Open Access Article
Open Access ArticleCooperative self-healing performance of shape memory polyurethane and Alodine-containing microcapsules
Weijie Fanab,
Weihua Li *a,
Yong Zhangc,
Wei Wang
*a,
Yong Zhangc,
Wei Wang a,
Xiaoying Zhanga,
Liying Songa and
Xiaojie Liua
a,
Xiaoying Zhanga,
Liying Songa and
Xiaojie Liua
aInstitute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, P. R. China. E-mail: wjf725@sina.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cQingdao Branch of Naval Aeronautical University, Qingdao 266041, P. R. China
First published on 3rd October 2017
Abstract
In this study, a method to prepare self-healing coatings by incorporating Alodine-containing microcapsules as fillers in Shape Memory Polyurethane (SMPU) was presented. Toluene diisocyanate (TDI) prepolymer was prepared for the microcapsule shells, while a healing agent (Alodine 5200) was selected for the core. SMPU with polyethylene glycol (PEG) as the soft segments and isophorone diisocyanate (IPDI) and 2,2-hydroxymethyl butyric acid (DMBA) as the hard segments were synthesized as main film-formers. A set of techniques including Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), electrochemical impedance spectroscopy (EIS), Scanning Kelvin Probe (SKP), and optical microscopy were used to completely characterize their self-healing performance on Al 7B04 T74 aluminum alloy surfaces. The study on the self-healing mechanism indicated that the self-healing process can be divided into two stages, and there was enormous potential for repairing large cracks with this technique.
1. Introduction
Organic coatings are widely used in metal corrosion protection because of their good properties and simple construction at a reasonable cost. At the same time, cracks in the coatings occur inevitably due to the existence of internal stresses and external damage.1 If the cracks are not repaired in time, the coatings soon lose their protective properties.2 Self-healing coating, which can be repaired the first time, has attracted the attention of numerous scholars all over the world. Various efforts have been made to develop crack-healing techniques by using new materials or designing new methods.3,4Microcapsules containing fillers such as inhibitors5,6 or film-forming reagents7–9 were used as self-healing additives in the coating systems. The coating damages could be repaired by the release of core. As described in literature,10,11 nano sizes microcapsules were the preferred choice for short term corrosion protection compared to long term. Therefore, a common problem in these systems is the inability to completely reinstall the large defects.
Shape memory polymers (SMP), an emerging class of smart materials, have been employed to prepare new types of self-healing coatings due to their potential to recover original shapes.12 Due to their functional groups, it is possible to create hydrogen bonds between the segments. When the coating is damaged, a physical self-healing is expected after an increase in the temperature between the glass-transition temperature of the hard segment and soft segment. Indeed, with the relaxation of the soft matrix, the polymer can fill the damaged area, while the hard segments maintain the mechanical properties.13 The shape memory is a completely physical process, there is no chemical bond breaking and recombination in the shape recovery process,2 and full repairs of the coatings' barrier properties remained challenging, due to the absence of filling and bonding. In this case, the corrosion reaction takes place on the metal surface before the shape reversion occurs. Thus, the presence of corrosion products between the metal and the healed coating will lead to a decrease in adhesion.
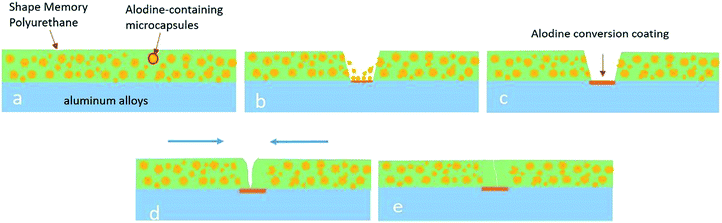
Combining the microcapsules with shape memory polymers may be a possible solution to the abovementioned problems. In the current study, we present a method to prepare self-healing coatings by incorporating Alodine-containing microcapsules as fillers in SMPU. Alodine conversion coating is widely used to improve the corrosion resistance and adhesion strength of Al/P film,14 which containing Ti and Zr ions can enable the aluminium alloy to have higher corrosion resistance and better adhesion. Once a crack occurs, the structure of the microcapsule breaks down. The encapsulation of the Alodine inside the microcapsule reacts with aluminium alloy or corrosion products at the bottom of the scratch as pre-treatment, and a dense conversion layer is formed on the substrate surface due to passivation. Further heating treatment activates the shape memory effect of SMPU, and two sides of the scratch move gradually towards each other. Finally, the healed coating has better corrosion resistance and adhesion strength due to the presence of the conversion layer.
2. Experimental
2.1 Materials
All chemicals, unless otherwise noted, were purchased from Sigma-Aldrich and used as received. Butanediol (BDO) and dibutyltin dilaurate were purchased from Aladdin. Polyethylene glycol (PEG) with Mn values of 2000 was dried overnight in vacuum (120 °C/5 mbar) over P2O5 before being used. N,N-dimethylformamide (DMF) was purified by vacuum distillation after being dried with anhydrous magnesium sulfate. Furthermore, Alodine 5200 was purchased from Henkel Company, which is a non-chrome titanium based conversion coating. The non-chrome treatments are designed to replace chromate conversion coatings in applications where they cannot be used for environmental/safety reasons. Aluminum alloys Al 7B04 T74 used in this study were kindly offered by AVIC Xi'an aircraft industry (group) company LTD and their chemical composition is shown in Table 1.| Element | Zn | Mg | Cu | Mn | Fe | Si | Ni | Cr | Ti | Al |
| w/% | 6.23 | 2.88 | 1.58 | 0.31 | 0.15 | 0.048 | <0.01 | 0.16 | 0.025 | Bal |
2.2 Preparation of microcapsules
Toluene diisocyanate (TDI) prepolymer was prepared for the microcapsule shells, while a healing agent (Alodine 5200) was selected for the core. The synthesis route were carried out by following the previous reports,15 as shown in Fig. 1a.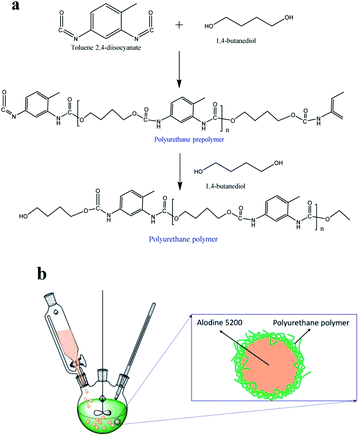 | ||
| Fig. 1 (a) Schematic of polyurethane polymer, (b) composition diagram of the Alodine-containing microcapsules. | ||
First, toluene 2,4-diisocyanate (TDI, Sigma-Aldrich, 10 g) was dissolved in 5 ml of cyclohexanone in a flask (250 ml). The mixture was kept in different temperature (70 °C, 75 °C, 80 °C) oil bath and stirred using a mechanical roaster. Dry nitrogen was introduced to the chamber with a low rate of 5.00 ml min−1. Then, 1,4-butanediol (BDO, 2.00 g), dibutyltin dilaurate (0.05 g), and dimethylolbutanoic acid (DMBA, 0.50 g) were dissolved in the TDI solution under agitation (1500 rpm). The reaction continued for 8 h before being distilled at 110 °C under vacuum for 6 h in order to remove excess materials. After that, Alodine 5200 (2.00 ml) was poured into the mixture to form an O/W emulsion. The oil bath was heated to 75 °C and butanediol (0.03 mol) was added as chain extender. After 45 min, the suspension of microcapsules was cooled, repeatedly rinsed with deionized water and dried under vacuum for 24 h. The composition diagram of the Alodine-containing microcapsules is shown in Fig. 1b.
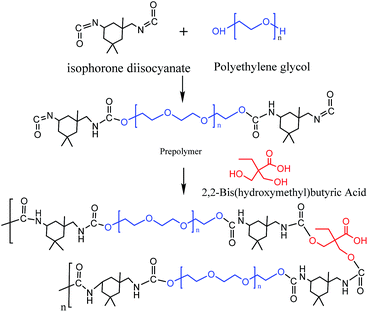
2.3 Preparation of shape memory polyurethane
The SMPU used in this study is constituted of PEG (Mn 2000) as the soft domains and polyurethane segments (PU) as the hard domains. The synthesis of the SMPU polymer containing 5.5 wt% of hard segment and coating application were performed as described in ref. 16. The schematic of shape memory polyurethane is shown in Fig. 2.At room temperature, PEG (20 g), which was dried overnight at 115 °C, dry acetone (10 ml), isophorone diisocyanate (IPDI, 5.3 g), and dibutyltin dilaurate (0.05 g) were mixed in a four-neck round-bottom flask (250 ml) with nitrogen purging. The mixture was then kept in different temperature (70 °C, 75 °C, 80 °C) oil bath and stirred using a mechanical roaster for 2 h. Afterwards, hydroxymethyl butyric acid (DMBA, 1 g) was dissolved in methyl formamide (DMF, 5 ml) and slowly added to reaction solution as chain extender. The reaction continued for 4–5 h before being cooled to room temperature. The pH was adjusted to approximately 7.0 using triethylamine. Finally, the SMPU was successfully synthesized and heat-treated at 90 °C for 1 hour to complete the polymerization reaction.
2.4 Preparation of the substrates
Al 7B04 T74 aluminum alloys were used as the substrate and first grounded by 240 grit abrasive paper to remove the surface oxide layer. Prior to all measurements, the aluminum samples (10 mm × 10 mm × 10 mm) were sealed in PVC pipes (outside diameter was 32 mm and length was 30 mm) with epoxy resin and triethylenetetramine (compounding ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The surface for tests was ground from 400 to 1000 grade SiC paper, rinsed with absolute ethyl alcohol, and finally dried in air.
1). The surface for tests was ground from 400 to 1000 grade SiC paper, rinsed with absolute ethyl alcohol, and finally dried in air.
To prepare the self-healing coatings, microcapsules and shape-memory polyurethane were mixed carefully by mechanical stirring. The sealed samples were coated by bar coating at 65 °C and cooled to room temperature before they were annealed for 24 h at 65 °C in a vacuum oven. The distance between the bar and the substrate was 100 mm.
2.5 Characterization
In order to identify the successful synthesis of microcapsules, the morphology was examined using optical stereomicroscopy (Hirox KH-7700) in the final emulsion and mixed with shape memory polyurethane. Thermal stability of the microcapsules was studied using thermogravimetric analysis. Small amounts of microcapsules (1–2 mg) were heated from 25 to 650 °C at a rate of 10 °C min−1 in a N2 environment.The shape-memory properties of the polyurethanes were investigated according to ref. 17. The specimens (average diameter was 5 mm and length was 58 mm) were first stretched into a length as long as possible at 70 °C, and the shapes could be fixed by cooling the specimens to room temperature rapidly. When the specimens were heated to 70 °C again, they started to recover to their permanent shape, and during this process the length between two sides was measured at regular intervals. The shape recovery ratio (R) was calculated using eqn (1):
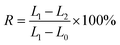 | (1) |
Fourier transform infrared spectroscopy (FT-IR) tests were performed using a Perkin Elmer 1600 FTIR to confirm the soft segments and hard segments of synthesized polyurethane, and the spectrum was recorded in 600–4000 cm−1 range.
The self-healing performance of the coatings was evaluated by typical corrosion tests. Scratches were applied manually using a razor blade on the prepared coatings and then the coatings were placed horizontally at room temperature for 3 hours in the first stage. After that, the sample was subsequently heated to 70 °C, kept for the next 2 hours, and allowed to heal itself. The cooperative self-healing performance of the reference group of polyurethane without microcapsules was also investigated. Electrochemical impedance spectroscopy (EIS) was measured to monitor the entire process using a PARSTAT 4000 electrochemical station. The frequency ranged from 105 Hz to 10−2 Hz, a total of 51 points. The entire electrochemical cell was placed in a Faraday cage to avoid interference from external electromagnetic fields and stray currents. Moreover, the surface morphologies of the coatings were observed by optical stereomicroscopy (Hirox KH-7700) during the entire self-healing process. The Scanning Kelvin Probe (SKP) technique was conducted to show the potential difference along the crevice in a measurement chamber, which kept the temperature and humidity stabilized. Corrosion potential maps of typically 2 mm × 2 mm covering a scratch were obtained with step of 100 μm using Uniscan Model 370. The results were presented as 3D images. It is worth to note that the low resolution was associated with faster acquisition rates to avoid the effects of time. To observe the compatibility of the Alodine coating and recovered polyurethane, the cross-section of the healed scratch was cut and carefully polished without causing further damage to the sample as demonstrated previously.18
3. Results and discussion
3.1 Characterization of Alodine-containing microcapsules
The optical microscopy and particle size distribution of the synthesized Alodine-containing microcapsules in the emulsion are shown in Fig. 3a and b. The capsules had spherical shape, a smooth and non-porous exterior shell wall, and ensured enough space for Alodine and easy dispersion into the coating, which could also be confirmed from Fig. 3b. The diameter of the microcapsule ranged from 10 μm to 80 μm. Microcapsules with different diameters can be seen to be filled with dark materials, and the objects were uniformly dispersed in polyurethane, as shown in Fig. 3.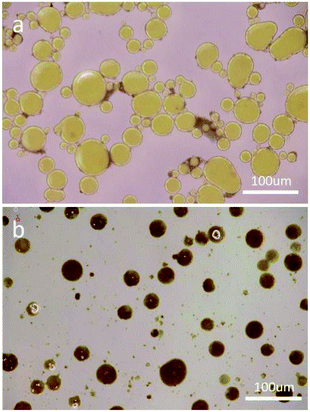 | ||
| Fig. 3 Optical microscopy images of microcapsules synthesized at 75 °C (a) in the emulsion obtained after addition of Alodine, and (b) mixed in shape memory polyurethane. | ||
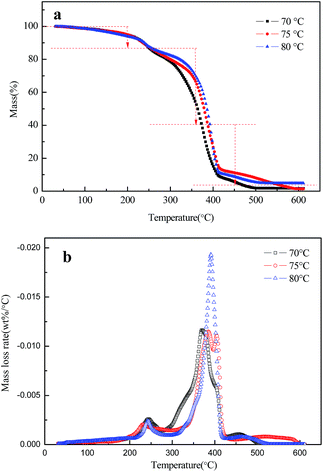
Thermogravimetric analysis (TGA) was applied to identify the thermal properties of filled microcapsules. Effects of different synthesis temperatures (70 °C, 75 °C, 80 °C) on the stability are depicted in Fig. 4a and b. The mass loss before 230 °C correlates to the physical loss of solvent.19 Large mass loss peaks at 380–400 °C (in Fig. 4a) were attributed to the decomposition of Alodine, whereas the final mass loss, which took place in the temperature over 450 °C, is indicative of the decomposition of the shell material, which is consistent with the literature.15 The percentage of Alodine in microcapsules was 74.9%, 67.6% and 73.4% respectively, which was calculated based on TGA analysis and mass loss rates. Therefore, the results demonstrate that there is a steady loading efficiency in different synthesis temperatures (ranging from 70 to 80 °C).
3.2 Characterization of shape memory polyurethane
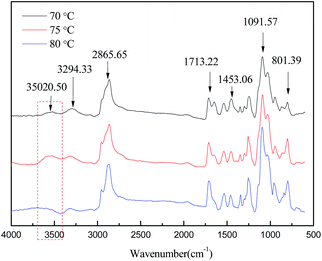
The chemical structure of the shape memory polyurethane was characterized by FT-IR. Fig. 5 shows the bending vibration peaks of the N–H group at 1537 cm−1 and the stretching vibration peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at 1713 cm−1,20 which validate the successful synthesis of the polyurethane.21 Maxima peaks found at 1091.57 cm−1 could be assigned as stretching vibration of the C–O–C group. These are usually attributed to the large number of repeating groups of soft segment and its large absorption coefficients. Another favorable evidence is that peaks at 1300 cm−1, 1349 cm−1 and 1453 cm−1 ascribed to the bending vibration of CH2 were observed. In addition, the stretching vibration peaks of the CH3 group of hard segment could be observed at 2865.65 cm−1. In the spectra obtained at a synthesis temperature of 80 °C, the peaks of O–H stretching vibration are red shifted.
O group at 1713 cm−1,20 which validate the successful synthesis of the polyurethane.21 Maxima peaks found at 1091.57 cm−1 could be assigned as stretching vibration of the C–O–C group. These are usually attributed to the large number of repeating groups of soft segment and its large absorption coefficients. Another favorable evidence is that peaks at 1300 cm−1, 1349 cm−1 and 1453 cm−1 ascribed to the bending vibration of CH2 were observed. In addition, the stretching vibration peaks of the CH3 group of hard segment could be observed at 2865.65 cm−1. In the spectra obtained at a synthesis temperature of 80 °C, the peaks of O–H stretching vibration are red shifted.
The shape-memory behavior of polyurethane was investigated by quantitative measurements. For quantitative investigation (Fig. 6), polyurethane was first stretched into a length as long as possible at a sufficiently high temperature (70 °C) for 1 min with a hair dryer, followed by cooling to room temperature for 5 min. Since the soft segments composed of poly diols undergo glass transition (Ttrans) at a relatively lower temperature, it can be deformed by an external force at 70 °C, which is higher than the Ttrans of the soft segment and lower than that of hard segment. Accordingly, the shape can be fixed with the temperature decreased below Ttrans of soft segment rapidly. Furthermore, the sample was recovered after heating to 70 °C for about 5 min due to the movement of soft segment, which indicated the shape memory properties of polyurethane. The shape memory recovery rate was 79.6%, as calculated by eqn (1).
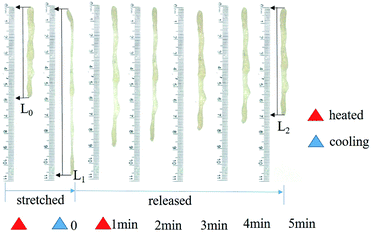 | ||
| Fig. 6 Shape memory properties of polyurethane (L0 initial length, L1 fixed longest length, L2 final recovery length). | ||
3.3 Self-healing performance of shape memory polyurethane loaded with microcapsules
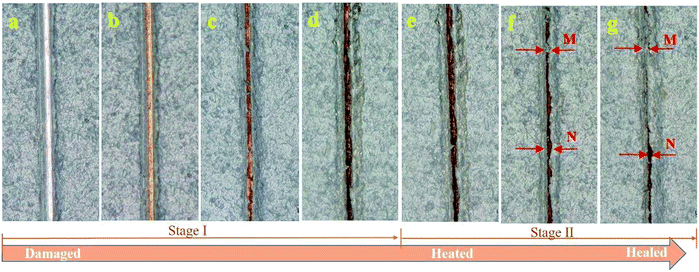
The self-healing process of the coatings was visualized via optical microscopy and divided into two stages. The original coating defect was generated by a razor blade, as shown in Fig. 7a, and the shiny surface at the bottom of the scratch indicated that the coating was cut through and the metal substrate was exposed. In the first healing stage without any treatment for 3 h, the shiny substrate became dark gradually, which can be observed by comparing Fig. 7b–d. This accounted for the generation of the novel Ti/Zr conversion coating, which enabled the Al alloy to have higher corrosion resistance22 and better adhesion strength.23 After the second healing stage at 75 °C, the shape memory effect of the polyurethane was triggered, recovering the wide scratch to close, as shown in Fig. 7e. On comparing Fig. 7f and g, we can find that the scratch (point M) is almost completely closed, except for some individual positions (point N) where even though the crack is not completely closed, the corrosion resistance will be improved10 and the adhesion strength of the coatings will be enhanced24 due to the existence of the passive film. Fig. 8 showed the cross section of the healed specimen. It is obvious that there is a dark film between the substrate and healed coating at the scratch site, not anywhere else. Thus, it can be inferred that this is the Alodine conversion coating. Moreover, there is no distinct interface between the Alodine coating and recovered polyurethane due to good compatibility. This good adhesion facilitates the Alodine coating to polyurethane, and thus improves scratch resistance.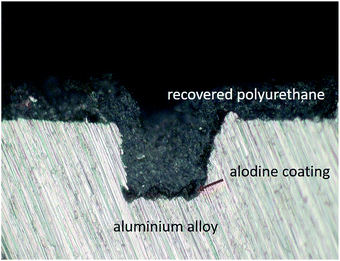 | ||
| Fig. 8 Optical microscope image of the Alodine coating and recovered polyurethane cross-section. Scale bar: 0.5 mm. | ||
The self-healing process could be described by a two-step “passivated-then-heal” mechanism as shown in Fig. 9. The Alodine-containing microcapsules were evenly dispersed in polyurethane and applied on surface of aluminum alloy (Fig. 9a). In the first step, some of the Alodine mainly containing hexafluorotitanic acid (H2TiF6) and hexafluorozirconic acid (H2ZrF6) was released from the microcapsule cores over time when the coating scratches were formed (Fig. 9b). They reacted with the aluminium alloy to produce Alodine conversion coating that could improve both the adhesion strength and the corrosion resistance of substrate (Fig. 9c). The mechanism of reaction between Alodine 5200 and the aluminum alloy was as follows according to the reports.22,25,26 Aluminum dissolves forming Al3+ ions during the initial stages (eqn (2)), and the oxygen reduction in the micro-cathode area produces OH− (eqn (3)).
| Al → Al3+ + 3e | (2) |
| O2 + 2H2O + 4e → 4OH− | (3) |
The above reactions create a local alkalinity adjacent to the intermetallics favouring the precipitation of TiO2, ZrO2 and Al2O3 (eqn (4)–(6)).
| Ti4+ + 4OH− → TiO2·2H2O | (4) |
| Zr4+ + 4OH− → ZrO2·2H2O | (5) |
| Al3+ + 6OH− → Al2O3·3H2O | (6) |
Thus, the main components of conversion coating are TiO2, ZrO2 and Al2O3.
The second step requires heating at T > Tg of soft segments, which activates the shape memory effect, releasing the restored strained energy in the deformed area and exerting a contracting force to close the defect (Fig. 9d). Furthermore, there was an Alodine conversion coating between the healed polyurethane and aluminium alloy (Fig. 9e). The conversion coating has better corrosion resistance than the substrate, while its surface roughness is related to the adhesion strength.
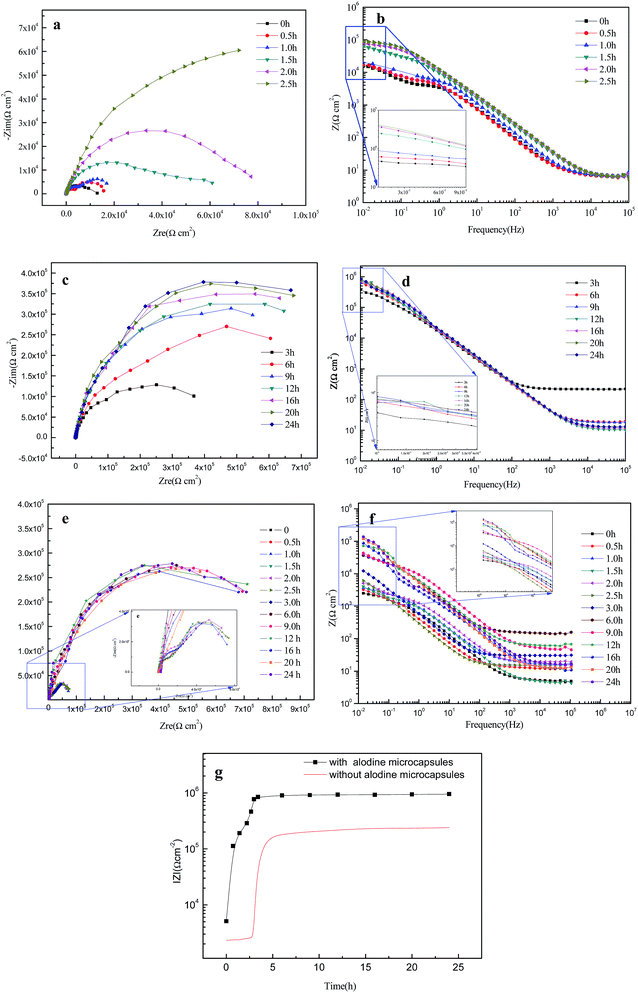
In order to verify the above mechanism, the possibility of Alodine release and the shape memory effect were investigated by the EIS measurements.27 Fig. 10a and b show the Nyquist and Bode plots of the EIS spectrum in the first stage of self-healing process for shape memory polyurethane loaded with microcapsules. Once the scratch was formed, the impedance amplitude decreased rapidly. According to literature,28 two small capacitive arcs on the Nyquist plot (Fig. 10a, 0 h) imply complete loss of coating properties and that the corrosion of the aluminum alloy occurred at the bottom of the scratch. Then, Alodine, released from microcapsules, reacted with aluminum alloy to form a conversion coating over time, which gradually slowed down the penetration of Cl ion (Fig. 10a, 0.5 h, 1 h). Accordingly, the constants in Bode plots (Fig. 10b, 0.5 h, 1.0 h) disappeared gradually in the low frequency range, which can be attributed to the gradual formation of the passive film. On comparing the capacitive arc at certain times (Fig. 10a), 1.5 h, 2.0 h, 2.5 h, we find a rapidly rising trend of radius, which indicates that the passive film is relatively complete over time and the resistance of the substrate is enhanced.29 The Bode plots (Fig. 10b) at 1.5 h, 2.0 h, 2.5 h show the capacitive response in the low frequency range due to the first stage of self-healing process.30 Fig. 10c and d show the EIS spectrum of coating after heating at second stage, in which the value of the impedance increased rapidly and the second capacitance arc completely disappeared (Fig. 10c). These results clearly confirmed that crack closure by the shape memory effect can, to some extent, enhance the resistance of coatings and reduce the penetration of corrosion.31 Eventually, the complete coating (Fig. 10c and d, 24 h) exhibits a strong barrier effect by showing one large capacitive arc (Fig. 10c); on the corresponding Bode plot, this is shown as a pure capacitive behavior.
By comparing Fig. 10a and e, two capacitive arcs on the Nyquist plot (Fig. 10e, 0–3 h) can be observed until polyurethane is heated, which implies the corrosion of the aluminum alloy. The second capacitive arc suddenly disappears with the start of heating. The rising trend of phase angle curves in the middle frequency range (Fig. 10f, 3–6 h) indicates that the resistance of the coatings is enhanced by the self-healing process. It can be seen that the capacitive response in the low frequency range is due to the two side walls of the scratched crevice touching each other.
As shown Fig. 10g, impedance modulus (|Z|) at a frequency of 0.01 Hz of each Bode plot was used to evaluate the corrosion resistance of coatings. The impedance modulus value (f = 0.01 Hz) in the curve of samples with microcapsules could be easily distinguished by two stages. The lowest value at 0 h accounts for the presence of the scratch, which causes the substrate to be exposed in the corrosion solution directly. Then, the value shows an increasing trend due to the formation of the passive film. Consequently, passivation reaction gradually slows down as the film thickness increases over time. At the second stage, shape memory effects made both sides of the scratch move to each other in the heating environment, and the formation of the physical shielding layer resulted in a rapid increase of the impedance modulus. In fact, the impedance moduli are relatively larger than those of the reference group. Furthermore, the impedance modulus curves of samples without microcapsules show different trends. There is no significant change in initial 3 h due to the absence of microcapsules. After heating, the impedance modulus increases rapidly and is stabilized at a relatively smaller value to the experimental group due to the shape recovery of polyurethanes. Thus, the difference in impedance value illustrates that the introduction of Alodine microcapsules into the polyurethane leads to significant effect on corrosion resistance or in other words, recovered polyurethane needs Alodine conversion coating to hinder the diffusion of Cl ions to substrate.32,33
Three equivalent circuits were used to fit the EIS data of the coating with Alodine-containing microcapsules at different times. The first, shown in Fig. 11a, was used to fit the impedance spectrum before the passivation film was fully formed. Furthermore, the second equivalent circuit (Fig. 11b) was suitable for the complete passivation film34 and the third one (Fig. 11c) indicated that the coating was completely repaired. The fitting results of different equivalent circuits are shown in Tables 2–4, where Rs is the resistance of NaCl solution, Rox and Qox represent the metal charge transfer resistance and double layer capacitance, respectively,35 Rf and Qf represent the resistance and capacitance of passivation film, respectively, Rpore represents pore solution resistance, and Rcoating and Ccoating represent the coating resistance and capacitance of coating, respectively.
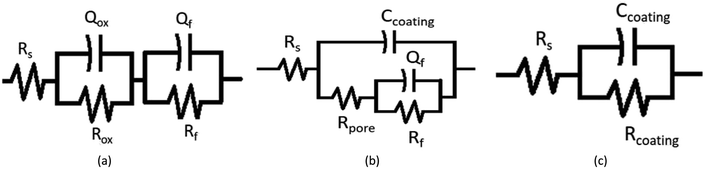 | ||
| Fig. 11 Equivalent circuit models used for the data analysis of the coatings, (a) before the passivation film was fully formed, (b) complete passivation film, and (c) the completely repaired coating. | ||
According to the results in Table 2, the increasing trend of the Rf value (from 3.615 to 11.58 kΩ cm−2) indicates that Alodine slowly reacts with aluminum to create the passivation film at the bottom of the scratch within 1 h. After immersion for 2.5 h, the continuous growth (from 41.63 to 64.85 kΩ cm−2) of Rf is related to the gradual integrity of the passive film, as shown in Table 3, and the increasing of pore solution resistance (from 2.271 to 2.973 kΩ cm−2) due to the size of pores in films getting smaller. However, the values of Qf gradually decreased (from 9.247 to 7.891 μF cm−2). The increasing trend of the Rc value (from 282.4 to 723.4 kΩ cm−2) in Table 4 indicates that the coating on the surface of the passivation film has good properties after 24 h. It is evidence that the shape memory properties play an important role in the self-healing process of the coating.
| Time (h) | Rs (Ω cm−2) | Qox (μF cm−2) | Rox (kΩ cm−2) | Qf (μF cm−2) | Rf (kΩ cm−2) |
|---|---|---|---|---|---|
| 0 | 7.463 | 12.96 | 4.677 | 11.64 | 3.615 |
| 0.5 | 7.453 | 17.71 | 4.912 | 17.27 | 6.821 |
| 1.0 | 7.516 | 19.22 | 4.861 | 19.40 | 11.58 |
| Time (h) | Rs (Ω cm−2) | Ccoating (μF cm−2) | Rpore (kΩ cm−2) | Qf (μF cm−2) | Rf (kΩ cm−2) |
|---|---|---|---|---|---|
| 1.5 | 7.318 | 5.610 | 2.271 | 9.247 | 41.63 |
| 2.0 | 7.124 | 5.054 | 2.728 | 8.239 | 62.74 |
| 2.5 | 6.962 | 4.986 | 2.973 | 7.891 | 74.85 |
| Time (h) | Rs (Ω cm−2) | Ccoating (μF cm−2) | Rcoating (kΩ cm−2) |
|---|---|---|---|
| 3 | 2.280 | 7.405 | 282.8 |
| 6 | 2.032 | 5.741 | 503.4 |
| 9 | 1.937 | 5.508 | 576.1 |
| 12 | 2.136 | 5.128 | 642.6 |
| 16 | 2.272 | 5.048 | 711.8 |
| 20 | 2.443 | 5.012 | 714.9 |
| 24 | 2.224 | 4.902 | 723.4 |
As shown in Fig. 12, the potential maps can be more intuitive to show the entire repair process of the scratched coating. A high and wide peak can be clearly observed in Fig. 12a, which could be explained by the direct exposure of the metal matrix under the scratch. However, this phenomenon changed quickly after a while as shown in Fig. 12b; the peak became weak and the potential difference with the surroundings decreased due to Alodine releasing from the microcapsule over time, and it reacted with aluminium alloy to produce the passive film at the bottom of the scratch. The formation of the passive film hindered the diffusion of corrosion products in aluminum alloy, and to some extent, it blocked the penetration of the corrosive Cl ions. Moreover, the dense film was conducive to the adhesion power of the coating.36 Fig. 12c shows that the peak of potential differences became much smaller after 2 h. Starting from 3rd hour, the width of peak apparently decreased with the progression in heating time, indicating that two sides of the scratch gradually moved towards each other, as shown in Fig. 12d–e. It was evident that the scratch was covered with more and more self-healing polyurethanes. Finally, there was no potential peak left in Fig. 12f indicating that the coating was completely repaired using a combination of the two technologies.
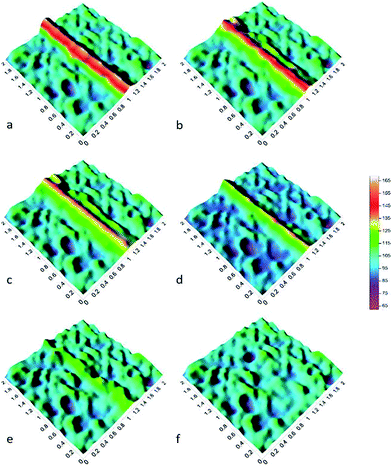 | ||
| Fig. 12 Potential maps obtained on surface of sample with the Kelvin probe ((a) 0 h, (b) 1 h, (c) 2 h, (d) 3 h, (e) 4 h, (f) 5 h). | ||
4. Conclusion
The combination of the microcapsules and shape memory polyurethane makes it possible to repair cracks. Alodine-containing microcapsules were synthesized by interfacial polymerization of polyurethanepre polymer. The results of thermogravimetric analysis demonstrated that there was a steady loading efficiency in different temperatures. The shape memory polyurethane containing polyethylene glycol (PEG) as the soft segments showed excellent recovery rate (79.6%). Following the results acquired in optical microscopy, EIS and SKP measurements, the entire self-healing process of shape memory polyurethane loaded with Alodine-containing microcapsules was divided into two different self-healing stages. In the first stage, Alodine released from microcapsules enabled the substrate to have higher corrosion resistance and better adhesion strength. After the second healing stage at 75 °C, the shape memory effect could make the wide scratch to close completely.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to gratefully thank the support of the Nantong science and technology plan (grant number GY12016046) and National Natural Science Foundation of China (Grant No. 51401185).References
- M. F. Montemor, Functional and smart coatings for corrosion protection: a review of recent advances, Surf. Coat. Technol., 2014, 258, 17–37 CrossRef CAS.
- J. B. Jorcin, G. Scheltjens and Y. V. Ingelgem, et al., Investigation of the self-healing properties of shape memory polyurethane coatings with the ‘odd random phase multisine’ electrochemical impedance spectroscopy, Electrochim. Acta, 2010, 55(21), 6195–6203 CrossRef CAS.
- P. Zhang and G. Li, Advances in healing-on-demand polymers and polymer composites, Prog. Polym. Sci., 2016, 57, 32–63 CrossRef CAS.
- Y. Meng, G. Fang and J. Wang, et al., Photosensitizer-Loaded pH-Responsive Hollow Gold Nanospheres for Single Light-Induced Photothermal/Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2015, 7(32), 17592 Search PubMed.
- M. Kopeć, K. Szczepanowicz and G. Mordarski, et al., Self-healing epoxy coatings loaded with inhibitor-containing polyelectrolyte nanocapsules, Prog. Org. Coat., 2015, 84, 97–106 CrossRef.
- Z. Zheng, X. Huang and M. Schenderlein, et al., Bioinspired nanovalves with selective permeability and pH sensitivity, Nanoscale, 2015, 7(6), 2409–2416 RSC.
- B. M. Bailey, Y. Leterrier and S. J. Garcia, et al., Electrically conductive self-healing polymer composite coatings, Prog. Org. Coat., 2015, 85, 189–198 CrossRef CAS.
- T. H. Tran, A. Vimalanandan and G. Genchev, et al., Regenerative nano-hybrid coating tailored for autonomous corrosion protection, Adv. Mater., 2015, 27(25), 3825 CrossRef CAS PubMed.
- W. Wang, T. Li and Z. Jin, Preparation of nanoiron/PMMA composite particles with core–shell structure through emulsion polymerization for nitrate removal, Abstracts of Papers, 234th ACS National Meeting, Boston, MA, United States, 19–23 August 2007, pp. 258–262 Search PubMed.
- L. Wang, L. Deng and D. Zhang, et al., Shape memory composite (SMC) self-healing coatings for corrosion protection, Prog. Org. Coat., 2016, 97, 261–268 CrossRef CAS.
- S. H. Boura, M. Peikari and A. Ashrafi, et al., Self-healing ability and adhesion strength of capsule embedded coatings—micro and nano sized capsules containing linseed oil, Prog. Org. Coat., 2012, 75(4), 292–300 CrossRef.
- T. Xie, Recent advances in polymer shape memory, Polymer, 2011, 52(22), 4985–5000 CrossRef CAS.
- Y. Wu, L. Wang and X. Zhao, et al., Self-healing supramolecular bioelastomers with shape memory property as a multifunctional platform for biomedical applications via modular assembly, Biomaterials, 2016, 104, 18 CrossRef CAS PubMed.
- M. Iannuzzi, J. Kovac and G. S. Frankel, A study of the mechanisms of corrosion inhibition of AA2024-T3 by vanadates using the split cell technique, Electrochim. Acta, 2007, 52(12), 4032–4042 CrossRef CAS.
- P. Kardar, Preparation of polyurethane microcapsules with different polyols component for encapsulation of isophorone diisocyanate healing agent, Prog. Org. Coat., 2015, 89, 271–276 CrossRef CAS.
- Y. González-García, J. M. C. Mol and T. Muselle, et al., A combined mechanical, microscopic and local electrochemical evaluation of self-healing properties of shape-memory polyurethane coatings, Electrochim. Acta, 2011, 56(26), 9619–9626 CrossRef.
- M. Xie, L. Wang and J. Ge, et al., Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering, ACS Appl. Mater. Interfaces, 2015, 7(12), 6772 CAS.
- F. Lei, M. Hamdi and P. Liu, et al., Scratch behavior of epoxy coating containing self-assembled zirconium phosphate smectic layers, Polymer, 2017, 112, 252–263 CrossRef CAS.
- Y. Zhu, J. Shi and W. Shen, et al., Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure, Angew. Chem., Int. Ed. Engl., 2005, 44(32), 5083–5087 CrossRef CAS PubMed.
- J. Zhou, H. Li and W. Liu, et al., A facile method to fabricate polyurethane based graphene foams/epoxy/carbon nanotubes composite for electro-active shape memory application, Composites, Part A, 2016, 91, 292–300 CrossRef CAS.
- L. Wang, X. Yang and H. Chen, et al., Design of triple-shape memory polyurethane with photo-cross-linking of cinnamon groups, ACS Appl. Mater. Interfaces, 2013, 5(21), 10520 CAS.
- X. Zuo, W. Li and S. Mu, et al., Investigation of composition and structure for a novel Ti–Zr chemical conversion coating on 6063 aluminum alloy, Prog. Org. Coat., 2015, 87, 61–68 CrossRef CAS.
- M. Iannuzzi, J. Kovac and G. S. Frankel, A study of the mechanisms of corrosion inhibition of AA2024-T3 by vanadates using the split cell technique, Electrochim. Acta, 2007, 52(12), 4032–4042 CrossRef CAS.
- A. Baldan, Adhesively-bonded joints and repairs in metallic alloys, polymers and composite materials: adhesives, adhesion theories and surface pretreatment, J. Mater. Sci., 2004, 39(1), 1–49 CrossRef CAS.
- J. H. Nordlien, J. C. Walmsley and H. Østerberg, et al., Formation of a zirconium-titanium based conversion layer on AA 6060 aluminium, Surf. Coat. Technol., 2002, 153(1), 72–78 CrossRef CAS.
- O. Lunder, C. Simensen and Y. Yu, et al., Formation and characterisation of Ti–Zr based conversion layers on AA6060 aluminium, Surf. Coat. Technol., 2004, 184(2–3), 278–290 CrossRef CAS.
- K. Zhang, L. Wang and G. Liu, Copper(II) 8-hydroxyquinolinate 3D network film with corrosion inhibitor embedded for self-healing corrosion protection, Corros. Sci., 2013, 75(7), 38–46 CrossRef CAS.
- I. A. Kartsonakis, A. C. Balaskas and E. P. Koumoulos, et al., Evaluation of corrosion resistance of magnesium alloy ZK10 coated with hybrid organic–inorganic film including containers, Corros. Sci., 2012, 65(12), 481–493 CrossRef CAS.
- W. Trabelsi, E. Triki and L. Dhouibi, et al., The use of pre-treatments based on doped silane solutions for improved corrosion resistance of galvanised steel substrates, Surf. Coat. Technol., 2006, 200(14–15), 4240–4250 CrossRef CAS.
- K. Aramaki, Preparation of chromate-free, self-healing polymer films containing sodium silicate on zinc pretreated in a cerium(III) nitrate solution for preventing zinc corrosion at scratches in 0.5 M NaCl, Corros. Sci., 2002, 44(6), 1375–1389 CrossRef CAS.
- C. Zhong, X. Tang and Y. F. Cheng, Corrosion of steel under the defected coating studied by localized electrochemical impedance spectroscopy, Electrochim. Acta, 2008, 53(14), 4740–4747 CrossRef CAS.
- S. Serajzadeh, S. R. Motlagh and S. M. H. Mirbagheri, et al., Deformation behavior of AA2017–SiCp in warm and hot deformation regions, Mater. Des., 2015, 67, 318–323 CrossRef CAS.
- S. Xiao, M. M. Hossain and P. Liu, et al., Scratch behavior of model polyurethane elastomers containing different soft segment types, Mater. Des., 2017, 132, 419–429 CrossRef CAS.
- G. Baranauskas, E. Maggiolini and E. Castagnola, et al., Carbon nanotube composite coating of neural microelectrodes preferentially improves the multiunit signal-to-noise ratio, J. Neural Eng., 2011, 8(6), 066013 CrossRef PubMed.
- X. Yuan, Z. F. Yue and X. Chen, et al., Effect of mixture ratio on water uptake and corrosion performance of silicone-epoxy hybrid coatings coated 2024 Al-alloy, Prog. Org. Coat., 2015, 78, 168–175 CrossRef CAS.
- K. Pacaphol and D. Aht-Ong, The influences of silanes on interfacial adhesion and surface properties of nanocellulose film coating on glass and aluminum substrates, Surf. Coat. Technol., 2017, 320, 70–81 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |