Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
1,3-Dipolar [3 + 3] cycloaddition of α-halohydroxamate-based azaoxyallyl cations with hydrazonoyl chloride-derived nitrile imines†
Hong-Wu Zhao *,
Yu-Di Zhao
*,
Yu-Di Zhao ,
Yue-Yang Liu
,
Yue-Yang Liu ,
Li-Jiao Zhao
,
Li-Jiao Zhao ,
Xiu-Qing Song,
Xiao-Qin Chen
,
Xiu-Qing Song,
Xiao-Qin Chen ,
Hai-Liang Pang
,
Hai-Liang Pang ,
Juan Du
,
Juan Du and
Ning-Ning Feng
and
Ning-Ning Feng
College of Life Science and Bio-engineering, Beijing University of Technology, No. 100 Pingleyuan, Chaoyang District, Beijing 100124, P. R. China. E-mail: hwzhao@bjut.edu.cn
First published on 5th December 2017
Abstract
Promoted by Et3N, the 1,3-dipolar [3 + 3] cycloaddition of α-halohydroxamate-based azaoxyallylcations with hydrazonoyl chloride-derived nitrile imines occurred efficiently, and furnished desired products in acceptable chemical yields. The chemical structure of the title compounds was firmly confirmed by an X-ray single crystal structure analysis.
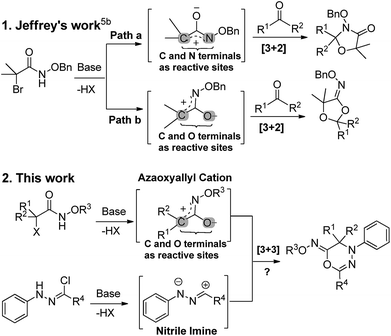
Azaoxyallylcations constitute a class of synthetically important and useful synthons, and their cycloaddition reactions serve as the main tools for the construction of structurally diverse and complex hetereocycles.1 Generally, the treatment of α-halohydroxamates with organic or inorganic bases can easily produce azaoxyallylcations. Pioneeringly, Jeffrey et al. reported the [4 + 3] cycloaddition of α-halohydroxamates with cyclic dienies.2 Since then, the synthetic methodology of azaoxyallylcations has experienced a wide and rapid development. Similarly, the Jeffrey, Wu and Liao research groups independently devised the [3 + 2] cycloaddition of azaoxyallylcations with indole derivatives for the synthesis of pyrroloindolines.3 The Chen research group designed the [3 + 1] and [3 + 2] cycloadditions of azaoxyallylcations with sulfurylides to produce β- and γ-lactams.4 Moreover, the Lin, Jeffrey and Wang research groups established the [3 + 2] cycloaddition of azaoxyallylcations with aldehydes or ketones to produce oxazolidin-4-ones.5 Wu and co-workers envisioned the [3 + 3] cycloaddition between azaoxyallylcations and isoquinoline N-oxides.6 Very recently, Lin and co-workers discovered [3 + 3] cycloaddition of azaoxyallylcations with 2-alkenylindoles to prepare tetrahydro-β-carbolinones.7 Concerning the above-mentioned cycloaddition reactions,1–7 the C and N terminals of the azaoxyallylcations are involved for the bond formations (e.g., Scheme 1(1), path a). Most importantly and elegantly, Jeffrey et al. recently disclosed that the azaoxyallylcations could utilize their C and O terminals to couple with ketones or aldehydes (Scheme 1(1), path b).5b Up to now, the cycloadditon of azaoxyallylcations using their C and O terminals as reactive sites has rarely been investigated.7
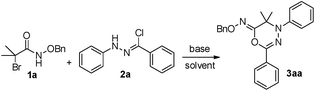
Motivated by Jeffrey's work,5b we first envisioned the 1,3-dipolar [3 + 3] cycloaddition of α-halohydroxamate-based azaoxyallylcations with synthetically important and useful hydrazonoylchloride-derived nitrile imines (Scheme 1(2)).8 Gratifyingly, we discovered that the in situ generated azaoxyallylcations readily utilized their C and O terminals to couple with the 1,3-dipolar nitrile imines in situ derived from the hydrazonoylchlorides, and produced structurally novel (Z)-4H-1,3,4-oxadiazin-6(5H)-imines in the acceptable chemical yields. Certainly, these new scaffolds can find some potential synthetic applications.9 To the best of our knowledge, such a work has not been reported in the literature to date.
Initially, in the presence of Et3N, we examined the solvent effect on the [3 + 3] cycloaddition of α-halohydroxamate 1a with hydrazonoyl chloride 2a as shown in entries 1–6 (Table 1). Use of CH3CN and DCM as solvents gave product 3aa in trace amounts after 48 h (entries 4–5). In contrast, the [3 + 3] cycloaddition did not take place in toluene at all (entry 6). Choice of HFIP, TFE and EtOH as solvents generated product 3aa in 13–60% chemical yields (entries 1–3). Basically, the protonic solvents provided better chemical yields than those obtained with the aprotonic solvents (entries 1–3 vs. 4–6). Subsequently, we explored the effect of the different bases on the [3 + 3] cycloaddition in HFIP as summarized in entries 7–18. Noticeably, the used bases affected the chemical yield of the [3 + 3] cycloaddition drastically. Use of NaHCO3 as a base delivered product 3aa in a trace amount (entry 11). In the case of Na2CO3 and MeONa as bases, the [3 + 3] cycloaddition produced product 3aa in 10% and 9% chemical yields, respectively (entries 7 & 12). In regard to the other bases tested, the chemical yield of 3aa widely ranged from 36% to 60% (entries 8–10 & 13–18). Obviously, among all the bases tested, Et3N behaved most efficiently, and gave product 3aa in the highest chemical yield (entry 1). Moreover, we checked the effect of the equivalent ratio of 1a/2a/Et3N on the [3 + 3] cycloaddition in the presence of Et3N in HFIP (see details in ESI†), and found that the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was the most optimal (entry 19). Finally, we attempted the [3 + 3] cycloaddition at varying reaction temperatures in 2
3 was the most optimal (entry 19). Finally, we attempted the [3 + 3] cycloaddition at varying reaction temperatures in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of 1a/2a/Et3N in HFIP, and found that the chemical yield of product 3aa did not increased as we expected (entries 20–21). Also, it should be noted that, in the [3 + 3] cycloaddition between 1a and 2a, the formation of major product 3aa usually was accompanied by the formation of a very polar and inseparable mixture even under the optimal reaction conditions, and that accounted for the moderate chemical yield of 3aa.
3 ratio of 1a/2a/Et3N in HFIP, and found that the chemical yield of product 3aa did not increased as we expected (entries 20–21). Also, it should be noted that, in the [3 + 3] cycloaddition between 1a and 2a, the formation of major product 3aa usually was accompanied by the formation of a very polar and inseparable mixture even under the optimal reaction conditions, and that accounted for the moderate chemical yield of 3aa.
| Entry | Solvent | Base | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Unless otherwise noted, reactions were carried out with 1a (0.15 mmol), 2a (0.1 mmol) in the presence of base (0.25 mmol) in the specified solvent (0.5 mL) at room temperature.b Isolated yield.c No reaction.d In 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of 1a/2a/Et3N.e Run at 60 °C.f Run at 0 °C. 3 ratio of 1a/2a/Et3N.e Run at 60 °C.f Run at 0 °C. |
||||
| 1 | HFIP | Et3N | 1.5 | 60 |
| 2 | TFE | Et3N | 48 | 21 |
| 3 | EtOH | Et3N | 48 | 13 |
| 4 | CH3CN | Et3N | 48 | Trace |
| 5 | DCM | Et3N | 48 | Trace |
| 6 | Toluene | Et3N | 48 | nrc |
| 7 | HFIP | Na2CO3 | 1.5 | 10 |
| 8 | HFIP | K2CO3 | 1.5 | 56 |
| 9 | HFIP | Cs2CO3 | 1.5 | 49 |
| 10 | HFIP | KOH | 1.5 | 57 |
| 11 | HFIP | NaHCO3 | 1.5 | Trace |
| 12 | HFIP | MeONa | 1.5 | 9 |
| 13 | HFIP | DBU | 1.5 | 56 |
| 14 | HFIP | DABCO | 1.5 | 36 |
| 15 | HFIP | Quinine | 1.5 | 50 |
| 16 | HFIP | DMAP | 1.5 | 59 |
| 17 | HFIP | DIPEA | 1.5 | 43 |
| 18 | HFIP | Pyridine | 1.5 | 39 |
| 19d | HFIP | Et3N | 1.5 | 67 |
| 20e | HFIP | Et3N | 1 | 53 |
| 21f | HFIP | Et3N | 2 | 64 |
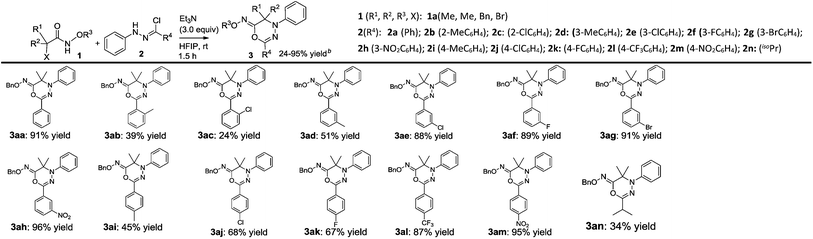
With the optimal reaction conditions in hand, we broaden the substrate scope of [3 + 3] cycloaddition by diversifying α-halohydroxamates 1 and hydrazonoyl chlorides 2 as outlined in Tables 2 and 3. Notably, the structural nature of substrates 1 and 2 affected the chemical yield of the [3 + 3] cycloaddition dramatically. As depicted in Table 2, the hydrazonoyl chlorides 2 (2a–2n) widely tolerated the variation of R4 group in the [3 + 3] cycloaddition with α-halohydroxamate 1a, and provided products 3 (3aa–3an) in the reasonable chemical yields. Generally, the substrates 2(2e–2h & 2J–l) possessing an electron-poor phenyl ring as R4 group tended to offer products 3 (3ae–3ah & 3aj–3al) in higher chemical yields; in contrast, the substrates 2 (2b, 2d & 2i) containing an electron-rich phenyl ring as R4 group preferred to furnish products 3 (3ab, 3ad & 3ai) in lower chemical yields.
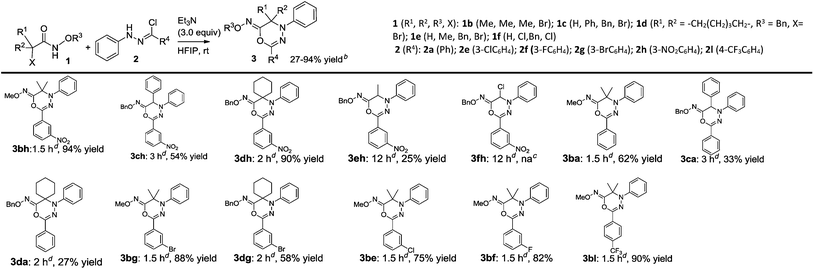
As summarized in Table 3, the [3 + 3] cycloaddition between the structurally varying α-halohydroxamates 1 (1b–1f) and hydrazonoyl chloride 2h proceeded quite differently, and furnished prodcuts 3 (3bh–3fh) in none to excellent chemical yields. Generally, the substrates 1 (1b & 1d) with a tertiary α-carbon center performed better than the substrates 1 (1c, 1e and 1f) bearing a secondary or primary α-carbon center in the [3 + 3] cycloaddition with 2h, and yielded products 3 (3bh & 3dh vs. 3ch, 3eh & 3fh) in excellent chemical yields. At last, we treated the substrates 1 (1b–1d) featuring a tertiary or secondary α-carbon center with the substrates 2 (2a, 2e–2g & 2l) possessing a phenyl ring or an electron-poor phenyl ring as R4 group, and the chemical yield of the [3 + 3] cycloaddition ranged from 27% to 90% (3ba, 3ca, 3da, 3bg, 3dg, 3be, 3bf & 3bl).
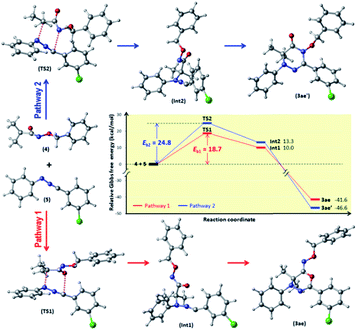
Moreover, the single crystal X-ray analysis firmly confirmed the chemical structure of 3ae, and disclosed that its 4H-1,3,4-oxadiazin-6(5H)-imine ring adopts a highly twisted conformation as illustrated in Fig. 1.10 Since the fact that the C and N or C and O terminals of azaoxyallylcation can serve as reactive sites in the cycloaddition,5b,7 we locked the two possible nonsynchronous concerted pathway 1 and pathway 2 for the [3 + 3] cycloaddition between α-halohydroxamate 1a and hydrazonoyl chloride 2e by conducting the DFT calculations at B3LYP/6-31+G(d) theoretical level in gas phase11 as shown in Fig. 2. Initially, upon treatment with Et3N, 1a provides azaoxyallylcation 4, and 2a gives nitrile imine 5. Subsequently, regarding pathway 1, through TS1 with an energy barrier of 18.7 kcal mol−1, 4 reacts with 5 using its C and O terminals to yield Int1, and then the formed Int1 barrierlessly transforms into product 3ae as demonstrated by the intrinsic reaction coordinate (IRC). As for pathway 2, according to TS2 bearing an energy barrier of 24.8 kcal mol−1, 4 performs the cycloaddition with 5 by employing its C and N terminals to deliver Int2 and subsequently the generated Int2 barrierlessly produces product 3ae′ as indicated by IRC. Overall, the pathway 1 is kinetically much more favorable than the pathway 2, and fully accounts for the formation of 3ae in the [3 + 3] cycloaddition between 1a and 2e. Also, we performed the DFT calculations for the possible pathways 1 and 2 at CPCM-B3LYP/6-311+G(d,p) level in HFIP, and found that the energy gap between TS1 and TS2 does not change substantially as compared with that obtained at B3LYP/6-31+G(d) theoretical level in gas phase (see details in ESI†). Certainly, the calculated energy gap between TS1 and TS2 is big enough to generate the observed selectivity between pathway 1 and pathway 2.12
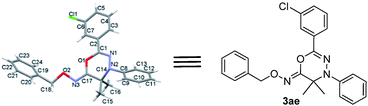 | ||
| Fig. 1 X-ray single crystal structure of 3ae (with thermal ellipsoils shown at the 50% probability level). | ||
In conclusion, the [3 + 3] cycloaddition of the in situ generated α-halohydroxamate-based azaoxyallylcations with in situ formed hydrazonoyl chloride-derived nitrile imines proceeded readily, and furnished the structurally novel (Z)-4H-1,3,4-oxadiazin-6(5H)-imines in the reasonable chemical yields. Furthermore, the exploration on the other novel cycloadditions between the α-halohydroxamate-based azaoxyallylcations and structurally diverse dipoles is ongoing in our organic lab, and will be reported in due course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Beijing Municipal Commission of Education (No. JC015001200902), Beijing Natural Science Foundation (No. 7102010, No. 2122008, No. 2172003), Basic Research Foundation of Beijing University of Technology (X4015001201101), Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (No. PHR201008025), Doctoral Scientific Research Start-up Foundation of Beijing University of Technology (No. 52015001200701) for financial supports.Notes and references
- For a review, see: K. L. Barnes, A. K. Koster and C. S. Jeffrey, Tetrahedron Lett., 2014, 55, 4690 CrossRef CAS.
- For selected examples, see: (a) C. S. Jeffrey, K. L. Barnes, J. A. Eickhoff and C. R. Carson, J. Am. Chem. Soc., 2011, 133, 7688 CrossRef CAS PubMed; (b) C. Jeffrey, A. Acharya and J. Eickhoff, Synthesis, 2013, 45, 1825 CrossRef.
- For selected examples, see: (a) A. Acharya, D. Anumandla and C. S. Jeffrey, J. Am. Chem. Soc., 2015, 137, 14858 CrossRef CAS PubMed; (b) M. C. DiPoto, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2015, 137, 14861 CrossRef CAS PubMed; (c) W. Ji, L. Yao and X. Liao, Org. Lett., 2016, 18, 628 CrossRef CAS PubMed.
- C. Li, K. Jiang, Q. Ouyang, T. Y. Liu and Y. C. Chen, Org. Lett., 2016, 18, 2738 CrossRef CAS PubMed.
- For selected examples, see: (a) K. Zhang, C. Yang, H. Yao and A. Lin, Org. Lett., 2016, 18, 4618 CrossRef CAS PubMed; (b) A. Acharya, K. Montes and C. S. Jeffrey, Org. Lett., 2016, 18, 6082 CrossRef CAS PubMed; (c) Q. Jia, Z. Du, K. Zhang and J. Wang, Org. Chem. Front., 2017, 4, 91 RSC.
- Y. An, H. Xia and J. Wu, Chem. Commun., 2016, 52, 10415 RSC.
- K. Zhang, X. Xu, J. Zheng, H. Yao, Y. Huang and A. Lin, Org. Lett., 2017, 19, 2596 CrossRef CAS PubMed.
- For selected examples, see: (a) L. K. Garve, M. Petzold, P. G. Jones and D. B. Werz, Org. Lett., 2016, 18, 564 CrossRef CAS PubMed; (b) S. M. Gomha, T. A. Farghaly, A. R. Sayed and M. M. Abdalla, J. Heterocycl. Chem., 2016, 53, 1505 CrossRef CAS; (c) H. Gazzeh, S. Boudriga, M. Askri, A. Khatyr, M. Knorr, C. Strohmann, C. Golz, Y. Rousselin and M. M. Kubicki, RSC Adv., 2016, 6, 49868 RSC; (d) A. Alizadeh and L. Moafi, Helv. Chim. Acta, 2016, 99, 457 CrossRef CAS; (e) Y. Miura and N. Yoshioka, Chem. Phys. Lett., 2015, 626, 11 CrossRef CAS; (f) M. Yıldırım and Y. Dürüst, Tetrahedron, 2011, 67, 3209 CrossRef; (g) A. L. Gerten, M. C. Slade, K. M. Pugh and L. M. Stanley, Org. Biomol. Chem., 2013, 11, 7834 RSC; (h) C. X. Guo, W. Z. Zhang, N. Zhang and X. B. Lu, J. Org. Chem., 2017, 82, 7637 CrossRef CAS PubMed; (i) M. P. Sibi, L. M. Stanley and C. P. Jasperse, J. Am. Chem. Soc., 2005, 127, 8276 CrossRef CAS PubMed; (j) A. Singh, A. L. Loomer and G. P. Roth, Org. Lett., 2012, 14, 5266 CrossRef CAS PubMed; (k) G. Wang, X. Liu, T. Huang, Y. Kuang, L. Lin and X. Feng, Org. Lett., 2013, 15, 76 CrossRef CAS PubMed.
- (a) T. Hashimoto, H. Kimura, Y. Kawamata and K. Maruoka, Angew. Chem., Int. Ed., 2012, 51, 7279 CrossRef CAS PubMed; (b) T. Soeta, K. Tamura and Y. Ukaji, Org. Lett., 2012, 14, 1226 CrossRef CAS PubMed; (c) V. FathiVavsari, G. MohammadiZiarani, S. Balalaie, A. Badiei, F. Golmohammadi, S. Ramezanpour and F. Rominger, ChemistrySelect, 2017, 2, 3496 CrossRef CAS.
- CCDC 1536335 contains the supplementary crystallographic data for compound 3ae.†.
- For selected examples, see: (a) A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS; (c) C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- (a) C. X. Yan, F. Yang, X. Yang, D. G. Zhou and P. P. Zhou, J. Org. Chem., 2017, 82, 3046 CrossRef CAS PubMed; (b) J. Wang, Z. Su, N. Yang and C. Hu, J. Org. Chem., 2016, 81, 6444 CrossRef CAS PubMed; (c) Y. Ran, M. Tang, Y. Wang, Y. Wang, X. Zhang, Y. Zhu, D. Wei and W. Zhang, Tetrahedron, 2016, 72, 5295 CrossRef CAS; (d) S. Emamian, RSC Adv., 2016, 6, 75299 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of NMR spectra for all products related to this article; X-ray single crystal structure analysis data for 3ad. CCDC 1536335. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra09766b |
| This journal is © The Royal Society of Chemistry 2017 |