Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design, synthesis, and herbicidal activity of pyrazole benzophenone derivatives†
Ying Fu ,
Meng-Xia Wang,
Dong Zhang,
Yu-Wen Hou,
Shuang Gao,
Li-Xia Zhao and
Fei Ye*
,
Meng-Xia Wang,
Dong Zhang,
Yu-Wen Hou,
Shuang Gao,
Li-Xia Zhao and
Fei Ye*
Department of Applied Chemistry, College of Science, Northeast Agricultural University, Harbin, 150030, P. R. China. E-mail: yefei@neau.edu.cn
First published on 4th October 2017
Abstract
4-Hydroxyphenylpyruvate dioxygenase is one of the most promising targets for herbicide discovery. A series of 1-acyl-3-phenyl-pyrazol benzophenones were designed and synthesized using 1,3-diphenylpropane-1,3-dione and dimethylformamide dimethylacetal as the starting materials. All of the compounds were characterized by IR, 1H NMR, 13C NMR, and HRMS. The configuration of 5f was determined by X-ray crystallography. The bioassay studies indicated that most of these derivatives exhibited herbicidal activity at least to a certain degree, in which compounds 5n and 5o displayed good herbicidal activity at a dosage of 0.05 mmol m−2, which were more potent than pyrazoxyfen against barnyard grass. In addition, compound 5o was also proved to be safer for maize than pyrazoxyfen. The binding free energy of compound 5o with HPPD was relatively low, and that agreed with the results of bioassay activity research. Therefore, compound 5o might be the lead compound for designing new HPPD inhibitors.
Introduction
4-Hydroxyphenylpyruvate dioxygenase (HPPD), a Fe(II)-dependent and non-heme dioxygenase, was founded by Zeneca Group PLC in 1982.1 HPPD catalyzes the conversion of 4-hydroxyphenylpyruvic acid (HPPA) to homogentisate (HGA) which involves decarboxylation, substituent migration and aromatic oxygenation in a single catalytic cycle.2–4 HGA is an important precursor for the biosynthesis of tocopherol and plastoquinone, which are crucial for the normal growth of plants. The plant will be injured severely when HPPD is inhibited, and the plant meristem will become bleached in sunlight and cause necrosis and finally death.5–7 Therefore, HPPD inhibitors are also termed bleaching herbicides.8,9 These herbicides have the advantages of a wide weed-control spectrum, flexibility for application time, and compatibility for tank mixes with other herbicides.10–13 Perennial broadleaf weeds in broad-leaf plant fields can also be weeded out by the HPPD inhibitor with high activities, low-residue, and application safety.11,12,14 HPPD inhibitors have been developed a number of different structures, such as triketone, pyrazole, isoxazole, diketonitrile and benzophenone.15,16 As reported, containing triketone quinolines derivatives were designed and showed good herbicidal activity of Arabidopsis thaliana HPPD (AtHPPD).17 And Yang et al. developed a series of triketone-based hybrid compounds which lengthening the aryl side chain with potent inhibitory activity.18The pyrazole and its derivatives have drawn wide attention in biological and pharmacological fields.19 As aromatic heterocyclic compounds, N-substituted pyrazole is important chemical scaffold, and a large number of pyrazole derivatives have been synthesized with extensive bioactivity. For example, N-substituted pyrazoles are good inhibitors of p38 mitogen-activated protein kinase for the treatment of cancer cells.20 It was reported that the pyrazole–benzimidazolone is a potential inhibitor to type I tyrosinemia.21 Furthermore, pyrazolone–quinazolone hybrids were confirmed as novel human 4-hydroxyphenylpyruvate dioxygenase inhibitors.22
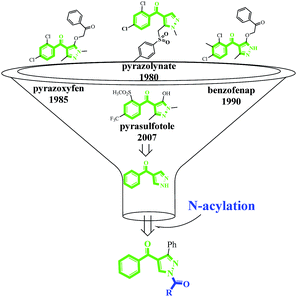
As shown in Fig. 1, there are four pyrazole HPPD inhibitors with good weed control containing pyrazolynate, pyrazoxyfen, benzofenap and pyrasulfotole. The common chemical subunit of these pyrazole inhibitors is 2-benzoylethen-1-ol. In order to search novel HPPD inhibitor analogues and continue to design and synthesis novel nitrogen-containing heterocycle pesticide,23–25 herein we reported the synthesis of a series of novel pyrazole derivatives via cyclization and acylation without any expensive reagent or catalyst (Scheme 1). Greenhouse experiments demonstrated that some of them exhibited promising herbicidal activity against barnyard grass at a rate of 0.05 mmol m−2.
Experimental
Chemicals and instruments
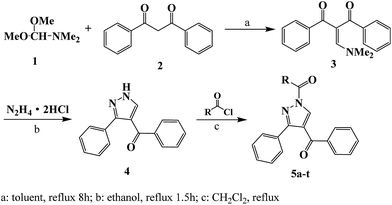
All the reagents were analytical grade and used without further purification. Analytical thin-layer chromatography (TLC) was performed on silica gel GF254 (Qingdao Haiyang Chemical Co. Ltd). The yields were not optimized. The melting point was measured using a Beijing Taike point apparatus (X-4) and the thermometer was not corrected. The infrared (IR) spectra were taken on a KJ-IN-27G infrared spectrophotometer (KBr). The NMR spectra were recorded on a Bruker AV600 spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. High-resolution mass spectrometry (HRMS) was obtained by FTICR-MS. Pyrazoxyfen were purchased from Tianjin Sigma Technology Co., Ltd.Synthesis of 2-dimethylaminomethylene-1,3-diphenylpropane-1,3-dione (3)
Compound 3 was synthesized according to the ref. 26. A mixture of 1,3-diphenylpropane-1,3-dione (2.24 g, 10 mmol) and dimethylformamide dimethylacetal (1.19 g, 10 mmol) in anhydrous toluene (50 mL) was heated and refluxed for 8 h, then cooled to room temperature. The compound 3 was obtained as faint yellow solid in 44% yield. Mp: 131–132 °C; 1H NMR (600 MHz, CDCl3): 2.83–3.14, (6H, –CH3); 7.59–7.16, (m, 10H, Ar–H); 7.64, (s, 1H, N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 42.3, 47.3, 111.5, 127.9 (4C), 129.0 (4C), 131.2 (2C), 141.0 (2C), 158.1, 194.9 (2C); IR (KBr, cm−1): 3051, 2923, 1651, 1584, 1562.
C); 13C NMR (150 MHz, CDCl3): 42.3, 47.3, 111.5, 127.9 (4C), 129.0 (4C), 131.2 (2C), 141.0 (2C), 158.1, 194.9 (2C); IR (KBr, cm−1): 3051, 2923, 1651, 1584, 1562.
Synthesis of phenyl-(3-phenyl-1H-pyrazol-4-yl) methanone (4)
Synthesis of compound 4 was based on the method presented earlier.26–29 Compound 3 (2.7 g, 10 mmol) was treated with N2H4·2HCl (1.04 g, 10 mmol) and refluxed in ethanol for 1.5 h, and appropriate distilled water was added to the solution before heating. The faint yellow solid product was obtained, filtered and recrystallized from ethanol. Compound 4 was obtained with the yield of 90%. Mp: 165–166 °C; 1H NMR (600 MHz, DMSO-d6): 7.38–7.78, (m, 10H, Ar–H); 8.10, (s, 1H, N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13.63, (br, 1H, NH); 13C NMR (150 MHz, DMSO-d6): 118.3, 128.6, 128.9 (2C), 129.1 (3C), 129.5 (4C), 132.8 (3C), 139.4, 190.1; IR (KBr, cm−1): 3345, 1651, 1562, 1433.
C); 13.63, (br, 1H, NH); 13C NMR (150 MHz, DMSO-d6): 118.3, 128.6, 128.9 (2C), 129.1 (3C), 129.5 (4C), 132.8 (3C), 139.4, 190.1; IR (KBr, cm−1): 3345, 1651, 1562, 1433.
General synthetic procedures for compounds 5a–t
Acyl chloride (12 mmol) were added dropwise to a solution of compound 4 (2.48 g, 10 mmol), and triethylamine (1.52 g, 15 mmol) as the attaching acid agent in CH2Cl2, and the mixture was refluxed for 4 h until the reaction was complete (indicated by TLC). The organic phase was washed with water until pH = 7. The organic layer was dried over anhydrous MgSO4 and vacuum distillation solvent. Compounds 5a–t was obtained by recrystallized with CH2Cl2 and light petroleum or column chromatography with CH2Cl2 and EtOH. The physical and spectra data of compounds 5a–t were as follows:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 122.4, 128.3 (2C), 28.4, 128.7 (2C), 128.9 (2C), 129.4 (2C), 129.7 (2C), 130.6, 131.1, 132.1 (2C), 133.4, 133.7, 135.4, 138.1, 155.8, 165.9, 189.8; IR (KBr, cm−1): 3154, 3062, 1706, 1654, 1596, 1446; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 353.1212, found 353.1286.
C); 13C NMR (150 MHz, CDCl3): 122.4, 128.3 (2C), 28.4, 128.7 (2C), 128.9 (2C), 129.4 (2C), 129.7 (2C), 130.6, 131.1, 132.1 (2C), 133.4, 133.7, 135.4, 138.1, 155.8, 165.9, 189.8; IR (KBr, cm−1): 3154, 3062, 1706, 1654, 1596, 1446; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 353.1212, found 353.1286.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 20.3, 122.7, 125.3, 128.3 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.7 (2C), 130.3, 131.0, 131.1, 131.3, 131.9, 133.5, 134.6, 138.0, 138.4, 155.9, 167.6, 189.8; IR (KBr, cm−1): 3124, 3031, 1713, 1655, 1599, 1449; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1443.
C); 13C NMR (150 MHz, CDCl3): 20.3, 122.7, 125.3, 128.3 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.7 (2C), 130.3, 131.0, 131.1, 131.3, 131.9, 133.5, 134.6, 138.0, 138.4, 155.9, 167.6, 189.8; IR (KBr, cm−1): 3124, 3031, 1713, 1655, 1599, 1449; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1443.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 21.5, 122.3, 128.2, 128.4 (2C), 128.7 (2C), 128.9 (2C), 129.3, 129.4, 129.7 (2C), 130.5, 131.2, 132.4, 133.4, 134.6, 135.5, 138.1, 138.2, 155.7, 166.2, 189.8; IR (KBr, cm−1): 3153, 3060, 1688, 1662, 1597, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1444.
C); 13C NMR (150 MHz, CDCl3): 21.5, 122.3, 128.2, 128.4 (2C), 128.7 (2C), 128.9 (2C), 129.3, 129.4, 129.7 (2C), 130.5, 131.2, 132.4, 133.4, 134.6, 135.5, 138.1, 138.2, 155.7, 166.2, 189.8; IR (KBr, cm−1): 3153, 3060, 1688, 1662, 1597, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1444.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 21.8, 122.1, 127.7, 128.3, 128.4, 128.6 (2C), 128.9, 129.1 (2C), 129.3, 129.6 (2C), 131.2, 132.1, 132.3, 132.5, 133.3, 135.4, 138.2, 144.8, 155.6, 165.7, 189.8; IR (KBr, cm−1): 3154, 3032, 1698, 1654, 1595, 1444; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1444.
C); 13C NMR (150 MHz, CDCl3): 21.8, 122.1, 127.7, 128.3, 128.4, 128.6 (2C), 128.9, 129.1 (2C), 129.3, 129.6 (2C), 131.2, 132.1, 132.3, 132.5, 133.3, 135.4, 138.2, 144.8, 155.6, 165.7, 189.8; IR (KBr, cm−1): 3154, 3032, 1698, 1654, 1595, 1444; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 367.1368, found 367.1444.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 123.2, 126.6, 128.3 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.6 (2C), 130.1, 130.4, 130.8, 132.2, 132.5, 132.6, 133.5, 133.9, 137.9, 156.2, 165.6, 189.6; IR (KBr, cm−1): 3126, 3032, 1735, 1662, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 387.0822, found 387.0895.
C); 13C NMR (150 MHz, CDCl3): 123.2, 126.6, 128.3 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.6 (2C), 130.1, 130.4, 130.8, 132.2, 132.5, 132.6, 133.5, 133.9, 137.9, 156.2, 165.6, 189.6; IR (KBr, cm−1): 3126, 3032, 1735, 1662, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 387.0822, found 387.0895.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 122.5, 128.4 (2C), 128.7 (4C), 128.8 (2C), 128.9, 129.5, 129.7 (2C), 130.9, 133.5, 133.6 (2C), 135.3, 138.0, 140.4, 156.0, 164.9, 189.7; IR (KBr, cm−1): 3145, 3036, 1707, 1659, 1589, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 387.0822, found 387.0896.
C); 13C NMR (150 MHz, CDCl3): 122.5, 128.4 (2C), 128.7 (4C), 128.8 (2C), 128.9, 129.5, 129.7 (2C), 130.9, 133.5, 133.6 (2C), 135.3, 138.0, 140.4, 156.0, 164.9, 189.7; IR (KBr, cm−1): 3145, 3036, 1707, 1659, 1589, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 387.0822, found 387.0896.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 115.5, 115.8, 122.3, 126.7, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.5 (2C), 129.7 (2C), 131.0, 133.5, 135.0, 135.2, 135.4, 138.0, 155.9, 164.6, 189.7; IR (KBr, cm−1): 3152, 3059, 1692, 1668, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 371.1118, found 371.1193.
C); 13C NMR (150 MHz, CDCl3): 115.5, 115.8, 122.3, 126.7, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.5 (2C), 129.7 (2C), 131.0, 133.5, 135.0, 135.2, 135.4, 138.0, 155.9, 164.6, 189.7; IR (KBr, cm−1): 3152, 3059, 1692, 1668, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 371.1118, found 371.1193.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 122.9, 125.2, 125.3, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.0, 129.6 (2C), 129.7 (2C), 130.8, 132.4 (2C), 133.6, 133.9, 134.6, 135.1, 137.9, 156.2, 164.9, 189.6; IR (KBr, cm−1): 3175, 3061, 1715, 1664, 1597, 1446; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 421.1086, found 421.1159.
C); 13C NMR (150 MHz, CDCl3): 122.9, 125.2, 125.3, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.0, 129.6 (2C), 129.7 (2C), 130.8, 132.4 (2C), 133.6, 133.9, 134.6, 135.1, 137.9, 156.2, 164.9, 189.6; IR (KBr, cm−1): 3175, 3061, 1715, 1664, 1597, 1446; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 421.1086, found 421.1159.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 123.2, 123.3 (2C), 128.5 (5C), 129.7 (2C), 129.7 (2C), 130.5, 133.0 (2C), 133.6, 134.8, 136.1, 137.8, 150.5, 156.5, 164.3, 189.5; IR (KBr, cm−1): 3134, 3051, 1715, 1657, 1596, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 398.1063, found 398.1129.
C); 13C NMR (150 MHz, CDCl3): 123.2, 123.3 (2C), 128.5 (5C), 129.7 (2C), 129.7 (2C), 130.5, 133.0 (2C), 133.6, 134.8, 136.1, 137.8, 150.5, 156.5, 164.3, 189.5; IR (KBr, cm−1): 3134, 3051, 1715, 1657, 1596, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 398.1063, found 398.1129.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 123.3, 127.0, 128.3 (2C), 128.7 (2C), 128.8 (2C), 129.6 (3C), 130.2, 130.5, 130.6, 131.4, 133.6, 133.7, 133.8, 137.8, 138.3, 156.3, 164.8, 189.49; IR (KBr, cm−1): 3148; 3056, 1727, 1660, 1581, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 421.0432, found 421.0505.
C); 13C NMR (150 MHz, CDCl3): 123.3, 127.0, 128.3 (2C), 128.7 (2C), 128.8 (2C), 129.6 (3C), 130.2, 130.5, 130.6, 131.4, 133.6, 133.7, 133.8, 137.8, 138.3, 156.3, 164.8, 189.49; IR (KBr, cm−1): 3148; 3056, 1727, 1660, 1581, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 421.0432, found 421.0505.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 10.86 (s, 1H, –OH); 13C NMR (150 MHz, DMSO-d6): 119.7, 122.0, 123.7, 126.1, 128.8 (2C), 129.0 (2C), 129.2 (2C), 129.7, 123.0 (2C), 130.4, 130.5, 131.4, 134.1, 136.2, 138.0, 153.3, 155.0, 165.0, 189.8; IR (KBr, cm−1): 3272, 3145, 3065, 1710, 1638, 1590, 1415; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 403.0771, found 403.0844.
C); 10.86 (s, 1H, –OH); 13C NMR (150 MHz, DMSO-d6): 119.7, 122.0, 123.7, 126.1, 128.8 (2C), 129.0 (2C), 129.2 (2C), 129.7, 123.0 (2C), 130.4, 130.5, 131.4, 134.1, 136.2, 138.0, 153.3, 155.0, 165.0, 189.8; IR (KBr, cm−1): 3272, 3145, 3065, 1710, 1638, 1590, 1415; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 403.0771, found 403.0844.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 44.4, 123.7, 125.6, 128.4 (2C), 128.8 (5C), 129.0, 129.7 (2C), 129.8, 130.3, 131.0, 133.4, 133.7, 137.4, 137.6, 144.1, 156.8, 164.3, 189.3; IR (KBr, cm−1): 3106, 2928, 1732, 1654, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 465.0598, found 465.0672.
C); 13C NMR (150 MHz, CDCl3): 44.4, 123.7, 125.6, 128.4 (2C), 128.8 (5C), 129.0, 129.7 (2C), 129.8, 130.3, 131.0, 133.4, 133.7, 137.4, 137.6, 144.1, 156.8, 164.3, 189.3; IR (KBr, cm−1): 3106, 2928, 1732, 1654, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 465.0598, found 465.0672.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 44.4, 123.5, 123.8, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.8, 130.1, 131.4, 132.7, 132.9, 133.5, 133.7, 137.6, 144.55, 147.9, 156.9, 163.6, 189.2; IR (KBr, cm−1): 3135, 2923, 1727, 1655, 1596, 1450; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 476.0838, found 476.0911.
C); 13C NMR (150 MHz, CDCl3): 44.4, 123.5, 123.8, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.8, 130.1, 131.4, 132.7, 132.9, 133.5, 133.7, 137.6, 144.55, 147.9, 156.9, 163.6, 189.2; IR (KBr, cm−1): 3135, 2923, 1727, 1655, 1596, 1450; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 476.0838, found 476.0911.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH
CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ); 8.72, (s, 1H, N–CH
); 8.72, (s, 1H, N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 113.0, 122.2, 126.0, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.5, 129.6 (2C), 131.1, 133.4, 134.8, 138.1, 144.3, 149.0, 154.4, 156.1, 189.5; IR (KBr, cm−1): 3155, 3066, 1696, 1652, 1595, 1445; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 343.1004, found 343.1077.
C); 13C NMR (150 MHz, CDCl3): 113.0, 122.2, 126.0, 128.4 (2C), 128.7 (2C), 128.8 (2C), 129.5, 129.6 (2C), 131.1, 133.4, 134.8, 138.1, 144.3, 149.0, 154.4, 156.1, 189.5; IR (KBr, cm−1): 3155, 3066, 1696, 1652, 1595, 1445; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 343.1004, found 343.1077.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 66.4, 115.0 (2C), 122.2, 123.0, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.7 (2C), 130.6, 132.9, 133.6 (2C), 137.8, 156.4, 157.7, 167.1, 189.4; IR (KBr, cm−1): 3137, 2924, 1774, 1647, 1600, 1419; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 383.1317, found 383.1390.
C); 13C NMR (150 MHz, CDCl3): 66.4, 115.0 (2C), 122.2, 123.0, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.7 (2C), 130.6, 132.9, 133.6 (2C), 137.8, 156.4, 157.7, 167.1, 189.4; IR (KBr, cm−1): 3137, 2924, 1774, 1647, 1600, 1419; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 383.1317, found 383.1390.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 13.2, 109.6, 123.0, 127.9 (2C), 128.2 (2C), 128.6 (2C), 128.7 (4C), 128.9, 129.4, 129.6 (2C), 130.1, 130.4, 133.4, 133.5, 137.7, 155.7, 161.2, 162.1, 175.5, 189.4; IR (KBr, cm−1): 3108, 3078, 1705, 1666, 1597, 1416; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 434.1426, found 434.1503.
C); 13C NMR (150 MHz, CDCl3): 13.2, 109.6, 123.0, 127.9 (2C), 128.2 (2C), 128.6 (2C), 128.7 (4C), 128.9, 129.4, 129.6 (2C), 130.1, 130.4, 133.4, 133.5, 137.7, 155.7, 161.2, 162.1, 175.5, 189.4; IR (KBr, cm−1): 3108, 3078, 1705, 1666, 1597, 1416; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 434.1426, found 434.1503.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 13.5, 111.4, 114.2, 114.4, 117.8, 117.9, 122.7, 125.3 (2C), 128.0 (2C), 128.6 (4C), 129.4, 129.6 (2C), 130.3, 131.2, 131.3, 133.5 (2C), 134.1 (2C), 137.8, 155.1, 159.7, 160.3, 161.4, 176.2, 189.5; IR (KBr, cm−1): 3135, 3064, 1717, 1660, 1598, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 486.0942, found 486.1015.
C); 13C NMR (150 MHz, CDCl3): 13.5, 111.4, 114.2, 114.4, 117.8, 117.9, 122.7, 125.3 (2C), 128.0 (2C), 128.6 (4C), 129.4, 129.6 (2C), 130.3, 131.2, 131.3, 133.5 (2C), 134.1 (2C), 137.8, 155.1, 159.7, 160.3, 161.4, 176.2, 189.5; IR (KBr, cm−1): 3135, 3064, 1717, 1660, 1598, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 486.0942, found 486.1015.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 8.94, (s, 1H, N–CH
C); 8.94, (s, 1H, N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 40.1, 111.3, 118.5, 122.3, 128.4 (2C), 128.7 (2C), 128.9 (2C), 129.6 (2C), 131.1, 133.5, 134.7, 137.9, 139.1, 144.5, 145.0, 156.1, 157.1, 189.4; IR (KBr, cm−1): 3162, 3062, 1707, 1655, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 425.1147, found 425.1220.
C); 13C NMR (150 MHz, CDCl3): 40.1, 111.3, 118.5, 122.3, 128.4 (2C), 128.7 (2C), 128.9 (2C), 129.6 (2C), 131.1, 133.5, 134.7, 137.9, 139.1, 144.5, 145.0, 156.1, 157.1, 189.4; IR (KBr, cm−1): 3162, 3062, 1707, 1655, 1598, 1448; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 425.1147, found 425.1220.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 20.5, 62.1, 123.0, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.7, 130.6, 132.9, 133.6, 137.8, 156.3, 165.9, 170.4, 189.4; IR (KBr, cm−1): 3130, 2952, 1759, 1659, 1596, 1449; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 349.1110, found 349.1187.
C); 13C NMR (150 MHz, CDCl3): 20.5, 62.1, 123.0, 128.5 (2C), 128.7 (2C), 128.8 (2C), 129.6 (2C), 129.7, 130.6, 132.9, 133.6, 137.8, 156.3, 165.9, 170.4, 189.4; IR (KBr, cm−1): 3130, 2952, 1759, 1659, 1596, 1449; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 349.1110, found 349.1187.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 13C NMR (150 MHz, CDCl3): 121.8, 128.3 (2C), 128.6 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.6 (2C), 129.7 (2C), 130.6, 133.5, 135.2, 135.5, 136.4, 137.8, 156.9, 189.3; IR (KBr, cm−1): 3148, 3058, 1652, 1596, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 389.0882, found 389.0955.
C); 13C NMR (150 MHz, CDCl3): 121.8, 128.3 (2C), 128.6 (2C), 128.7 (2C), 128.9 (2C), 129.4, 129.6 (2C), 129.7 (2C), 130.6, 133.5, 135.2, 135.5, 136.4, 137.8, 156.9, 189.3; IR (KBr, cm−1): 3148, 3058, 1652, 1596, 1447; HRMS (ESI): m/z [M + H+] calculated for monoisotopic mass 389.0882, found 389.0955.X-ray diffraction
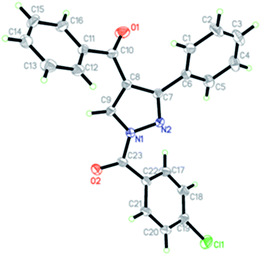
The suitable single crystal of 5f was recrystallized from a mixture of ethyl acetate and petroleum ether (Fig. 2). The X-ray data were collected on a Rigaku RAXIS-RAPID diffractometer (Japan) with Mo-Kα radiation (λ = 0.71073 Å) at 293(2) K. A total of 5577 reflections were measured, of which 3266 independent reflections (Rint = 0.0382) were obtained in the range of 3.17° < θ < 25.00° (h, −9 to 9; k, −12 to 10; l, −13 to 11), and 1805 observed reflections with I > 2σ(I) were used in refinement on F2. The structure was solved by direct method using SHELXS-97 crystallographic software package.30 The full matrix least squares refinement based on F2 gave final values of R1 = 0.1277, ωR2 = 0.3374 and ω = 1/[σ2(F02) + (0.0782P)2 + 9.9560P], where P = (F02 + 2Fc2)/3 with (Δ/σ)max = 0.983 and S = 1.019.Biological assays
The greenhouse experiment was employed to measure the influences of target compounds to barnyard grass and maize. The concentration was set to 0.05 mmol m−2 after initial screening with pyrazoxyfen, and pyrazoxyfen was also used as the reference substance. After 15 days, all the compounds solutions were sprayed to the leaves and stems. Then 10 days later, chlorophyll content of barnyard grass was tested (Table 1).| Compound | R | Chlorophyll a content (mg g−1) | Chlorophyll b content (mg g−1) |
|---|---|---|---|
| a Note: CK is for water treated. | |||
| 5a |  |
130.44 ± 1.64 | 54.75 ± 1.53 |
| 5b | 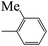 |
132.92 ± 2.23 | 57.72 ± 0.56 |
| 5c | 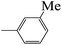 |
133.01 ± 2.43 | 59.45 ± 1.36 |
| 5d | 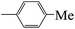 |
130.61 ± 1.94 | 55.32 ± 0.28 |
| 5e | 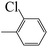 |
132.78 ± 2.97 | 57.19 ± 0.45 |
| 5f | 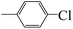 |
131.65 ± 2.56 | 56.61 ± 1.09 |
| 5g | 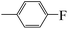 |
90.78 ± 2.34 | 38.48 ± 0.23 |
| 5h | 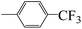 |
115.53 ± 2.36 | 48.64 ± 0.27 |
| 5i | 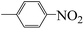 |
132.89 ± 2.23 | 58.46 ± 1.38 |
| 5j | 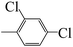 |
132.19 ± 1.57 | 57.05 ± 0.34 |
| 5k | 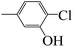 |
131.76 ± 2.01 | 54.29 ± 0.79 |
| 5l | 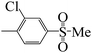 |
132.99 ± 1.08 | 58.97 ± 1.19 |
| 5m | 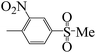 |
133.13 ± 1.34 | 59.69 ± 1.79 |
| 5n | 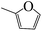 |
85.65 ± 1.87 | 34.49 ± 0.94 |
| 5o | 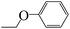 |
96.56 ± 1.09 | 40.32 ± 1.31 |
| 5p | 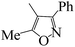 |
124.15 ± 2.09 | 52.82 ± 1.65 |
| 5q | 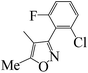 |
119.35 ± 2.28 | 50.12 ± 0.97 |
| 5r | 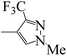 |
127.95 ± 2.09 | 53.62 ± 1.05 |
| 5s | 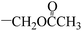 |
113.61 ± 1.26 | 50.59 ± 1.83 |
| 5t | 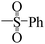 |
121.18 ± 1.83 | 53.89 ± 1.48 |
| CK | 132.16 ± 1.37 | 59.72 ± 0.94 | |
| Pyrazoxyfen | 128.29 ± 1.28 | 54.97 ± 1.39 | |
The safety to maize of the selected compounds was also determined. The pyrazoxyfen concentration was set to 1.25 mmol m−2 in order to make it injury to maize. The spraying treatment was conducted at the same dosage with pyrazoxyfen when the maize had reached the two-leaf stage. After 7 days, the chlorophyll content of the maize was determined (Table 2).
| Compound | Chlorophyll a content (mg g−1) | Chlorophyll b content (mg g−1) |
|---|---|---|
| a Note: CK is for water treated. | ||
| 5g | 44.57 ± 1.65 | 15.19 ± 0.09 |
| 5h | 54.56 ± 1.82 | 17.92 ± 0.27 |
| 5n | 35.34 ± 0.97 | 12.72 ± 0.69 |
| 5o | 64.70 ± 1.56 | 20.49 ± 1.06 |
| 5q | 55.32 ± 1.59 | 17.63 ± 1.12 |
| 5s | 50.65 ± 0.93 | 16.02 ± 0.58 |
| 5t | 60.13 ± 1.41 | 18.63 ± 0.83 |
| CK | 109.73 ± 1.27 | 31.50 ± 1.13 |
| Pyrazoxyfen | 43.86 ± 0.99 | 12.94 ± 0.88 |
Computational methods
Compounds were constructed and optimized using SYBYL-X 2.0 and Gasteiger–Huckel charges were calculated for them.31 The crystal structure of AtHPPD was obtained from the Protein Data Bank (PDB ID 1TFZ). Before docking, the protein structure was prepared in Accelrys Discovery Studio 2.5 (DS 2.5) to remove the water and some other co-crystallized small molecules. After the protein prepared, the active site of the protein was defined based on volume occupied by the known ligand pose already in an active site. During the docking process top 10 conformations were generated for each ligand based on docking score value after the energy minimization using the smart minimize method in DS 2.5.Results and discussion
Chemistry
In this research, compound 4 was synthesized with 1,3-diphenylpropane-1,3-dione, dimethylformamide dimethylacetal and N2H4·2HCl as the starting materials, and dry toluene as solvent. It should be noted that the yield of compound 4 was promoted to 89.5% when reaction time prolonged to 1.5 h and appropriate distilled water added compared with the method of the ref. 26. It might due to that the N2H4·2HCl dissolved adequately while adding distilled water.The procedure to compounds 5a–t was N-acylation reaction. The substitute structure affected the yields significantly due to the electronic effect. Compounds 5a–5d with benzoyl chloride or Me substituted benzoyl chloride used as acylated reagent were obtained in the high yield, such as the yield of compound 5b was increased to 90%. While p-substituted phenyl with electron-withdrawing groups like –Cl, –F, –CF3 and –NO2, the yields were decreased largely, especially the yield of compound 5i was decrease to 30%.
The structures of all the synthesized compounds were confirmed by 1H NMR, 13C NMR, and HRMS analyses. All target compounds showed similar spectroscopic characteristics due to the structural similarity. Single peak present at around 8.50–8.80 ppm in 1H NMR spectra correspond to the proton of pyrazole. The carbonyl connected to the 4 position of the pyrazole showed a shift at 189 ppm in the 13C NMR spectrum, and the shift around 167 ppm correspond to carbonyls connected to N substituted of the pyrazole.
Structure analysis
The molecular structure of 5f was shown in Fig. 2. Compound 5f contained three benzene rings and a pyrazole ring. The pyrazole ring and benzene ring are not coplanar with the dihedral angle being 46.726 (279)°. In addition, the second benzene ring [C11, C12, C13, C14, C15, and C16] is almost vertical to the first one [C1, C2, C3, C4, C5, and C6] with the dihedral angle being 74.64 (2)°. No significant π–π interactions were found in the crystal structure.Biological activity
At present, HPPD inhibitory activity was evaluated through different assays. The enzyme assay in vitro is the most common method which tested by monitoring the production of maleylacetoacetate at 318 nm.17,18 However, the most pyrazole HPPD inhibitors could not directly inhibit the HPPD activity in vitro. In fact, pyrazoxyfen have to metabolized to 4-(2,4-dichlorobenzoyl)-1,3-dimethyl-5-hydroxypyrazole in plant. The HPPD assay revealed that pyrazoxyfen inhibited the enzyme activity with the IC50 value of 7.5 μM while the values of its metabolite is 13 nM. These data strongly suggest that the pyrazole herbicide inhibit HPPD after conversion to the herbicidally active metabolite in plants.32The synthesized pyrazole benzophenone derivatives didn't inhibit the growth of barnyard grass or maize in the first 7 days, and then it showed typical bleaching injury symptoms to plants until the plants withered. The post-emergent herbicidal activities of compounds 5a–t were tested against barnyard grass. The commercial pyrazole herbicide pyrazoxyfen was selected as a positive control. As showed in Table 1, most of the compounds showed some extent herbicidal activities via decreasing the concentration of chlorophyll a and chlorophyll b, such as compounds 5a–j, 5l–q, 5s, 5t. Most surprisingly, compounds 5g, 5h, 5n, 5o, 5q, 5s, 5t displayed the highest herbicidal activity which were superior to pyrazoxyfen. Comparing the activities of the compounds revealed that substituent R was the primary group which played a crucial role in the activity of the compounds. As can be seen from Table 1, compounds 5n, 5q and 5p with an aromatic five-member ring at R displayed increasing herbicidal activity compared to compounds with benzoyl at R. It is very interesting that compound 5n (furan) showed most strong herbicidal activity. Furthermore, compounds 5o, 5s and 5t with ethoxy substitution also found to have herbicidal activity against the barnyard grass. In contrast, compounds with benzoyl at R exhibited much lower herbicidal activity, even if compounds 5g and 5h showed more potent inhibition against barnyard grass, superior to pyrazoxyfen.
Compounds 5g, 5h, 5n, 5o, 5q, 5s, 5t were selected to determine the safety to maize after herbicidal test. As shown in Table 2, some compounds were safe compared with pyrazoxyfen. Compound 5n exhibited the best herbicidal activities against barnyard grass, unfortunately, it showed poor security to maize. Therefore, compound 5o may be the candidate as a herbicide for weed control in maize, and it was selected for further crop selectivity testing. The binding free energy of compound 5o with HPPD was relatively low, and that was agreed with the results of bioassay activity research (Table 3).
| Compound | Binding free energy (kJ mol−1) | Compound | Binding free energy (kJ mol−1) | Compound | Binding free energy (kJ mol−1) |
|---|---|---|---|---|---|
| 5a | −7.723 | 5h | −8.554 | 5o | −8.982 |
| 5b | −4.242 | 5i | −7.607 | 5p | −7.495 |
| 5c | −6.008 | 5j | −9.309 | 5q | −4.472 |
| 5d | −8.354 | 5k | −5.283 | 5r | −4.538 |
| 5e | −5.654 | 5l | −9.180 | 5s | −4.654 |
| 5f | −7.746 | 5m | −2.312 | 5t | −6.985 |
| 5g | −8.652 | 5n | −9.236 |
Compound 5n was selected to docking with AtHPPD for it showed the best herbicidal activity. The important interactions of 5n with the active site of AtHPPD were presented in Fig. 3. The bidentate coordination of the pyrazoxyfen part with Fe2+ and the sandwiched π–π interaction of the benzene ring with Phe403 and Phe360 were mainly two interactions of AtHPPD with inhibitor. Compound 5n bind to HPPD as the same configuration as the DAS869. The oxygen of furan and carbonyl were responsible for forming a bidentate coordination with the Fe2+. Meanwhile, benzene ring which links to 4-position of pyrazole formed a favorable sandwich π–π interaction with residues Phe360 and Phe403 increasing hydrophobic interaction with amino acid of AtHPPD in the active pocket. The result also indicated that compound 5n might be a promising herbicide candidate.
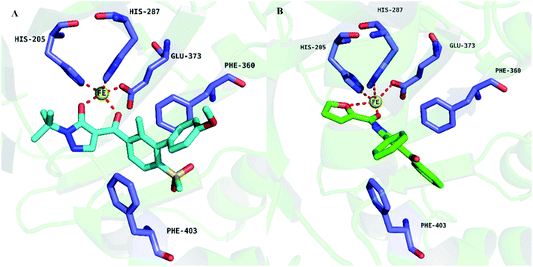 | ||
| Fig. 3 Binding modes of ligand DAS869 (PDB ID: 1TFZ) (A) and compound 5n with 1TFZ (B). (DAS869 is shown in cyan sticks and compound 5n is shown in green sticks). | ||
Conclusion
In conclusion, a series of novel pyrazole derivatives were designed and identified as potent HPPD inhibitors. Most of the synthesized compounds displayed excellent herbicidal activities, some of them even superior to the commercial herbicide pyrazoxyfen. Much to our delight, compounds 5g, 5h, 5n, 5o, 5p, 5q, 5s, 5t displayed promising herbicidal activities at a rate of 0.05 mmol m−2. It is inspired that compounds 5o showed better safety to maize. Therefore, the herbicidal activity of compound 5o was better than pyrazoxyfen. Besides, it was safe when used on maize. These results indicated that pyrazole derivatives could be novel lead compounds for novel herbicide discovery.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We are very grateful to the National Natural Science Foundation of China (31572042), the Natural Science Foundation of Heilongjiang Province (ZD2017002), and the Research Science Foundation in Technology Innovation of Harbin (2015RAYXJ010).References
- D. L. Lee, M. P. Prisbylla, T. H. Cromartie, D. P. Dagarin, S. W. Howard, W. M. Provan, M. K. Ellis, T. Fraser and L. C. Mutter, Weed Sci., 1997, 45, 601–609 CAS.
- G. R. Moran, Arch. Biochem. Biophys., 2005, 433, 117–128 CrossRef CAS PubMed.
- D. W. Wang, H. Y. Lin, R. J. Cao, Z. Z. Ming, T. Chen, G. F. Hao, W. C. Yang and G. F. Yang, Pest Manage. Sci., 2015, 71, 1122–1132 CrossRef CAS PubMed.
- C. Raspail, M. Graindorge, Y. Moreau, S. Crouzy, B. Lefebvre, A. Y. Robin, R. Dumas and M. Matringe, J. Biol. Chem., 2011, 286, 26061–26070 CrossRef CAS PubMed.
- A. Santucci, G. Bernardini, D. Braconi, E. Petricci and F. Manetti, J. Med. Chem., 2017, 60, 4101–4125 CrossRef CAS PubMed.
- S. R. Norris, T. R. Barrette and D. DellaPenna, Plant Cell, 1995, 7, 2139–2149 CrossRef CAS PubMed.
- H. Maeda and D. DellaPenna, Curr. Opin. Plant Biol., 2007, 10, 260–265 CrossRef CAS PubMed.
- F. E. Dayan, S. O. Duke, A. Sauldubois, N. Singh, C. McCurdy and C. Cantrell, Phytochemistry, 2007, 68, 2004–2014 CrossRef CAS PubMed.
- A. S. Godar, V. K. Varanasi, S. Nakka, P. V. V. Prasad, C. R. Thompson and J. Mithila, PLoS One, 2015, 10, 1–17 Search PubMed.
- A. J. Jhala, L. D. Sandell, N. Rana, G. R. Kruger and S. Z. Knezevic, Weed Technol., 2014, 28, 28–38 CrossRef CAS.
- M. J. Walsh, K. Stratford, K. Stone and S. B. Powles, Weed Technol., 2012, 26, 341–347 CrossRef CAS.
- J. D. Bollman, C. M. Boerboom, R. L. Becker and V. A. Fritz, Weed Technol., 2008, 22, 666–674 CrossRef CAS.
- M. M. Williams and J. K. Pataky, Weed Sci., 2010, 58, 289–294 CrossRef CAS.
- S. Lindstedt, E. Holme, E. A. Lock, O. Hjalmarson and B. Strandvik, Lancet, 1992, 340, 813–817 CrossRef CAS.
- A. J. Woodyard, G. A. Bollero and D. E. Riechers, Weed Technol., 2009, 23, 513–518 CrossRef CAS.
- M. Baalouch, A. De Mesmaeker and R. Beaudegnies, Tetrahedron Lett., 2013, 54, 557–561 CrossRef CAS.
- D. W. Wang, H. Y. Lin, R. J. Cao, T. Chen, F. X. Wu, G. F. Hao, Q. Chen, W. C. Yang and G. F. Yang, J. Agric. Food Chem., 2015, 63, 5587–5596 CrossRef CAS PubMed.
- D. W. Wang, H. Y. Lin, B. He, F. X. Wu, T. Chen, Q. Chen, W. C. Yang and G. F. Yang, J. Agric. Food Chem., 2016, 64, 8986–8993 CrossRef CAS PubMed.
- C. D. Duarte, E. J. Barreiro and C. A. M. Fraga, Mini-Rev. Med. Chem., 2007, 7, 1108–1119 CrossRef CAS PubMed.
- A. Y. Shaw, H. H. Liau, P. J. Lu, C. N. Yang, C. H. Lee, J. Y. Chen, Z. G. Xu and G. Flynn, Bioorg. Med. Chem., 2010, 18, 3270–3278 CrossRef CAS PubMed.
- Y. L. Xu, H. Y. Lin, X. Ruan, S. G. Yang, G. F. Hao, W. C. Yang and G. F. Yang, Eur. J. Med. Chem., 2015, 92, 427–438 CrossRef CAS PubMed.
- Y. L. Xu, H. Y. Lin, R. J. Cao, Z. Z. Ming, W. C. Yang and G. F. Yang, Bioorg. Med. Chem., 2014, 22, 5194–5211 CrossRef CAS PubMed.
- F. Ye, S. L. Wu, L. X. Zhao, H. T. Qu, S. Gao and Y. Fu, Heterocycles, 2015, 91, 1256–1268 CrossRef CAS.
- Y. Fu, Y. N. Sun, K. H. Yi, M. Q. Li, H. F. Cao, J. Z. Li and F. Ye, Molecules, 2017, 22, 959 CrossRef PubMed.
- Y. Fu, Y. N. Sun, H. F. Cao, K. H. Yi, X. Z. Li, J. Z. Li and F. Ye, Comb. Chem. High Throughput Screening, 2017, 20 DOI:10.2174/1386207320666170622073738.
- M. A. Al-Shiekh, Org. Prep. Proced. Int., 2005, 37, 223–230 CrossRef CAS.
- G. Menozzi, L. Merello, P. Fossa, S. Schenone, A. Ranise, L. Mosti, F. Bondavalli, R. Loddo, C. Murgioni and V. Mascia, Bioorg. Med. Chem., 2004, 12, 5465–5483 CrossRef CAS PubMed.
- P. Schenone, L. Mosti and G. Menozzi, J. Heterocycl. Chem., 1982, 19, 1355–1361 CrossRef CAS.
- D. J. Hogenkamp, T. B. C. Johenstion and K. W. Gee, WO Pat., 2005/108347, 2005.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- SYBYL, Version 6.9, Tripos Search PubMed.
- H. Matsumoto, ACS Symp. Ser., 2005, 892, 161–171 CrossRef CAS.
Footnote |
| † CCDC 1530820. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra09858h |
| This journal is © The Royal Society of Chemistry 2017 |