Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
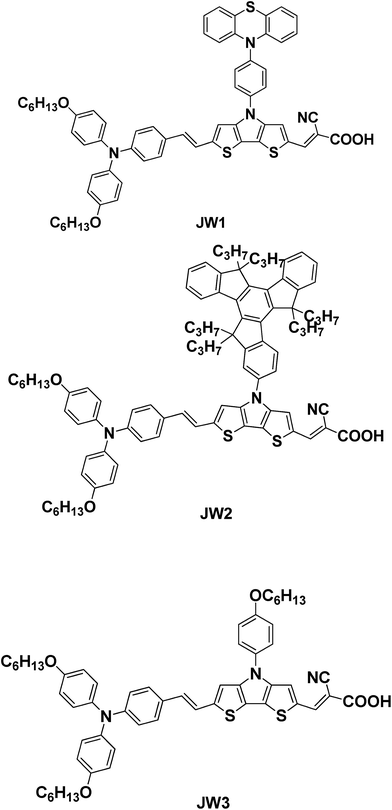
New D–π–A dyes incorporating dithieno[3,2-b:2′,3′-d]pyrrole (DTP)-based π-spacers for efficient dye-sensitized solar cells†
Jingwen Jia,
Yu Chen *,
Liangsheng Duan,
Zhe Sun
*,
Liangsheng Duan,
Zhe Sun ,
Mao Liang
,
Mao Liang and
Song Xue*
and
Song Xue*
Tianjin Key Laboratory of Organic Solar Cells and Photochemical Conversion, School of Chemistry & Chemical Engineering, Tianjin University of Technology, Tianjin 300384, China. E-mail: cytjut@163.com; xuesong@ustc.edu.cn; Fax: +86-22-60214252
First published on 26th September 2017
Abstract
Three D–π–A sensitizers incorporating dithieno[3,2-b:2′,3′-d]pyrrole (DTP)-based π-linkers were synthesized for fabricating efficient dye-sensitized solar cells (DSSCs). The DTP-based π-spacers were attached by 10-phenyl-10H-phenothiazine (JW1), 5,5,10,10,15,15-hexapropyl-10,15-dihydro-5H-diindeno[1,2-a:1′,2′-c]fluorene (JW2) and 4-hexyloxyphenyl (JW3). The bulky groups and alkyl chains are introduced to suppress dye aggregation as well as reduce electron recombination. An additional double bond spacer was introduced between the arylamine donor and DTP-based π-spacer in the studied dyes. Based on the theoretical calculation results, all of the dye molecules display good electron transport. The photovoltaic performances of device based on JW1, JW2 and JW3 were measured by J–V characterizations in Co-phen based electrolyte, achieving power conversion efficiencies of 7.09%, 7.87% and 6.50%, respectively. The incident photon-to-current conversion efficiency (IPCE) follows the order JW2 > JW1 > JW3, which is accordance with their photovoltaic performances. IMVS results indicate that the three dyes incorporating the modified DTP-based π-spacers have an influence on the CB shift of TiO2 and the inhibition of charge recombination. Therefore, the DTP-based π-spacer is a promising building block for developing new dyes to build highly efficient DSSCs.
1. Introduction
Dye-sensitized solar cells (DSSCs) have drawn much attention since the work of O'Regan and Grätzel in 1991.1,2 DSSCs can reduce the production cost and exhibit good photovoltaic performances so as to become a good substitute for expensive silicon solar cells. To date, various sensitizers have been used to fabricate highly efficient DSSCs, involving metal complex dyes3–8 and metal-free organic dyes.9–14 Metal-free organic dyes are amenable to structure modification and easy to purify. The power conversion efficiency (PCE) of DSSCs based on metal-free organic dyes has been greatly improved by structure design. Kakiage and co-workers reported that DSSCs co-sensitized by ADKEA-1 and LEG-4 exhibited a highest PCE of over 14%.15–17 Recently, it has been demonstrated by Freitag and co-workers that a DSSC achieved a very high PCE of 28.9% under ambient light conditions, using two organic sensitizers coded D35 and XY1.18 Therefore, metal-free organic dyes show promising advantages in fabricating highly effective DSSCs.The design of metal-free organic dyes can focus on molecular engineering of the electron donor, π-spacer, and acceptor for good light response, large anchoring amount and suitable energy levels.19–21 Suitable molecular energy levels of organic dyes to match electrolyte are beneficial to achieve an efficient regeneration of the oxidized dyes.22 Triarylamines are mostly used as effective electron donor groups in the sensitizer molecules, which can be feasibly synthesized and modified. Cyanoacetic acid is widely used as end groups with good electron accepting ability. The π-spacers of the organic dyes have a great influence on electron transfer between donors and acceptors in a dye molecule. In recent years, π-conjugated spacers containing thiophene rings have been reported showing positive effects on improving photovoltaic performances of organic sensitizers, such as thieno[3,2-b]thiophene,23 cyclopenta[1,2-b:5,4-b′]dithiophene24–26 and dithieno[3,2-b:2′,3′-d]pyrrole (DTP).27–29
DTP is accessible with a yield in one step up to 80%, and the N-position provides great opportunity for the control of dye aggregation, which makes it good for DSSC applications. As reported in our previous study,30 DTP can be easily modified by the introduction of long-chain alkyl groups on the bridging nitrogen, and DSSCs fabricated by these DTP-based dyes achieved a PCE of 8.14%. Wang and co-workers reported a series of arylamine organic dyes bearing N-heterocycle-substituted DTP spacers displayed a PCE of 8.20%.31 Polander and co-workers synthesized two donor–π-spacer–acceptor (D–π–A) dyes based on the DTP π-bridge and compared with their cyclopenta[1,2-b:5,4-b′]dithiophene (CPDT) analogues.32 Based on their results, DTP-based device exhibited an increased open circuit photovoltage values and improved charge-transfer kinetics relative to the CPDT systems. The present researches suggest DTP has a great potential for the design of efficient metal-free organic sensitizers in the field of DSSCs. The effects of N-substituents on dyes properties and device performances are worthy to explore.
In this work, three new D–π–A metal-free organic dyes were developed for DSSC application (JW1, JW2 and JW3). JW1 and JW2 have been synthesized by introducing bulky groups of 10-phenyl-10H-phenothiazine and 5,5,10,10,15,15-hexapropyl-10,15-dihydro-5H-diindeno[1,2-a:1′,2′-c]fluorene (truxene) onto the N-position of DTP spacers, respectively. JW3 was prepared by incorporating 4-hexyloxyphenyl as a reference compound. In the three dyes, the triphenylamine substituted by hexyloxy groups were used as electron donating group, and 2-cyanoacrylic acid was used as electron accepting group. A double bond was inserted between triphenylamine donor and DTP spacer, aiming to enhance π-conjugation length of dye molecules.33 The UV-vis absorption, optimized geometrical and electronic structures by density functional theory (DFT) calculations, and cyclic voltammetry behaviors of the three D–π–A dyes were characterized. DSSCs based on JW1, JW2 and JW3 were fabricated by using Co-phen based (phen = 1,10-phenanthroline) electrolytes with 4-tert-butylpyridine (TBP). The photovoltaic performances of DSSCs based on the studied dyes are measured by J–V characteristics, and the corresponding incident photon-to-current conversion efficiency (IPCE) spectra of JW1, JW2 and JW3 were recorded. The results show JW1 and JW2 bearing bulky groups exhibit a more positive influence on enhancing efficiency of DSSCs than JW3. The dyes effect on device performance was further discussed by conducting the measurements of the controlled intensity modulated photovoltage spectroscopy (IMVS).
2. Experimental
2.1. Instrument and materials
The chemical structures of target products JW1, JW2 and JW3 are shown in Fig. 1. BINAP, P(t-Bu)3, t-BuONa, Pd2(dba)3 and PPh3·HBr were purchased from J&K Chemical. Cyanoacetic acid was purchased from Energy Chemical (China). The solvents and chemicals used in this work were analytical grade and purchased from Energy Chemical (China). The reagents for reactions and spectral measurements were dried and distilled before use. The treatment on solvents and reactants is shown in ESI.†1H NMR and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer. The reported chemical shifts were against TMS. High resolution mass spectra were obtained with a Micromass GCT-TOF mass spectrometer. The melting point was taken on a RY-1 thermometer and the temperatures were uncorrected.
2.2. Photophysical properties and electrochemical properties
The absorption spectra of dyes and sensitized films were measured by SHIMADZU UV-2600 spectrophotometer. Fluorescence measurements were carried out with a HITACHI F-4500 fluorescence Spectrophotometer.Cyclic voltammetry (CV) measurements for sensitized films were measured by a Zennium electrochemical workstation (ZAHNER, Germany), with sensitized electrodes as the working electrode, Pt-wires as the counter electrode, and an Ag/AgCl electrode as the reference electrode at a scan rate of 25 mV s−1. Tetrabutylammonium perchlorate (TBAP, 0.1 M) and acetonitrile were used as supporting electrolyte and solvent, respectively. Ferrocene was used as standard in the measurements.
Charge densities at open circuit and intensity modulated photovoltage spectroscopy (IMVS) were performed on a Zennium electrochemical workstation (ZAHNER, Germany), which includes a green light emitting diode (LED, 525 nm) and the corresponding control system. Charge densities and IMVS were measured on a two electrode system with the TiO2-photoanode as a working electrode and the photocathode as the counter/reference electrodes. The intensity-modulated spectra were measured at room temperature with light intensity ranging from 5 to 75 W m−2, in modulation frequency ranging from 0.1 Hz to 10 kHz, and with modulation amplitude less than 5% of the light intensity.
2.3. The DSSCs fabrication and characterization
A TiO2 electrode (0.156 cm2) was developed on a precleaned fluorine-doped tin oxide (FTO) conducting glass (Nippon Sheet Glass, Hyogo, Japan, sheet resistance of 14 Ω sq−1), and the detailed fabrication procedure was included in ESI.† The dye was loaded by immersing it into a 300 μM dye solution (ethanol/CH2Cl2 = 2/3, v/v) for 12 h at room temperature. Then washed the dye coated titania electrode with dry ethanol. The prewashed FTO glass was deposited with Pt catalyst by coating with H2PtCl6 solution (40 mM in ethanol), and used the heat treatment sinter at 395 °C for 15 min to get photocathode. The sensitized electrode and Pt-counter electrode were assembled into a sandwich type cell by a 25 mm-thick Surlyn (DuPont) hot-melt gasket and sealed up by heating. The Co-phen electrolyte is composed of 0.25 M [Co(II) (phen)3](PF6)2, 0.05 M [Co(III) (phen)3](PF6)3, 0.5 M TBP and 0.1 M LiTFSI in acetonitrile.The photocurrentevoltage (J–V) characteristics of the solar cells based on the studied dyes were carried out using a Keithley 2400 digital source meter controlled by a computer and a standard AM1.5 solar simulator-Oriel 91160-1000 (300 W) SOLAR SIMULATOR 2 × 2 BEAM. The light intensity was calibrated by an Oriel reference solar cell. The action spectra of monochromatic incident photon-to-current conversion efficiency (IPCE) for solar cell were performed by using a commercial setup (QTest Station 2000 IPCE Measurement System, CROWNTECH, USA).
2.4. Synthesis
The synthesis route for JW1, JW2 and JW3 are shown in Scheme 1. The intermediates of compounds 1–5 have been reported in literatures.34–36 The 1H NMR and 13C NMR data of 7a–c, 8a–c, JW1, JW2 and JW3 are listed in ESI.†3. Results and discussion
The synthetic routes for JW1, JW2 and JW3 were shown in Scheme 1. The aldehyde intermediates (6a–c) play a key role in obtaining the target dye molecules. All intermediates and final products are purified and characterized by NMR spectra. The spectroscopic and electrochemical properties of JW1, JW2 and JW3 were tested and analyzed, respectively.3.1. Absorption spectra
Absorption and emission spectra of JW1, JW2 and JW3 were studied by UV-vis spectroscopy and fluorescence spectroscopy, and the optical band gaps (E0–0) of the three dyes are calculated from the intersections of normalized absorption and emission spectra. Fig. 2(a) shows the normalized absorption and emission spectra of JW1, JW2 and JW3 in dichloromethane. The maximum absorption wavelength in dichloromethane of JW1, JW2 and JW3 show little difference, which are 510 nm, 515 nm and 516 nm, respectively. This suggests the bulky groups are not key point for increasing the light response, while the basic D–π–A skeleton is a more important factor for their absorption ability. Subsequently, the molar extinction coefficients of the dye solution with the concentration of 1.5 × 10−5 M were measured. As listed in Table 1, the corresponding molar extinction coefficients of JW1, JW2 and JW3 in dichloromethane are 3.23 × 104 M−1 cm−1, 4.63 × 104 M−1 cm−1 and 3.48 × 104 M−1 cm−1, respectively. JW2 shows the highest molar extinction among the three dyes in solution. The intersections of normalized absorption and emission spectra of JW1, JW2 and JW3 are at 614 nm, 622 nm and 620 nm, respectively. Thus, the E0–0 values of JW1, JW2 and JW3 are 2.20 eV, 2.17 eV, and 2.16 eV, separately.37 The multi-alkyl groups of JW2 and JW3 relatively reduce the E0–0 value as compared to JW1. The absorption spectra of the studied dyes anchored on mesoporous TiO2 were also investigated, as shown in Fig. 2(b). Three dyes absorbing on TiO2 display obvious absorption extended to 700 nm. While the weaker absorbance of JW2 on TiO2 due to its larger molecular size than JW1 and JW3 causing a decrease of absorbed amount on TiO2 per square centimeter. The absorbed amounts of JW1, JW2 and JW3 on 3 μm-thick TiO2 films are 4.6 × 10−6 mol cm−2, 1.6 × 10−6 mol cm−2 and 3.1 × 10−6 mol cm−2, respectively. The spectroscopic data are summarized in Table 1.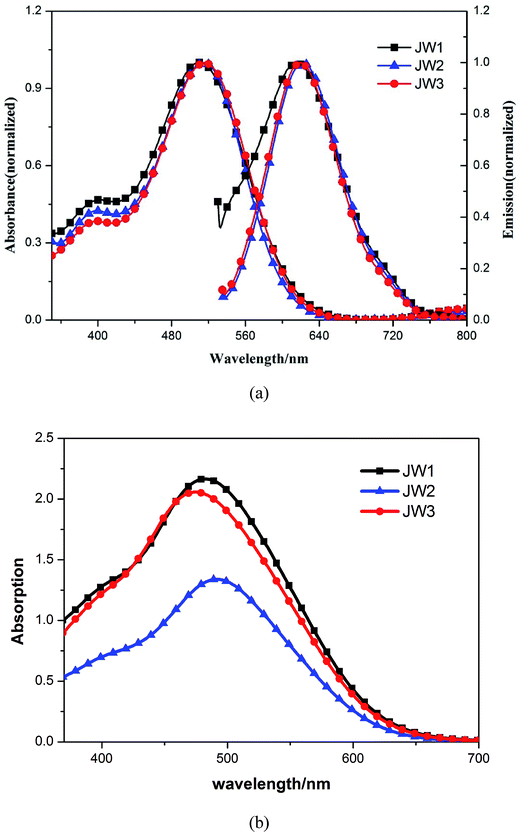 | ||
| Fig. 2 The absorption and emission spectra of JW1, JW2 and JW3 (a) in dichloromethane and (b) on TiO2 films. | ||
| Dye | λmax,sol a (nm) | ε (104 M−1 cm−1) | λmax,filmb (nm) | λintc (nm) | Adsorbed dyed 10−6 mol cm−2 | HOMOe/vs. NHE (eV) | LUMOf/vs. NHE (eV) | E0–0c (eV) |
|---|---|---|---|---|---|---|---|---|
| a The maximum absorption wavelength of UV-vis spectra measured in dichloromethane.b The maximum absorption wavelength of UV-vis spectra measured on TiO2.c λint is the intersections of normalized absorption and emission spectra in dichloromethane; E0–0 values were estimated from E0–0 = 1240/λint.d The adsorbed amount of dye on the TiO2 surface.e HOMO (vs. NHE) were measured in acetonitrile and calibrated with ferrocene (0.63 V vs. NHE).f LUMO (vs. NHE) were estimated from calculated from LUMO = HOMO–E0–0. | ||||||||
| JW1 | 510 | 3.23 | 482 | 614 | 4.6 | 1.014 | −1.186 | 2.20 |
| JW2 | 515 | 4.63 | 490 | 622 | 1.6 | 0.996 | −1.174 | 2.17 |
| JW3 | 516 | 3.48 | 472 | 620 | 3.1 | 0.914 | −1.246 | 2.16 |
3.2. Electrochemical properties and theoretical calculations
The structure effect on energy levels has a great impact on getting a suitable drive force for dye regeneration in DCCSs. Cyclic voltammograms (CV) was conducted to determine the accurate highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of JW1, JW2 and JW3. The CV curves of three dyes in acetonitrile are shown in Fig. 3(a). The electrochemical data and related calculation methods are given in Table 1. The HOMO levels of JW1, JW2 and JW3 corresponding to the first oxidation potentials was 1.104 eV, 0.996 eV and 0.914 eV (vs. NHE), respectively. The LUMO levels of JW1, JW2 and JW3 are −1.186 eV, −1.174 eV and −1.246 eV (vs. NHE), respectively. The LUMO levels of the three dyes are more negative than the conduction band (CB) of the TiO2 electrode (−0.5 V vs. NHE), making it easily for electron injection into the CB of TiO2. Based on the spectroscopic and electrochemical data, the energy-level diagram of JW1, JW2 and JW3 are depicted in Fig. 3(b). The energy levels of the studied dyes are well matched with the CB of TiO2 and the redox energy of the selected Co-phen electrolyte.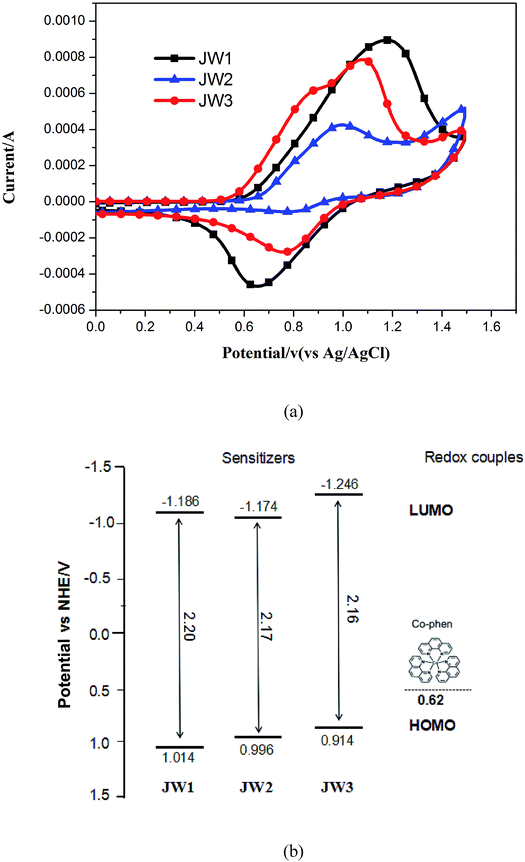 | ||
| Fig. 3 (a) Cyclic voltammograms of JW1, JW2 and JW3 measured in acetonitrile solutions; (b) energy levels of dyes and redox couples. | ||
To gain insight into the electronic properties and geometries of JW1, JW2 and JW3, quantum chemical calculations were performed. Their geometric structures were optimized on the basis of density functional theory (DFT) at the B3LYP/6-31G level. All structural optimization and energy calculations were performed using the GAUSSIAN 09 program. The optimized structures and the Frontier orbitals of molecules of JW1, JW2 and JW3 are shown in Fig. 4. The HOMOs of JW1, JW2 and JW3 are extended over the whole backbone of dye molecules, and the LUMOs are effectively transferred from the arylamine donor to the acceptor part unit via the DTP spacer. The optimized structures of JW1, JW2 and JW3 are shown in Fig. S1 and S2,† and the corresponding data on the representative dihedral angles and bond lengths are listed in Table S1 (ESI).† In Fig. S2,† the dihedral angles between DTP and its chains for JW1, JW2 and JW3 are 126.9°, 125.9° and 126.9°, respectively. The DTP spacer on JW2 exhibits a little more twisted structure due to the larger size of truxene unit. Based on the photovoltaic performances discussed on Section 3.4, the little twisted change on JW2 has a positive effect on reducing charge recombination on interfaces in DSSCs.
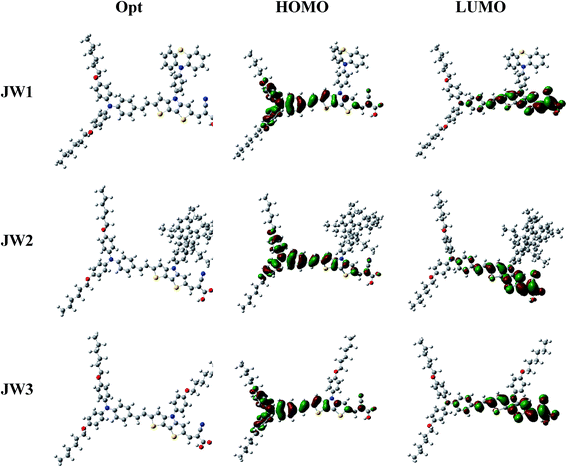 | ||
| Fig. 4 The Frontier molecular orbital distributions of JW1, JW2 and JW3 optimized by DFT calculations at the B3LYP/6-31G level. | ||
3.3. Photovoltaic performances of DSSCs
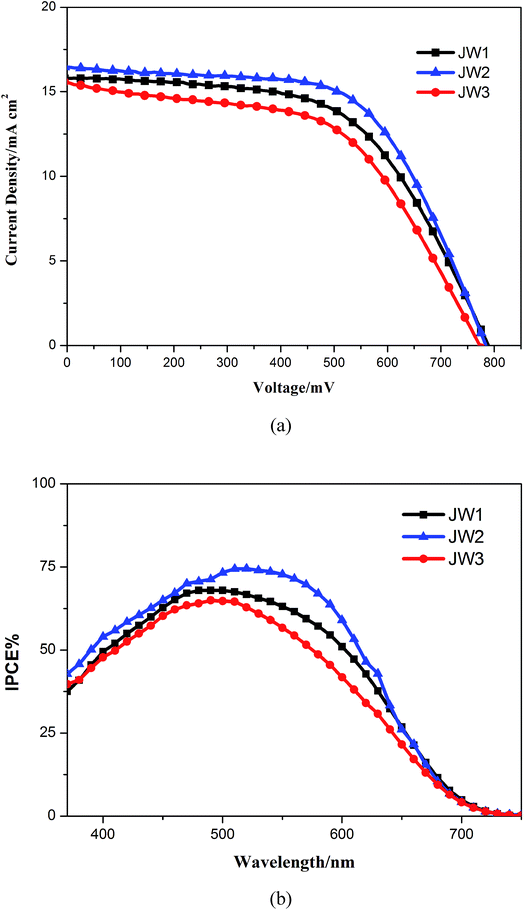
DSSCs based on JW1, JW2 and JW3 were fabricated with Co-phen electrolyte. The photovoltaic performances of devices based on JW1, JW2 and JW3 were investigated by photocurrent–voltage (J–V) measurements. The J–V curves are shown in Fig. 5(a), and the corresponding photovoltaic parameters are listed in Table 2. In Fig. 5(a), devices based on JW1, JW2 and JW3 show a PCE of 7.09%, 7.87% and 6.50%, respectively. JW2-based DSSCs exhibit the highest PCE, yielding a Jsc of 16.45 mA cm2, a Voc of 784 mV and a FF of 0.61. As shown in Fig. 3(b), the suitable HOMO–LUMO energy gap of JW2 can both strengthens electron injection from dye to TiO2 and reduces driving force for the regeneration of oxidized dye among the three dyes, making the corresponding device based on JW2 display the highest Jsc value. In Fig. 5(b), the corresponding incident photon-to-current conversion efficiency (IPCE) spectra of DSSCs fabricated by JW1, JW2 and JW3 were recorded. Correspondingly, IPCE values of JW1 and JW2-based DSSCs are higher than that of JW3-based DSSCs. As listed in Table 1, the absorbed JW1, JW2 and JW3 on TiO2 are 4.6 × 10−6 mol cm−2, 1.6 × 10−6 mol cm−2 and 3.1 × 10−6 mol cm−2, respectively. Although the amount of JW2 absorbed on TiO2 is the least among the three dyes due to the large size of truxene unit, JW2-based cells achieved the best performance.| Dye | Jsc/MA cm2 | Voc/mV | FF | PCE% |
|---|---|---|---|---|
| JW1 | 15.79 | 788 | 0.57 | 7.09 |
| JW2 | 16.45 | 784 | 0.61 | 7.87 |
| JW3 | 15.57 | 773 | 0.54 | 6.50 |
In order to further study the relationship between dye structures and device performances, the charge density (dn) at open circuit and the electron lifetime (τ) as a function of charge density were measured by controlled intensity modulated photovoltage spectroscopy (IMVS).38–40 The results indicate that the introduction of 10-phenyl-10H-phenothiazine and truxene onto DTP spacers of JW1 and JW2 is benefit to enhance the photovoltaic performances of DSSCs by more efficiently reduce electron recombination, as compared to the introduction of 4-hexyloxyphenyl onto DTP spacers of JW3. As shown in Fig. 6(a), TiO2 grafted with JW1, JW2 and JW3 displays an increased Voc value in the order of JW2 < JW3 < JW1 at a fixed dn, indicating the introduction of bulky groups on DTP has an obvious impact on the CB shift of TiO2.27,41 TiO2 grafted with JW2 caused a downward CB shift of TiO2 compared to JW1 and JW3. However, the LUMO level of JW2 is low enough to make sure efficient electron injection from JW2 to TiO2. Therefore, the different bulky groups feasibly regulate HOMO–LUMO energy gap of the studied sensitizers, the suitable energy levels have a positive effect on fabricating highly-efficient DSSCs. As shown in Fig. 6(b), the τ value of JW2 exhibits a certain increase than those of devices based on JW1 and JW3, which may be more favorable to reduce charge recombination in the device. Truxene is a good building block for the modification on π-spacers of sensitizers in DSSCs.
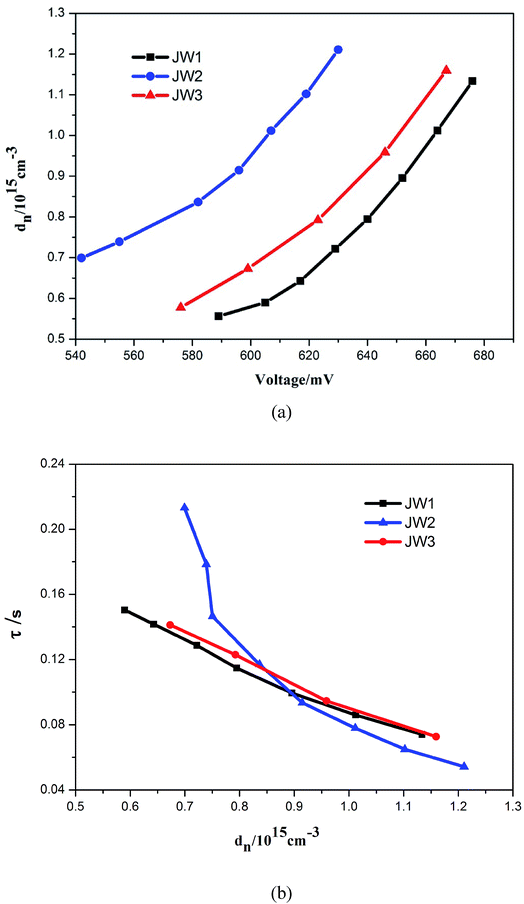 | ||
| Fig. 6 (a) Charge density as the open circuit and (b) electron lifetime as the charge density for DSSCs based on the dyes JW1, JW2 and JW3. | ||
4. Conclusions
New D–π–A organic dyes with dithieno[3,2-b:2′,3′-d]pyrrole (DTP) π-spacers (JW1, JW2 and JW3) were synthesized for fabricating efficient DSSCs in Co-phen based electrolyte. Different bulky groups were introduced onto the DTP unit, and the structure–efficiency relationship was investigated by theoretical calculations, spectroscopic, electrochemical and photovoltaic measurements. The three dyes display strong absorption at the range of 400–700 nm both in solution and on TiO2 film. CV measurements show that JW2 incorporating DTP with truxene shows modest energy levels for reducing drive force for electron injection and dye regeneration. Based on J–V results, DSSCs fabricated by JW2 exhibit the highest PCE of 7.87%, and that of cells based on JW1 and JW3 are 7.09% and 6.50%. Devices based on JW1 and JW2 with lager bulky units yield higher Jsc and Voc values than that based on JW3. IMVS results indicate the three dyes have an obvious influence on the CB shift of TiO2 via the introduction of different substitutes on DTP π-spacer, and differs the inhibition ability of charge recombination in device. Therefore, suitable design of dye molecules has a great effect on improve the photovoltaic performances of DSSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21376179, 21506164).References
- B. O'regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353, 737–740 CrossRef.
- Y. Z. Wu, W. H. Zhu, S. M. Zakeeruddin and M. Grätzel, Insight into D–A–π–A structured sensitizers: a promising route to highly efficient and stable dye-eensitized solar cells, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CAS.
- L. Wei, Y. Na, Y. L. Yang, R. Q. Fan, P. Wang and L. Li, Efficiency of ruthenium dye sensitized solar cells enhanced by 2,6-bis[1-(phenylimino)ethyl]pyridine as a co-sensitizer containing methyl substituents on its phenyl rings, Phys. Chem. Chem. Phys., 2015, 17, 1273–1280 RSC.
- H. Ozawa, T. Sugiura, T. Kuroda, K. Nozawa and H. Arakawa, Highly efficient dye-sensitized solar cells based on a ruthenium sensitizer bearing a hexylthiophene modified terpyridine ligand, J. Mater. Chem. A, 2016, 4, 1762–1770 CAS.
- H. Ozawa, S. Honda, D. Katano, T. Sugiura and H. Arakawa, Novel ruthenium sensitizers with a dianionic tridentate ligand for dye-sensitized solar cells: the relationship between the solar cell performances and the electron-withdrawing ability of substituents on the ligand, Dalton Trans., 2014, 43, 8026–8036 RSC.
- H. Cheema, A. Islam, L. Han and A. El-Shafei, Monodentate pyrazole as a replacement of labile NCS for Ru(II) photosensitizers: Minimum electron injection free energy for dye-sensitized solar cells, Dyes Pigm., 2015, 120, 93–98 CrossRef CAS.
- H. Cheema, L. Ogbose and A. El-Shafe, Structure–property relationships: steric effect in ancillary ligand and how it influences photocurrent and photovoltage in dye-sensitized solar cells, Dyes Pigm., 2015, 113, 151–159 CrossRef CAS.
- E. Schönhofer, B. Bozic-Weber, C. J. Martin, E. C. Constable, C. E. Housecroft and J. A. Zampese, Surfaces-as-ligands, surfaces-as-complexes, strategies for copper(I) dye-sensitized solar cells, Dyes Pigments, 2015, 115, 154–165 CrossRef.
- M. Liang and J. Chen, Arylamine organic dyes for dye-sensitized solar cells, Chem. Soc. Rev., 2013, 42, 3453–3488 RSC.
- C. P. Lee, R. Y. Lin, L. Y. Lin, C. T. Li, T. C. Chu, S. S. Sun, J. T. Lin and K. C. Ho, Recent progress in organic sensitizers for dye-sensitized solar cells, RSC Adv., 2015, 5, 23810–23825 RSC.
- S. Ahmad, E. Guillén, L. Kavan, M. Grätzel and M. K. Nazeeruddin, Metal free sensitizer and catalyst for dye sensitized solar cells, Energy Environ. Sci., 2013, 6, 3439–3466 CAS.
- A. L. Capodilupo, L. D. Marco, G. A. Corrente, R. Giannuzzi, E. Fabiano, A. Cardone, G. Gigl and G. Ciccarella, Synthesis and characterization of a new series of dibenzofulvene based organic dyes for DSSCs, Dyes Pigm., 2016, 130, 79–89 CrossRef CAS.
- M. Grishina, O. Bol'shakov, A. Potemkin and V. Potemkin, Benzo[1,2,5]thiadiazole dyes: Spectral and electrochemical properties and their relation to the photovoltaic characteristics of the dye-sensitized solar cells, Dyes Pigm., 2017, 144, 80–93 CrossRef CAS.
- C. Maglione, A. Carella, C. Carbonara, R. Centore, S. Fusco, A. Velardo, A. Peluso, D. Colonna, A. Lanuti and A. D. Carlo, Novel pyran based dyes for application in dye sensitized solar cells, Dyes Pigm., 2016, 133, 395–405 CrossRef CAS.
- K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen and M. Unno, An achievement of over 12 percent efficiency in an organic dye-sensitized solar cel, Chem. Commun., 2014, 50, 6379–6381 RSC.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. I. Fujisawa and M. Hanaya, Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes, Chem. Commun., 2015, 51, 15894–15897 RSC.
- W. H. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z. Wang and H. Tian, Organic D–A–π–A solar cell sensitizers with improved stablility and soectral response, Adv. Funct. Mater., 2011, 1, 756–763 CrossRef.
- M. Freitag, J. Teuscher, Y. Saygili, X. Zhang, F. Giordano, P. Liska, J. Hua, S. M. Zakeeruddin, J. E. Moser, M. Grätzel and A. Hagfeldt, Dye-sensitized solar cells for efficient power generation under ambient lighting, Nat. Photonics, 2017, 11, 372–378 CrossRef CAS.
- T. Hao, Y. Saygili, J. Cong, A. Eriksson, W. Yang, J. Zhang, E. Polanski, K. Nonomura, M. Z. ZakeeruddinShaik, M. Grätzel, A. Hagfeldt and G. Boschloo, Novel blue organic dye for dye-sensitized solar cells achieving high efficiency in cobalt-based electrolytes and by co-sensitization, ACS Appl. Mater. Interfaces, 2016, 8, 32797–32804 Search PubMed.
- C. Y. Lo, D. Kumar, S. H. Chou, C. H. Chen, C. H. Tsai, S. H. Liu, P. T. Chou and K. T. Wong, Highly twisted dianchoring D–π–A sensitizers for efficient dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2016, 8, 27832–27842 CAS.
- P. Dai, H. Dong, M. Liang, H. Cheng, Z. Sun and S. Xue, Understanding the role of electron donor in truxene dye sensitized solar cells with cobalt electrolytes, ACS Sustainable Chem. Eng., 2017, 5, 97–104 CrossRef CAS.
- Y. K. Eom, J. Y. Hong, J. Kim and H. K. Kim, Triphenylamine-based organic sensitizers with π-spacer structural engineering for dye-sensitized solar cells: synthesis, theoretical calculations, molecular spectroscopy and structure-property-performance relationships, Dyes Pigm., 2017, 136, 496–504 CrossRef CAS.
- S. M. Fernandes, M. Mesquit, L. Andrade, A. Mendes and M. Raposo, Synthesis and characterization of novel thieno[3,2-b]thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells, Dyes Pigm., 2017, 1, 46–53 CrossRef.
- A. Yella, H. B. Robin, B. F. E. Curchod, N. A. Astani, J. Teuscher, L. E. Polander, S. Mathew, J. E. Moser, I. Tavernelli, U. Rothlisberger, M. Grätzel, M. K. Nazeeruddin and J. Frey, Molecular engineering of a fluorene donor for dye-sensitized solar cells, Chem. Mater., 2013, 25, 2733–2739 CrossRef CAS.
- P. Gao, H. N. Tsao, M. Grätzel and M. K. Nazeeruddin, Fine-tuning the electronic structure of organic dyes for dye-sensitized solar cell, Org. Lett., 2012, 14, 4330–4333 CrossRef CAS PubMed.
- X. Zhang, Y. Xu, F. Giordano, M. Schreier, N. Pellet, Y. Hu, C. Yi, N. Robertson, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, Molecular engineering of potent sensitizers for very efficient light harvesting in thin-film solid-state dye-sensitized solar cells, J. Am. Chem. Soc., 2016, 138, 10742–10745 CrossRef CAS PubMed.
- Z. Wang, M. Liang, H. J. Yu, Y. Zhang, L. Wang, Z. Sun and S. Xue, Influence of the N-heterocycle substituent of the dithieno[3,2-b:2′,3′-d]pyrrole (DTP) spacer as well as sensitizer adsorption time on the photovoltaic properties of arylamine organic dyes, J. Mater. Chem. A, 2013, 1, 11809–11819 CAS.
- Y. Xie, W. Wu, H. Zhu, J. Liu, W. Zhang, H. Tian and W. H. Zhu, Unprecedentedly targeted customization of molecular energy levels with auxiliary-groups in organic solar cell sensitizers, Chem. Sci., 2016, 7, 544–549 RSC.
- F. S. Li, Y. Chen, X. P. Zong, W. H. Qiao, H. Y. Fan, M. Liang and S. Xue, New benzothiadiazole-based dyes incorporating dithieno[3,2-b:2′,3′-d]pyrrole (DTP) π-linker for dye-sensitized solar cells with different electrolytes, J. Power Sources, 2016, 332, 345–354 CrossRef CAS.
- H. Dong, M. Liang, C. Zhang, Y. Wu, Z. Sun and S. Xue, Twisted fused-ring thiophene organic dye-sensitized solar cells, J. Phys. Chem. C, 2016, 120, 22822–22830 CAS.
- Z. Wang, M. Liang, Y. Hao, Y. Zhang, L. Wang, Z. Sun and S. Xue, Influence of the N-heterocycle substituent of the dithieno[3,2-b:2′,3′-d]pyrrole (DTP) spacer as well as sensitizer adsorption time on the photovoltaic properties of arylamine organic dyes, J Mater. Chem. A, 2013, 1, 11809–11819 CAS.
- L. E. Polander, A. Yella, J. Teuscher, R. Humphry-Bake, B. Curchod, N. A. Astani, P. Gao, J. E. Moser, I. Tavernelli, U. Rothlisberger, M. Grätzel, M. K. Nazeeruddin and J. Frey, Unravelling the potential for dithienopyrrole sensitizers in dye-sensitized solar cells, Chem. Mater., 2013, 25, 2642–2648 CrossRef CAS.
- D. H. Lee, M. J. Lee, H. M. Song, B. J. Song, K. D. Seo, M. Pastore, C. Anselmi, F. D. Angelis and M. K. Nazeeruddin, Organic dyes incorporating low-band-gap chromophores based on π-extended benzothiadiazole for dye-sensitized solar cell, Dyes Pigm., 2011, 91, 192–198 CrossRef CAS.
- T. Ganesh, H. M. Nguyen, R. S. Mane, N. Kim, D. V. Shinde and S. S. Bhande, Promising ZnO-based DSSC performance using HMP molecular dyes of high extinction coefficients, Dalton Trans., 2014, 43, 11305–11308 RSC.
- J. Liu, D. Zhou, M. Xu, X. Jing and P. Wang, The structure–property relationship of organic dyes in mesoscopic titania solar cells: only one double-bond difference, Energy Environ. Sci., 2011, 4, 3545–3551 CAS.
- W. H. Nguyen, C. D. Bailie, J. Burschka, T. Moehl, M. Grätzel and M. D. Mcgehee, Molecular engineering of organic dyes for improved recombination lifetime in solid-state dye-sensitized solar cells, Chem. Mater., 2013, 25, 1519–1525 CrossRef CAS.
- M. Grishina, O. Bol'shakov, A. Potemkin and V. Potemkin, Benzo[1,2,5]thiadiazole dyes: Spectral and electrochemical properties and their relation to the photovoltaic characteristics of the dye-sensitized solar cells, Dyes Pigm., 2017, 144, 80–93 CrossRef CAS.
- Z. S. Huang, L. H. Feng, X. F. Zang, Z. Iqbal, H. Zeng and D. B. Kuang, Dithienopyrrolobenzothiadiazole-based organic dyes for efficient dye-sensitized solar cells, J. Mater. Chem. A, 2014, 2, 15365–15376 CAS.
- J. H. Lee, C. H. Park, J. P. Jung and J. H. Kim, Worm-like mesoporous TiO2 thin films templated using comb copolymer for dye-sensitized solar cells with polymer electrolyte, J. Power Sources, 2015, 298, 14–22 CrossRef CAS.
- Y. Q. Wang, J. Lua, J. Yina, G. Lü, Y. M. Cui, S. S. Wang, S. Y. Deng, D. Shan, H. L. Tao and Y. M. Sun, Influence of 4-tert-butylpyridine/guanidinium thiocyanate co-additives on band edge shift and recombination of dye-sensitized solar cells: experimental and theoretical aspects, Electrochim. Acta, 2015, 185, 69–75 CrossRef CAS.
- X. L. Hao, M. Liang, X. B. Cheng, X. Q. Pian, Z. Sun and S. Xue, Organic dyes incorporating the benzo[1,2-b:4,5-b′]dithiophene moiety for efficient dye-sensitized solar cells, Org. Lett., 2011, 13, 5424–5427 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08965a |
| This journal is © The Royal Society of Chemistry 2017 |