accordance with the BCR sequential extraction method.
(1) Morphological analysis. The BCR 3 level 4 step extraction method was used to analyze the arsenic speciation (The European Community Bureau of Reference 1992).17–19 The method is an improvement of the Tessier sequential extraction process. The salt obtained by the extraction process is lower in amount than that obtained by the Tessier sequential extraction method, which will help in the subsequent determination and has a better combined effect.
The steps used in the BCR leaching extraction method are shown in Fig. 2.
(2) The leaching solution. Using the Chinese “Solid waste-extraction procedure for leaching toxicity – sulfuric acid & nitric acid method (SNP)(HJ/T299-2007)” national standard to perform the leaching test, we obtained the arsenic element leaching concentration (mol L−1) to estimate the effect of the stabilizers.
The steps used to determine the As leaching concentration in this experiment were as follows.
Each 10.00 g arsenic waste slag sample was crushed into powder (<180 μm) using a grinder. According to the solid–liquid ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (g mL−1), the arsenic waste slag samples and the leach liquor were added into a conical flask (250 mL). The leach liquor was a mixed solution of concentrated sulfuric acid and concentrated nitric acid (mass ratio = 2
10 (g mL−1), the arsenic waste slag samples and the leach liquor were added into a conical flask (250 mL). The leach liquor was a mixed solution of concentrated sulfuric acid and concentrated nitric acid (mass ratio = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The sample solution pH was adjusted to 3.20 ± 0.05, and the solution was then sealed in the conical flask. The conical flasks were fixed to a horizontal oscillating device; after the previous preparation was completed, each sample was mixed at a vibration rate of 110 ± 10 min−1 for 18 h at room temperature to ensure that the leach liquor and samples were mixed thoroughly and reacted completely. Finally, the concentration of arsenic in the leaching solution was measured after centrifugal filtration.
1). The sample solution pH was adjusted to 3.20 ± 0.05, and the solution was then sealed in the conical flask. The conical flasks were fixed to a horizontal oscillating device; after the previous preparation was completed, each sample was mixed at a vibration rate of 110 ± 10 min−1 for 18 h at room temperature to ensure that the leach liquor and samples were mixed thoroughly and reacted completely. Finally, the concentration of arsenic in the leaching solution was measured after centrifugal filtration.
2.3 Chemical analysis method
Atomic fluorescence spectrometry was used to measure the total arsenic content in accordance with the “Analysis of total arsenic contents in soils (GB/T 22105-2008)” national standard. The solid waste-determination of arsenic–silver diethyldithiocarbamate spectrophotometric method (GB/T 15555.3-1995) was used to determine the As leaching concentration.3 Results and discussion
3.1 The acidification effects of iron-containing materials on arsenic waste slag
Since FeCl2 or FeCl3 is acidic, use of FeCl2 or FeCl3 as a stabilizer to stabilize arsenic waste slag causes the entire reaction system pH to decrease. The change in pH along with the increasing Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio is shown in Fig. 3; after the FeCl2 or FeCl3 treatment, the pH of the arsenic waste slag mixed solution decreased, and the greater the amount of the stabilizer added, the greater the pH decrease. However, despite the fact that both FeCl2 and FeCl3 lead to a reduction in the pH, the pH reduction rate of arsenic waste slag upon treatment with FeCl3 is higher than that with FeCl2; this indicates that FeCl3 is more effective in the acidification of arsenic waste slag.
As molar ratio is shown in Fig. 3; after the FeCl2 or FeCl3 treatment, the pH of the arsenic waste slag mixed solution decreased, and the greater the amount of the stabilizer added, the greater the pH decrease. However, despite the fact that both FeCl2 and FeCl3 lead to a reduction in the pH, the pH reduction rate of arsenic waste slag upon treatment with FeCl3 is higher than that with FeCl2; this indicates that FeCl3 is more effective in the acidification of arsenic waste slag.
 | ||
Fig. 3 The effects of the FeCl2 and FeCl3 stabilizers with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratios on the mixed solution pH. As molar ratios on the mixed solution pH. | ||
3.2 The effects of the mixed solution pH with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) As molar ratios on the As leaching concentration
As molar ratios on the As leaching concentration
Iron and aluminum have good effects on the stabilization of arsenic.20–25 The effects of the mixed solution pH with different Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratios on the As leaching concentration observed using the FeCl2 and FeCl3 stabilizers are shown in Fig. 4.
As molar ratios on the As leaching concentration observed using the FeCl2 and FeCl3 stabilizers are shown in Fig. 4.
 | ||
Fig. 4 The effects of the mixed solution pH with different molar ratios of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As on the As leaching concentration observed using (a) FeCl2 and (b) FeCl3. As on the As leaching concentration observed using (a) FeCl2 and (b) FeCl3. | ||
As shown in Fig. 4(a), the application of FeCl2 as a stabilizer with a Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 0–4.0 was studied. At pH = 4.5, 6.5, 7.5, and 8.5, the As leaching concentration decreased as the amount of ferrous ion increased. When the Fe
As molar ratio = 0–4.0 was studied. At pH = 4.5, 6.5, 7.5, and 8.5, the As leaching concentration decreased as the amount of ferrous ion increased. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was ≥1.0, the As leaching concentration was lower than 2.5 mg L−1 at pH = 6.5 and 7.5. At pH = 9.5, the As leaching concentration initially decreased and then increased with the addition of FeCl2.
As molar ratio was ≥1.0, the As leaching concentration was lower than 2.5 mg L−1 at pH = 6.5 and 7.5. At pH = 9.5, the As leaching concentration initially decreased and then increased with the addition of FeCl2.
FeCl3 was also investigated as a stabilizer, as shown in Fig. 4(b). At pH = 4.5, 6.5, 7.5, and 8.5, the As leaching concentration decreased with an increase in the amount of ferric iron. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was ≥1.0, the As leaching concentration was lower than 2.5 mg L−1. When the pH = 9.5, the As leaching concentration initially improved and then decreased with the addition of FeCl3.
As molar ratio was ≥1.0, the As leaching concentration was lower than 2.5 mg L−1. When the pH = 9.5, the As leaching concentration initially improved and then decreased with the addition of FeCl3.
Both excessive alkalinity and acidity may lead to a steady decline in the stabilization effect mainly because the Fe(OH)3 colloid decomposes under excessively acidic conditions. Generally, the Fe(OH)3 colloid has a positive charge; however, under excessively alkaline conditions, a large number of OH− ions are adsorbed by the Fe(OH)3 colloid, and this results in a negatively charged species. AsO33− and AsO43− are hard to stabilize, even when adsorbed via a physical reaction. Therefore, a high pH does not favor the stabilization of arsenic.
The results indicated that the stabilization effect of As was not only related to the dosage of the iron salt, but also closely related to the pH. The reasons for the different variation tendencies observed between FeCl2 and FeCl3 were investigated in the following experiments.
3.3 The effects of the FeCl2 or FeCl3 stabilizers at different pH values on the As leaching concentration
When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 1.0, the As leaching concentration is lower than 2.5 mg L−1 in most cases, as shown in Fig. 4, and a further increase in the dosage of the iron salt does not have a big impact. Thus, we chose an Fe
As molar ratio = 1.0, the As leaching concentration is lower than 2.5 mg L−1 in most cases, as shown in Fig. 4, and a further increase in the dosage of the iron salt does not have a big impact. Thus, we chose an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 1.0 as a basis condition, and deionized water was added to achieve a solid–liquid ratio = 3
As molar ratio = 1.0 as a basis condition, and deionized water was added to achieve a solid–liquid ratio = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pH of the reaction system was adjusted to 1, 3, 4, 5, 6, 7, 8, 9, 10, and 11 using a 40% H2SO4 solution or 20% NaOH solution. The effect of pH on the As leaching concentration was studied. The results are shown in Fig. 5, and the best stabilizing effect pH range observed for FeCl2 and FeCl3 is not the same. When FeCl2 was used as a stabilizer, at pH = 6.5–7.5, the As leaching concentration was at its lowest level; this indicated that the FeCl2 stabilizer displayed a good stabilizing effect under neutral conditions. When FeCl3 was used as a stabilizer, at pH = 4.0–5.0, the As leaching concentration reached its lowest; this suggested that the FeCl3 stabilizer had a good stabilizing effect under acidic conditions. pH has a great influence on the FeCl2 or FeCl3 stabilizer. The different stabilizers have different optimal pH ranges.
1. The pH of the reaction system was adjusted to 1, 3, 4, 5, 6, 7, 8, 9, 10, and 11 using a 40% H2SO4 solution or 20% NaOH solution. The effect of pH on the As leaching concentration was studied. The results are shown in Fig. 5, and the best stabilizing effect pH range observed for FeCl2 and FeCl3 is not the same. When FeCl2 was used as a stabilizer, at pH = 6.5–7.5, the As leaching concentration was at its lowest level; this indicated that the FeCl2 stabilizer displayed a good stabilizing effect under neutral conditions. When FeCl3 was used as a stabilizer, at pH = 4.0–5.0, the As leaching concentration reached its lowest; this suggested that the FeCl3 stabilizer had a good stabilizing effect under acidic conditions. pH has a great influence on the FeCl2 or FeCl3 stabilizer. The different stabilizers have different optimal pH ranges.
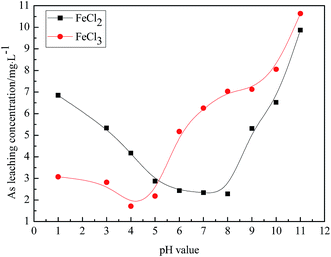 | ||
| Fig. 5 The effects of the FeCl2 and FeCl3 stabilizers at different pH values on the As leaching concentration. | ||
3.4 The effects of the FeCl2 or FeCl3 stabilizers on As speciation
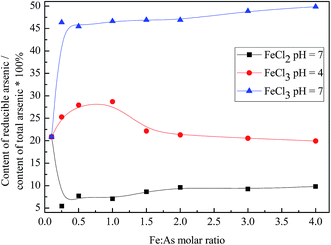
In the BCR extraction process, the As species were divided into acid-soluble arsenic, reducible arsenic, oxidized arsenic, and residual arsenic. After testing, the contents of acid-soluble arsenic, reducible arsenic, oxidized arsenic, and residual arsenic in the tested arsenic waste slag were 4.13 mg kg−1 (10.17%), 8.47 mg kg−1 (20.85%), 15.64 mg kg−1 (38.53%), and 12.36 mg kg−1 (30.45%), respectively.The acid-soluble arsenic is the exchangeable and carbonate-binding state. The reducible arsenic is the Fe/Mn oxide-combined state. The oxidized arsenic is the organic and sulfide binding state. The residual arsenic is the product of the reaction with aqua regia. The first three species are the extractable and effective species. We studied the effect of the FeCl2 or FeCl3 stabilizers at different stages.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 0.5, the FeCl2 and arsenic waste slag mixed solution at pH = 7, and the FeCl3 mixed solution in pH = 4 and pH = 7, the content of the acid-soluble arsenic decreased by 78.66%, 92.58%, and 93.52%, respectively. The effect of the FeCl3 stabilizer was better than that of the FeCl2 stabilizer. As the amount of the stabilizer increased, the acid-soluble arsenic content decreased in the Fe
As molar ratio = 0.5, the FeCl2 and arsenic waste slag mixed solution at pH = 7, and the FeCl3 mixed solution in pH = 4 and pH = 7, the content of the acid-soluble arsenic decreased by 78.66%, 92.58%, and 93.52%, respectively. The effect of the FeCl3 stabilizer was better than that of the FeCl2 stabilizer. As the amount of the stabilizer increased, the acid-soluble arsenic content decreased in the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio range of 0–2.0. When the Fe
As molar ratio range of 0–2.0. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was >2.0, addition of the stabilizer did not decrease the acid-soluble arsenic content obviously. When the Fe
As molar ratio was >2.0, addition of the stabilizer did not decrease the acid-soluble arsenic content obviously. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 4.0, the acid-soluble arsenic content decreased by 97.61%, 95.48%, and 96.00%, respectively. In this case, the FeCl2 stabilizer has a better effect on the reduction of acid-soluble arsenic.
As molar ratio = 4.0, the acid-soluble arsenic content decreased by 97.61%, 95.48%, and 96.00%, respectively. In this case, the FeCl2 stabilizer has a better effect on the reduction of acid-soluble arsenic.
Under the conditions of the FeCl2 mixed solution at pH = 7 and the FeCl3 mixed solution at pH = 4 and pH = 7, the effect of the FeCl2 or FeCl3 stabilizer on the reducible arsenic was studied. As shown in Fig. 7, the use of the FeCl2 stabilizer at pH = 7 decreased the content of reducible arsenic instantly. However, upon increasing the amount of the stabilizer, the tendency showed a slight change. The use of the FeCl3 stabilizer at pH = 7 increased the content of reducible arsenic. Moreover, when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 4.0, the content of reducible arsenic increased nearly 138.97%. At pH = 4, as the dosage of FeCl3 increased, the content of reducible arsenic increased initially in the Fe
As molar ratio = 4.0, the content of reducible arsenic increased nearly 138.97%. At pH = 4, as the dosage of FeCl3 increased, the content of reducible arsenic increased initially in the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio range of 0–0.5 and then decreased when the Fe
As molar ratio range of 0–0.5 and then decreased when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was >0.5. When the ratio of Fe
As molar ratio was >0.5. When the ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As was 0.5, the content of reducible arsenic reached its highest, which increased by 37.52% when compared with that of the sample without a stabilizer. Until the Fe
As was 0.5, the content of reducible arsenic reached its highest, which increased by 37.52% when compared with that of the sample without a stabilizer. Until the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 3.0, the content of reducible arsenic was lower than that of the sample without the stabilizer. When the Fe
As molar ratio = 3.0, the content of reducible arsenic was lower than that of the sample without the stabilizer. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 4.0, the content of reducible arsenic decreased by 4.43%. From the abovementioned data, we could predict that the stable mechanisms of the effects of different valence iron salt stabilizers on the reducible arsenic were different under various pH conditions. Under neutral conditions, the treatment of arsenic waste slag with the FeCl2 stabilizer can decrease the reducible arsenic and convert it into a more stable form. The FeCl3 stabilizer has little contribution to the reduction of reducible arsenic. Thus, the addition of the FeCl2 stabilizer under neutral conditions helps to lower the content of the harmful reducible arsenic.
As molar ratio = 4.0, the content of reducible arsenic decreased by 4.43%. From the abovementioned data, we could predict that the stable mechanisms of the effects of different valence iron salt stabilizers on the reducible arsenic were different under various pH conditions. Under neutral conditions, the treatment of arsenic waste slag with the FeCl2 stabilizer can decrease the reducible arsenic and convert it into a more stable form. The FeCl3 stabilizer has little contribution to the reduction of reducible arsenic. Thus, the addition of the FeCl2 stabilizer under neutral conditions helps to lower the content of the harmful reducible arsenic.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio range of 0–0.5 and then decreased when the Fe
As molar ratio range of 0–0.5 and then decreased when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was >0.5. When the Fe
As molar ratio was >0.5. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 0.5, the oxidized arsenic content was at its highest level, which increased by 27.88%. At pH = 4, the use of FeCl3 initially decreased the oxidized arsenic content in the Fe
As molar ratio = 0.5, the oxidized arsenic content was at its highest level, which increased by 27.88%. At pH = 4, the use of FeCl3 initially decreased the oxidized arsenic content in the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio range of 0–0.5 and then increased when the Fe
As molar ratio range of 0–0.5 and then increased when the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio was >0.5. When the Fe
As molar ratio was >0.5. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 0.5, the oxidized arsenic content was at its lowest level, which decreased by 3.36%. When the Fe
As molar ratio = 0.5, the oxidized arsenic content was at its lowest level, which decreased by 3.36%. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio = 4.0, the oxidized arsenic content was at its highest level, which increased by 16.53%. When the pH = 7, the use of FeCl3 increased the content of oxidized arsenic. However, upon increasing the dosage of iron, the amplitude gradually decreased. The content of oxidized arsenic increased by 26.34% at most.
As molar ratio = 4.0, the oxidized arsenic content was at its highest level, which increased by 16.53%. When the pH = 7, the use of FeCl3 increased the content of oxidized arsenic. However, upon increasing the dosage of iron, the amplitude gradually decreased. The content of oxidized arsenic increased by 26.34% at most.
 | ||
Fig. 8 The effects of the FeCl2 or FeCl3 stabilizers with different molar ratios of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As on the oxidized arsenic content. As on the oxidized arsenic content. | ||
From the abovementioned data, we can predict that in the stabilization process, ferrous and ferric iron can transform the other forms of arsenic into oxidized arsenic, which is more stable. Through this reaction mechanism, arsenic in the waste slag became stable; this reduced the chance of its contact with the environment and also reduced its biological toxicity.
3.5 Characterization of the samples
 | ||
| Fig. 10 The FTIR spectra of the arsenic waste slag samples obtained before and after carrying out the stabilization step using FeCl2 and FeCl3. | ||
The stretching vibration absorption peak of –OH appeared at 3417 cm−1, and the variable angle absorption peak of crystal water appeared at 1635 cm−1. This indicated that there existed crystalline water and hydroxyl groups, and the crystal water molecules did not produce intermolecular hydrogen bonds except while participating in the coordination with metal ions. Moreover, three distinct peaks were observed at 795 cm−1, 586 cm−1, and 638 cm−1, which proved the existence of iron oxyhydroxide FeOOH. The absorption peak of Fe2O3 appeared at 533 cm−1. These absorption peak data can be used to explain the stability effect of the iron compounds and hydroxyl groups in the process of the stabilization treatment. The anti-symmetric stretching vibration absorption peak of AsO43− appeared at 878 cm−1, and the stretching vibration absorption peak of As–O appeared at 840 cm−1. This indicated that there existed As–O bonding after the stabilization treatment process, and a relatively stable structure was formed. For better understanding the chemical compounds of arsenic species, the X-ray diffraction analysis was conducted.
As can be seen from Fig. 11, the XRD diffraction patterns of the samples before and after the stabilization step were basically the same because of arsenic infusion. There were only some changes in the peak intensity. This shows that the addition of stabilizers has no obvious effect on the crystal structure, the stabilizer does not affect the crystal structure of arsenic waste slag after the stabilization step, and a new crystal form is not formed by the reaction. According to the PDF card, a better crystal union and amorphous ferric arsenate or iron hydroxide FeOOH exist in the waste slag. The results are consistent with the FTIR results. During the whole diffraction pattern analysis, the peak intensity was sorted into (a) > (c) > (b). The higher the peak intensity, the less the impurity content, and the simpler the structure. Therefore, it can be explained that FeCl2 makes the arsenic slag structure simpler and have a better stability.
3.6 Mechanism of the FeCl2 or FeCl3 stabilizer
The experimental results show that at an Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio >1.0, the As leaching concentrations are below 2.5 mg L−1, which meet the safety requirements for landfills. Both the FeCl2 or FeCl3 stabilizers reduced the content of acid-soluble arsenic, thereby reducing the activity and biological toxicity of the arsenic waste slag and the threat to the environment. Under different conditions used for the FeCl2 or FeCl3 stabilizers, the transformation among the four forms of arsenic species was also slightly different. Therefore, pH was an important factor affecting the transformation of arsenic.
As molar ratio >1.0, the As leaching concentrations are below 2.5 mg L−1, which meet the safety requirements for landfills. Both the FeCl2 or FeCl3 stabilizers reduced the content of acid-soluble arsenic, thereby reducing the activity and biological toxicity of the arsenic waste slag and the threat to the environment. Under different conditions used for the FeCl2 or FeCl3 stabilizers, the transformation among the four forms of arsenic species was also slightly different. Therefore, pH was an important factor affecting the transformation of arsenic.
We speculate that the mechanism mainly includes the following 3 stages.
(1) The majority of the iron compound surface charge changes with the environmental pH, and there exists a pHpzc. The adsorption and desorption of H+ and OH− ions on the surface of iron oxide and the chemical behavior of the surface hydroxyl groups make the surface charge, and the main groups are: Fe–OH2, Fe–OH, and FeO−. The pHpzc of Fe(OH)3 is 7.9. Iron oxide is positively charged due to its the Fe–OH2 surface groups, and electrostatic attraction occurs between arsenate anions; moreover, an outer layer is formed on the surface of the iron oxide complex, which is non-obligate adsorption. The existence of an iron material influences the pH of the arsenic waste slag and thus affects the morphology of arsenic and surface charge of the soil colloid, which is advantageous towards arsenic stabilization.26
(2) The iron compound surface has functional groups (Fe–OH); moreover, –COO− is a bidentate ligand with metal ions (chelation) or connection bridge-type coordination. When adding the iron salt materials, the iron oxide adsorption of arsenic mainly takes the arsenate anion to the oxide surface or micropores via non-specific adsorption approach at first. Then, the Fe–OH and Fe–OH2 ligands of the multi-core dentate complexation ions [Fe(H2O)6]3+, [Fe2(OH)3]3+, and [Fe2(OH)2]4+ on the iron oxide surface exchange with them to form a Fe–O–AsO(OH)–O–Fe and Fe–O–As(OH)–O–Fe dual-core bridging inner complex. The arsenic will be fixed in the double electric layer.27–29
(3) Arsenic directly reacts with Fe2+ or Fe3+ in the waste slag to generate stable iron–arsenic compounds.
The reaction mechanism is shown in Fig. 12.
4 Conclusions
(1) Both the FeCl2 and FeCl3 stabilization treatments can significantly reduce the acid-soluble arsenic content. When the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) As molar ratio >1.0, the As leaching concentrations are below 2.5 mg L−1, which meet the safety requirements for landfills. This shows that both the FeCl2 and FeCl3 stabilizers display good performance towards the stabilization of arsenic from waste slag.
As molar ratio >1.0, the As leaching concentrations are below 2.5 mg L−1, which meet the safety requirements for landfills. This shows that both the FeCl2 and FeCl3 stabilizers display good performance towards the stabilization of arsenic from waste slag.
(2) Upon increasing the amount of iron materials, the FeCl2 stabilizer converts the acid-soluble arsenic and reducible arsenic into residual arsenic, and the FeCl3 stabilizer converts the acid-soluble arsenic into residual arsenic and oxidized arsenic. When FeCl2 or FeCl3 is used as a stabilizer, the arsenic waste slag should be stabilized at its most appropriate range of pH to achieve the best effect. The optimal pH of the FeCl2 or FeCl3 stabilizer is pH = 7 and pH = 4, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (KKGL201222003, No. 51368026) and the National Development and Reform Commission for Heavy Metal Treatment Foundation of China (2014-GH-03).References
- C. A. Impellitteri, Sci. Total Environ., 2005, 345, 175–190 CrossRef CAS PubMed.
- A. Krysiak and A. Karczewska, Sci. Total Environ., 2007, 379, 190–200 CrossRef CAS PubMed.
- S. Bozkurt, L. Moreno and I. Neretnieks, Sci. Total Environ., 2000, 250, 101–121 CrossRef CAS PubMed.
- J. C. Ng, J. Wang and A. Shraim, Chemosphere, 2003, 52, 1353–1359 CrossRef CAS PubMed.
- M. Jayasumana, P. Paranagama, M. Amarasinghe, K. Wijewardane, K. Dahanayake, S. Fonseka, K. Rajakaruna, U. Samarasinghe and V. Senanayake, J. Nat. Sci. Res., 2013, 3, 64–73 Search PubMed.
- J. Caruso, B. Klaue, B. Michalke and D. Rocke, Ecotoxicol. Environ. Saf., 2003, 56, 32–44 CrossRef CAS PubMed.
- C.-H. Tseng, Atherosclerosis, 2008, 199, 12–18 CrossRef CAS PubMed.
- X. Cao, L. Q. Ma and A. Shiralipour, Environ. Pollut., 2003, 126, 157–167 CrossRef CAS PubMed.
- L. W. Jacobs, J. K. Syers and D. R. Keeney, Soil Sci. Soc. Am. J., 1970, 34, 750–754 CrossRef CAS.
- G. Akinci and D. E. Guven, Desalination, 2011, 268, 221–226 CrossRef CAS.
- J.-f. Peng, Y.-h. Song, P. Yuan, X.-y. Cui and G.-l. Qiu, J. Hazard. Mater., 2009, 161, 633–640 CrossRef CAS PubMed.
- D. E. Voigt, S. L. Brantley and R. J. C. Hennet, Appl. Geochem., 1996, 11, 633–643 CrossRef CAS.
- T. J. Moore, C. M. Rightmire and R. K. Vempati, J. Soil Contam., 2000, 9, 375–405 CrossRef CAS.
- W. Hartley, R. Edwards and N. W. Lepp, Environ. Pollut., 2004, 131, 495–504 CrossRef CAS PubMed.
- M. Sadiq, Water, Air, Soil Pollut., 1997, 93, 117–136 CAS.
- I. A. Katsoyiannis and A. I. Zouboulis, Water Res., 2002, 36, 5141–5155 CrossRef CAS PubMed.
- P. Quevauviller, G. Rauret, J. F. López-Sánchez, R. Rubio, A. Ure and H. Muntau, Sci. Total Environ., 1997, 205, 223–234 CrossRef CAS.
- G. Ouzounidou, M. Čiamporová, M. Moustakas and S. Karataglis, Environ. Exp. Bot., 1995, 35, 167–176 CrossRef CAS.
- I. U. Umoren, A. P. Udoh and I. I. Udousoro, Environmentalist, 2007, 27, 241–252 CrossRef.
- Y. Liang, X. Min, L. Chai, M. Wang, W. Liyang, Q. Pan and M. Okido, Chemosphere, 2017, 168, 1142–1151 CrossRef CAS PubMed.
- A. J. Bora, S. Gogoi, G. Baruah and R. K. Dutta, J. Environ. Chem. Eng., 2016, 4, 2683–2691 CrossRef CAS.
- K. Yang, B. Chul Kim, G. Yu and K. Nam, Applicability of Stabilization with Iron Oxides for Arsenic-Contaminated Soil at the Forest Area near the Former Janghang Smelter Site, 2016 Search PubMed.
- L. Huang, Z. Liu, H. Li and Y. Liu, Guangdong Chem. Ind., 2017, 44(6), 59–65 Search PubMed.
- J. M. R. Antoine, L. A. H. Fung and C. N. Grant, Toxicol. Rep., 2017, 4, 181–187 CrossRef CAS PubMed.
- S. K. Karn, X. Pan and I. R. Jenkinson, 3 Biotech, 2017, 7, 50 CrossRef PubMed.
- N. Bolan, S. Mahimairaja, A. Kunhikrishnan and R. Naidu, J. Hazard. Mater., 2013, 261, 725–732 CrossRef CAS PubMed.
- Z. Hongshao and R. Stanforth, Environ. Sci. Technol., 2001, 35, 4753–4757 CrossRef CAS PubMed.
- J. Kumpiene, A. Lagerkvist and C. Maurice, Waste Manag., 2008, 28, 215–225 CrossRef CAS PubMed.
- L. Luo, S. Zhang, X. Q. Shan, W. Jiang, Y. G. Zhu, T. Liu, Y. N. Xie and R. G. McLaren, Environ. Toxicol. Chem., 2006, 25, 3118–3124 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |