Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Complex study of the physical properties of a poly(lactic acid)/poly(3-hydroxybutyrate) blend and its carbon black composite during various outdoor and laboratory ageing conditions†
Katarína Mosnáčková a,
Martin Danko
a,
Martin Danko a,
Alena Šiškováa,
Lorena M. Falco
a,
Alena Šiškováa,
Lorena M. Falco a,
Ivica Janigováa,
Štefan Chmelaa,
Zuzana Vanovčanováb,
Leona Omaníkováb,
Ivan Chodáka and
Jaroslav Mosnáček
a,
Ivica Janigováa,
Štefan Chmelaa,
Zuzana Vanovčanováb,
Leona Omaníkováb,
Ivan Chodáka and
Jaroslav Mosnáček *a
*a
aPolymer Institute, Slovak Academy of Sciences, Dúbravská cesta 9, 845 41 Bratislava, Slovakia. E-mail: jaroslav.mosnacek@savba.sk; Tel: +421-2-3229 4353
bDepartment of Plastics, Rubber and Fibres, Faculty of Chemical and Food Technology, Slovak University of Technology in Bratislava, Radlinského 9, 821 37 Bratislava, Slovakia
First published on 6th October 2017
Abstract
Ageing of foils made from poly(lactic acid)/poly(3-hydroxybutyrate) PLA/PHB blends, unfilled or filled with 1 wt% of carbon black is presented. The foils were either buried in the soil or exposed to the sunlight for 4 months to imitate the behavior of the material when applied as mulching foils. In addition, UV-vis irradiation and hydrolysis over water vapor were studied under laboratory conditions. Finally, the effect of the ageing under the mentioned conditions on mineralization of the foils at room temperature was investigated in order to simulate plowing the material after its application. The degradation was studied by gel permeation chromatography and structural changes were investigated using differential scanning calorimetry. The changes in mechanical properties were investigated by dynamic-mechanical thermal analysis and a tensile test. Addition of carbon black to PLA/PHB resulted in improved photochemical stabilization of the foils, preventing supplemental crystallization during ageing and provided longer retention of the mechanical properties during ageing.
1. Introduction
Plastics are widely used in packaging and agriculture, however, most of them are resistant to microbiological attack, leading to an accumulation of plastic waste in landfills. Biodegradable polymers represent a suitable solution for this problem due to the chemical structure of the polymer containing oxygen which enables enzymatic hydrolysis to occur and they are prone to natural recycling by biological processes. Environmentally friendly materials, such as poly(hydroxyalkanoates) (PHAs) and poly(lactic acid) (PLA) represent two families of biodegradable polymers derived from renewable resources. PLA is the first polymer based on renewable materials commercialized at a large scale due to its thermoplastic behaviour, good processability, biocompatibility and some interesting physical properties e.g. transparency after processing.1 Unfortunately, amorphous PLA is rigid and brittle due to the glass transition temperature (Tg) above RT, being in the range of 50–60 °C, which limits the application of PLA materials. Therefore, considerable efforts have been made to improve the plastic deformation ability. To enhance the flexibility of PLA polymers the citrate esters,2 poly(ethylene glycol),3 glycerol4 and triacetine5 are commonly used as the plasticizers. On the other hand, the addition of plasticizers generally results in lower strength and Young's modulus of PLA.6 For these purposes the nano-structured carbon fillers have been used to enhance the mechanical properties such as modulus and tensile strength of the composites.7PHAs belong to a family of poly(hydroxyesters) of 3-, 4-, 5- and 6-hydroxyalkanoic acids. Structurally the simplest and generally the most frequently investigated PHA is poly(3-hydroxybutyrate) (PHB) synthesized in bacteria as an energy storage material.8 PHB is fully biodegradable thermoplastic polyester, however, its broader application is hindered by its brittleness and low thermal resistance leading to extensive thermal degradation during processing. Partial solution of this problem is represented by synthesis of copolymers, such as poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV), introduction of additive or preparation of blends with PLA.9 It's well known, that preparation of blends using certain weight ratio of polymers may have synergistic effect and resulting mechanical properties could be greatly modified.10 The mechanical and thermal stability are very important for packaging as well as agriculture applications (mulching foils or tunnels). Under these applications blends will be exposed to sunlight and to other weather elements such as heat and moisture. Thus, it becomes of great importance to elucidate how PLA/PHB blends and also its composites with carbon black (CB) will respond when exposed to these conditions.
In the natural environment, the degradation of PLA proceeds either through hydrolytic or enzymatic chain scission of the ester bonds resulting in fragmented low molecular weight residues which are more vulnerable for bacterial attack.11 Generally, degradation of PLA proceeds readily in compost environment, which is characterized by relatively high temperatures.12–14 Agarwal et al.15 reported that microorganisms do not enhance PLA degradation and that polymer cleavage proceeds solely through abiotic hydrolysis of ester linkages in the presence or absence of microorganisms.
Weng et al.16 found that the degradation of PHA is caused by bacteria catalyzed erosion from the surface to the interior, while PLA is hydrolytically degraded, resulting in different degradation rates in different depths of soil due to different humidity and microbial strains influence on the polymers. It was shown that PLA buried 40 cm in the depth of soil undergoes to degradation more rapidly than in aerobic conditions. The finding, that abiotic chemical hydrolysis at elevated temperatures in the presence of water is primarily responsible for PLA chain cleavage and consecutive degradation was confirmed by many works published recently.17–22 Moreover, carboxyl end groups initially present or formed during the chain cleavage may participate in auto-catalyzed ester hydrolysis.23,24
The (bio)degradation behavior of PLA/PHAs blends has been investigated by many researchers. While published papers have been focused on the (bio)degradation of PHA, PLA, or their blends under composting conditions25–28 as well as on photodegradation27,29–31 and laboratory simulations testing,32,33 the degradation behavior of PLA/PHB and PLA/PHB/carbon black materials in real soil environments has not been reported so far.
The aim of the study presented in this paper is to offer better understanding and enrich the existing knowledge about the long-term degradation of PLA/PHB blends and their composites with 1 wt% carbon black loading under conditions of agricultural weathering. The hydrolytic and (bio)degradation behavior in the natural soil environment was studied by small scale flower pot experiments and by respiration test on the simulated soil burial experiments under controlled laboratory conditions. To compare the results of natural ageing caused by sunlight, the PLA/PHB/CB blend was also exposed to artificial UV radiation at different irradiation times. The thermal and mechanical properties were evaluated on aged composite foils and compared to unfilled PLA/PHB foils exposed to the same ageing treatment.
2. Materials and methods
2.1. Materials
Commercial blend PLA/PHB Nonoilen® pellets were provided by Panara s.r.o. (Nonoilen contained PLA, PHB polymers and citrate ester as plasticizer). Delivered pellets were converted into two types of film using film blowing technology. The first one was prepared directly from original material and the second one, containing 1 wt% of carbon black (N220, supplied by Corax®), was prepared with addition of carbon black masterbatch. The carbon black masterbatch containing 30 wt% of carbon black was prepared by melt mixing of PLA/PHB pellets with appropriated amount of carbon black at 175 °C and 40 rpm using Plasti-corder Brabender PLE 331 equipped with 30 mL chamber (Germany). Film blowing was performed at 190 °C, the thickness of the resulted foil was approximately 40 μm. A soil was collected in an agricultural region at South Slovakia near Danube River. The pH of the soil was determined according to the standard STN ISO 10390 and was 7.5. Its moisture hold capacity (MHC) represents the moisture in soil that keeps inside against the gravity and was established on 0.85. Expanded Perlit EP 200 for agricultural purposes has grain diameter 0.5/4 mm and was purchased from LB Minerals, a.s. Slovakia. Deionized water in Millipore® system with 18 mΩ/25 °C was used for all solutions needed for titrations.2.2. Outdoor weathering
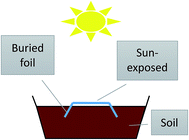
The foils were cut into 10 × 30 cm specimens and their weathering was carried out in the standard flower pots (45 × 20 × 20 cm) by the manner simulated the real conditions in the field as mulches (Fig. 1). Part of the samples was buried in the soil and part of the foil was located on the top of soil (exposed to the sunlight), while the samples were watered regularly to imitate the agricultural treatment. To follow the changes during weathering, the samples were washed by warm water, dried at room temperature in the dark overnight, stored in the dark and tested within one week. In all used testing methods (all except tensile test) at least 2–3 samples from various places were cut and tested to check the reproducibility.2.3. UV-vis irradiation
The UV-vis irradiation was performed using the merry-go-round type set up, equipped with a medium pressure 250 W mercury arc with luminophore envelope (RVL, Tesla Holešovice, Czech Republic) as source of irradiation. The temperature of photooxidation was 35–40 °C. The course of photooxidation was followed by GPC measurements monitoring the decrease of average molecular weights.2.4. Mineralization test under laboratory controlled conditions
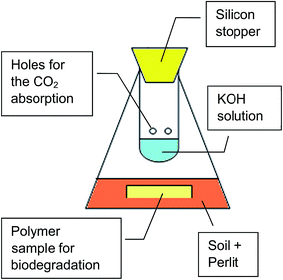
Mineralization test was carried out according to standards STN 17556-2012 and ASTM D5988-12 (ref. 34 and 35) in soil under aerobic conditions in the reactor schematically showed in Fig. 2. Carbon content in tested PLA/PHB foil determined by elemental analysis was 52.15%. Polymer foil was buried in mixed Perlit and soil equivalent amount (totally 200 g) in 500 mL Erlenmayer flask. Flask was closed by perforated glass tube fulfilled with 20 mL of KOH 0.5 M solution and equipped by rubber stopcock. CO2 produced by mineralization was absorbed in the KOH solution. Samples of the trap solution were opened periodically at least every 10 days for 1 hour to tend free respiration of microorganisms in the soil and to establish the amount of absorbed CO2. 1 mL of KOH solution from the trap was titrated with 0.05 M HCl with phenolphthalein as indicator. For new period the new 20 mL of KOH was added to the trap. All samples were processed by triplicate. Flasks with only Perlit and soil were used as control and empty flasks were used as blank, respectively, to measure production of the CO2 by the microorganisms itself and to measure CO2 present in the air, respectively.2.5. Hydrolysis with water vapor
Hydrolysis was carried out using sample with dimension 4 × 4 cm placed approximately 1 cm over the water level in sealed 250 mL vessels containing 120 mL of water. The vessels were placed in an oven and kept at 60 °C for various times.2.6. Analyses
The tensile properties were measured using a Dynamometer Instron 4301 on strip shape specimens cut directly from foils at room temperature. Five specimens of 15 mm width were tested for each foil sample according to STN EN ISO 527-3.36 The mechanical tests were performed at cell load of 5 kN while up to 0.5% deformation the speed of 1 mm min−1 was used and above 0.5% deformation the speed changed to 50 mm min−1. Gripping distance of 50 mm and initial length of 100 mm was used at room temperature in respect to STN EN ISO 527-3. Tensile tests were measured always in the blow molding direction that were more regular compared to transverse direction. Average values of Young's modulus E, elongation at break εB and tensile strength σM were calculated from stress–strain plots.The Dynamic Mechanical Thermal Analysis (DMTA) was performed using the Dynamic Mechanical Analyser DMA Q800 in the temperature range of −20 °C to 160 °C and with heating rate of 3 °C min−1. A stress in tensile mode was applied to the sample at 1 Hz with deformation amplitude of 40 μm. The samples were cut out in the blow molding direction with specimen dimension of 30 mm (length) × 5 mm (width) × 0.08 (thickness). The storage modulus (E′), loss modulus (E′′), and loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = E′/E′′) were determined for at least three specimens of each sample.
δ = E′/E′′) were determined for at least three specimens of each sample.
Differential scanning calorimetry (DSC) was performed in a Mettler-Toledo DSC 821e differential scanning calorimeter under nitrogen atmosphere. The calibration of temperature and heat of fusion was performed using indium. The DSC measurements were performed in the range of 0–200 °C at a heating rate of 10 °C min−1. For each type of foil three specimens from different positions of the foil were measured. This process represents first heating cycle, which reflects the actual state (change in crystalline fraction) of samples after outdoor weathering, hydrolysis with water vapor and UV-vis irradiation.
GPC system consisted of P102 pump from Watrex (Czech Republic) and evaporative light scattering detectors (ELSD) model 1000 from PL-Agilent Technologies, UK-USA. Temperature of evaporators was set at 80 °C and gas flow rate was 1.5 mL min−1. GPC column was TSKgel GMH HR – M, 300 × 7.5 mm from Tosoh Corporations. The GPC measurements were performed at ambient temperature with flow rate 1 mL min−1. Chloroform CHROMASOLV®, HPLC grade, with purity ≥ 99.8%, amylene stabilized from Sigma-Aldrich was used as GPC eluent. Calibration was based on polystyrene standards. Data were collected and processed with Clarity software from DataApex (Czech Republic).
The morphology of the foils was analyzed by Jeol JSM 7600F Schotky field emission scanning electron microscope (Jeol Ltd. Tokyo, Japan) at accelerated voltage 2 kV and working distance 15 mm. The samples were broken in liquid nitrogen and sputtered with a thin layer of gold before analysis.
3. Results and discussion
Conflicting results by different researchers concerning the mechanism of PLA degradation have been found in literature. Some studies have argued that PLA is degraded entirely by abiotic processes, whereas others have claimed that microorganisms or enzymes play a vital role in PLA degradation.37 Generally, the abiotic hydrolysis of PLA proceeds first until the molecular weight of PLA decrease sufficiently to be assimilated by microorganisms. For potential application of biodegradable materials such as PLA/PHB blends as a mulching foils we studied the effect of various factors, such as UV-light, water vapor, microorganisms, presence of carbon filler and/or their combination, on degradation of PLA/PHB foils. Thus following experiments were done for PLA/PHB blend and PLA/PHB/carbon black composite: (1) UV-vis irradiation with mercury lamp, (2) hydrolysis with water vapor, (3) outdoor weathering of the foil, while the foil was either buried in the soil or laid on the soil (exposed to the sunlight) (see Fig. 1), and (4) microbial degradation at 20 °C under laboratory controlled conditions. From the point of view of applicability of these materials as mulching foils, the studies were done in order to obtain information whether the mulching foils are able to retain sufficiently their mechanical properties during one season with subsequent removing of the foil for composting. Other borderline case can be that an extensive degradation will be obtained with possibility of subsequent plowing the residues in the soil, where the microbial and hydrolytic degradation would be followed through.3.1. GPC analysis
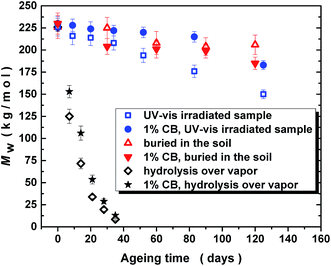
Fig. 3 compares the changes in molar mass (Mw) of PLA/PHB blend and its 1 wt% carbon black composite before and after various times of UV-vis irradiation, weathering in soil and hydrolysis in water vapors at 60 °C. Presence of carbon black affected the UV-stability and the decrease in molar mass after 125 days of UV-vis irradiation was lower for carbon black filled PLA/PHB composite compared to unfilled PLA/PHB blend. The decrease in molar mass was accompanied with broadening of molar mass dispersity from about 1.7 for original PLA/PHB blend to 1.9 for PLA/PHB blend UV-vis irradiated for 125 days (data not shown).Only slight decrease in molar mass was observed for samples, either unfilled or filled with carbon black, buried in the soil. This could indicate that under outdoor weathering conditions the effect of microorganism and water on changes of molar mass is minimal compared to effect of UV-vis radiation. Interestingly in the case of sun exposed samples, regardless on presence or absence of carbon black filler, the decrease in molar mass was similar as in the case of samples buried in the soil. Thus the molar masses of unfilled and carbon black filled PLA/PHB after 120 days of sun exposure were 207 and 192 kg mol−1, respectively. As can be seen, there was a lower decrease in molar mass for unfilled PLA/PHB exposed outdoor to the sunlight compared to that one exposed in laboratory to UV-vis irradiation (150 kg mol−1 after 125 days of irradiation). The reason could be higher average temperature during UV-vis irradiation (permanently in the range of 35–40 °C) than the average outdoor temperature (in the range of 15–40 °C – cooling down also by regular watering).
A significant decrease of molar mass was observed during hydrolysis laboratory test performed with water vapors at 60 °C. It is worth to mention that the steep decrease in molecular weight occurs from very beginning, so that after 5 days the decrease is represented by about 30% reduction what is sufficient to influence significantly a number of physical properties of the blend. Already after 32 days, the decrease of molar mass was found to be down to about 10 kg mol−1 due to chains scissions and apparently no difference was found between hydrolysis rate for blends with or without carbon blacks addition. These results show that at temperature common in compost the materials undergo degradation in a relatively short time even in the absence of bacteria. At 60 °C, i.e. at temperature over Tg of PLA, the hydrolysis of PLA seems to proceed via bulk erosion mechanism giving significant changes in GPC traces. Unlike that at room temperature the hydrolysis of the PLA seems to proceed preferably from the surface (surface erosion) providing water soluble oligomers and low molecular weight compounds from hydrolyzed PLA38 in addition to PLA chains with almost unchanged molar mass, similarly as it was recently observed for PCL.39 In this case only the later is detected in GPC traces and the low molecular weight compounds can stay within the soil.
3.2. DSC measurements
DSC analysis was used to follow changes caused by cold crystallization characterized by temperature (Tcc) and enthalpy (ΔHcc), and melting process represented by melting temperature (Tm) and enthalphy (ΔHm). As described previously,40 Tcc and Tm of neat PLA are at 60.3 °C and 150 °C, respectively. Likewise, the neat PHB shows Tcc and Tm at 90 °C and 180 °C, respectively. The PLA/PHB blend containing plasticizer exhibited only one Tg at 41.7 °C indicating good miscibility in the system.40 In addition, two melting peaks at 149 and 169 °C for PLA and PHB crystals, respectively, were observed during the first heating cycle (Fig. 4a, Table 1).| CODE | Time (hours) | Tcc (°C) | ΔHcc (J g−1) | Tm1 (°C) | ΔHm1 (J g−1) | Tm2 (°C) | ΔHm2 (J g−1) | Tm3 (°C) | ΔHm3 (J g−1) |
|---|---|---|---|---|---|---|---|---|---|
| PLA/PHB | 0 | 70.3 | 10.5 | 148.9 | 24.3 | 168.5 | 11.8 | — | — |
| 800 | 80.9 | 1.8 | 149.7 | 21.4 | 164.5 | 10.3 | 178.9 | 1.8 | |
| 1250 | 82.3 | 2.2 | 150.5 | 24.7 | 169.1 | 12.2 | — | — | |
| 2000 | 91.8 | 0.7 | 150.8 | 24.2 | 169.5 | 12.0 | — | — | |
| 3000 | 95.7 | 0.5 | 151.5 | 23.6 | 170.5 | 11.8 | — | — | |
| PLA/PHB/CB | 0 | 67.5 | 7.6 | 147.6 | 22.6 | 167.5 | 10.8 | — | — |
| 800 | 80.8 | 0.4 | 149.8 | 22.7 | 169.4 | 10.8 | — | — | |
| 1250 | 83.9 | 0.4 | 150.1 | 22.9 | 169.9 | 11.0 | — | — | |
| 2000 | 86.8 | 0.1 | 150.0 | 23.1 | 169.8 | 10.9 | — | — | |
| 3000 | 90.3 | 0.2 | 151.8 | 25.5 | 170.8 | 12.2 | — | — |
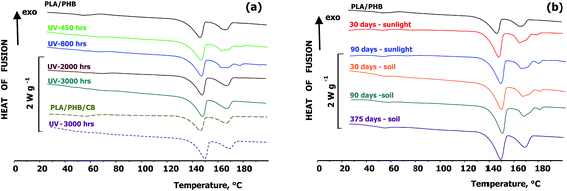
DSC traces of the blends and composites, recorded after ageing under various conditions are shown in Fig. 4 and Tables 1 and 2. Ageing under both UV-vis irradiation and outdoor weathering led to a decrease in the extent of cold crystallization characterized by ΔHcc. Tm1 and ΔHm1 from PLA mostly did not change with ageing. Similarly the ageing only minimally affected the ΔHm2 of PHB. It can be however also seen that during UV-vis irradiation of unfilled PLA/PHB blends foils Tm2 was split in a double peak and Tm3 at approximately 179 °C was formed. The presence of double melting peak could correspond to the melting of formed and recrystallized PHB crystallites. According to Ikehara et al.41 the crystallization behaviour of miscible crystalline/crystalline blends depends on the difference in the melting point of both components. When the difference of melting temperatures is large enough, the higher Tm component crystallizes first and its spherulites usually fill the whole volume. The component with lower Tm crystallizes at lower temperature in space limited region inside the existing spherulites. This agree with previous study by Arrieta et al.,42 who stated that the PHB can act as nucleating agent for PLA resulting in an improvement of oxygen barrier properties. The occurrence of additional melting peaks could be also related to unstable lamellar layers that are present generally on the surface of crystals as a results of chain scission. According to Janigová et al.43 an increase in crystallinity during UV-vis irradiation was due to higher chain mobility and better packing of segments. It should be however noted that the formation of Tm3 was observed also in the PLA/PHB blend stored for 1 year in the dark at RT. Thus, it is a result of physical ageing rather than of changes caused by irradiation. Interestingly, prolonged irradiation for more than 52 days (1250 hours), accompanied with decrease of molar mass due to chain cleavage, led to disappearing of the splitted PHB melting temperature.
| CODE | Time (days) | Tcc (°C) | ΔHcc (J g−1) | Tm1 (°C) | ΔHm1 (J g−1) | Tm2 (°C) | ΔHm2 (J g−1) | Tm3 (°C) | ΔHm3 (J g−1) |
|---|---|---|---|---|---|---|---|---|---|
| PLA/PHB | 0 | 70.3 | 10.5 | 148.9 | 24.3 | 168.5 | 11.8 | — | — |
| PLA/PHB buried in soil | 30 | 71.6 | 3.3 | 148.8 | 23.5 | 167.2 | 9.0 | 179.0 | 0.9 |
| 60 | 80.2 | 3.1 | 148.8 | 21.8 | 167.3 | 9.4 | 179.6 | 1.4 | |
| 90 | 79.5 | 2.9 | 150.2 | 22.9 | 167.4 | 10.0 | 179.6 | 1.4 | |
| 375 | 78.5 | 6.7 | 150.9 | 25.1 | 167.0 | 11.7 | — | — | |
| PLA/PBH exposed to sunlight | 30 | 70.5 | 2.7 | 149.6 | 23.4 | 167.2 | 10.6 | 179.5 | 1.1 |
| 60 | 71.2 | 3.3 | 150.0 | 21.7 | 167.0 | 9.2 | 179.6 | 1.5 | |
| 90 | 82.7 | 5.6 | 159.5 | 24.5 | 166.3 | 8.8 | 179.2 | 1.7 | |
| PLA/PBH in vapor | 30 | 68.2 | 0.6 | 150.9 | 31.2 | 167.7 | 12.1 | — | — |
| PLA/PLA/CB | 0 | 67.5 | 7.6 | 147.6 | 22.6 | 167.5 | 10.8 | — | — |
| PLA/PHB/CB buried in soil | 30 | 86.0 | 5.4 | 150.6 | 22.6 | 170.1 | 11.3 | — | — |
| 90 | 90.6 | 3.0 | 150.5 | 22.8 | 169.8 | 11.2 | — | — | |
| PLA/PBH exposed to sunlight | 30 | 68.5 | 3.2 | 149.9 | 22.2 | 169.7 | 10.9 | — | — |
| 90 | 69.8 | 3.0 | 150.1 | 22.9 | 169.6 | 11.6 | — | — | |
| PLA/PBH/CB in vapor | 30 | 70.6 | 0.9 | 151.0 | 31.5 | 167.4 | 11.3 | — | — |
Similar splitting of Tm2 and formation of Tm3 was observed also during outdoor weathering of the unfilled PLA/PHB foils regardless whether they were exposed to the sunlight or buried in the soil. This observation further confirms that the splitting is probably a result of physical ageing processes. In these cases the splitting did not disappeared until 90 days of ageing, but after prolonged ageing in the soil for 1 year the formation only one Tm2 was again observed. In the case of PLA/PHB composites filled with 1 wt% carbon black the splitting of Tm2 or formation of Tm3 was not recorded. The reason can be that carbon black serves as a nucleation agent preventing supplemental crystallization during physical ageing.
3.3. Dynamic mechanical thermal analysis
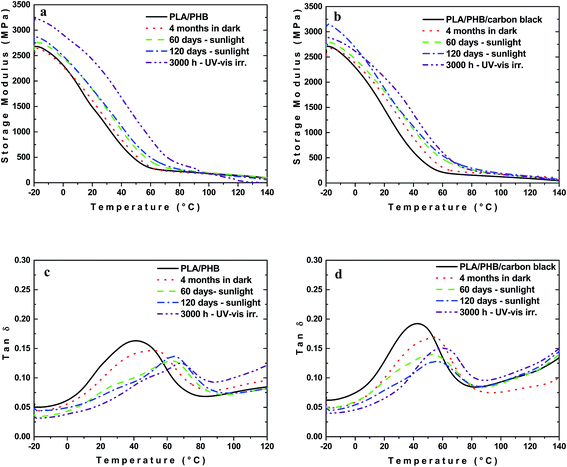
DMTA is a sensitive characterization test in which the response of a material to a periodic stimulus is recorded. The total response includes the contributions of the molar mass, as well as the phase or molecular structure within polymeric material such as branching, crosslinking and crystallinity. The behavior of storage modulus and loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) for both unfilled PLA/PHB blend and composites with 1 wt% of carbon black after different times of various types of ageing are shown in Fig. 5 and 6.
δ) for both unfilled PLA/PHB blend and composites with 1 wt% of carbon black after different times of various types of ageing are shown in Fig. 5 and 6.
Incorporation of carbon black into PLA/PHB had originally only marginal effect on E′ and Tg. It is known that during storage, ageing of PHB is accompanied by an increase in the brittleness within a few months after the thermal processing.44 Some extent of physical ageing manifested itself by a slight shifting of the glass transition and drop in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ maximal intensity was observed also in the case of the PLA/PHB blend and its composite (Fig. 5c and d).
δ maximal intensity was observed also in the case of the PLA/PHB blend and its composite (Fig. 5c and d).
Unlike the samples stored in the dark at RT, a progressive increase in E′ was observed with time of either exposition to sunlight or UV-vis irradiation, regardless if PLA/PHB blend or composite was used (Fig. 5a and b). Both, UV-vis irradiation and sunlight exposure were accompanied by the elevated temperature, which may cause the enhanced mobility of amorphous phase resulting in increased rate of physical ageing. This effect is connected with the crystallinity increase since, although the crystallinity needs not to change substantially, it occurs to significant extent in tie molecules resulting in a reduction of the mobility of the chain segments45 and thus leads to the increase of the storage modulus and embrittlement of the material. In general, the polymer crystallization is manifested by shifting the glass transition to higher temperature as a result of the constraints imposed on the amorphous chains by the crystals (Fig. 5c and d).46 For PLA/PHB blend and its composite the shifting and broadening of the glass transition region could be clearly observed in the dynamic mechanical spectra.
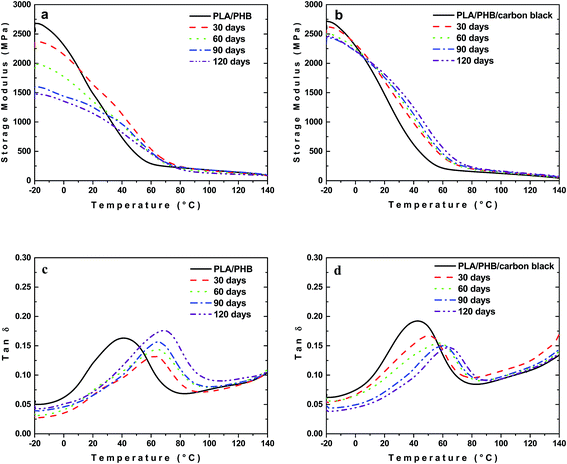
Different situation was observed for samples buried in the soil. In the case of neat PLA/PHB blend the increasing time of burial caused pronounced drop in E′ in glassy state (Fig. 6a). At the same time the glass transition temperature (Tg) shifted to higher values with increasing time of burial (Fig. 6c). The increase of height of the dynamic loss peak with time of burial corresponded to the enhanced mobility of amorphous phase. This behavior may be related to certain decrease in the molar mass. Although the decrease is small, it may play a significant role in the mechanical properties due to preferential scission of longer chains and especially those which are under mechanical stress created during cooling and crystallization of the material after melt processing.45
The properties however changed differently for composite foils. The decrease in E′ with time of burial for PLA/PHB/carbon black foils was less significant compared to unfilled PLA/PHB foils (Fig. 6b). A decrease in E′, observed for unfilled PLA/PHB blends, can be in composites probably compensated by nucleation effect of the filler. Similarly to unfilled PLA/PHB blend, also in the case of composite, the most significant shift of glass transition temperature was observed after 120 days of burial (Fig. 6d). In this case however the peak height was lower confirming enhanced brittleness of the material and the lower mobility of the polymer chains probably due to presence of filler serving as a nucleating agent.
3.4. Testing of mechanical properties
It is well known that ageing upon storage results in an increase in Young's modulus accompanied by a decrease in elongation at break, which translates into a pronounced increase in brittleness of the material.44 Generally, the most significant changes of mechanical properties proceed during the first fourteen days after thermal processing. After this time the process of ageing is much less pronounced.47 This time dependence influences mainly elongation at break (εB), Young's modulus (E) and toughness (Γ), which relate to the cold crystallization and physical ageing.48 The results of mechanical properties represented by E, εB and tensile strength (σM) before and after 120 days of storage in dark at room temperature are summarized in Table 3.As expected the presence of carbon black resulted in an increase of σM, but surprisingly also εB, compared to unfilled PLA/PHB blend. This increase of εB after addition of carbon black could be explained by nucleation effect of the carbon black,49 which manifests itself in a small but significant drop in Tm, indicating thinner lamellae. It is well known that finer structure of crystallites leads to more ductile material even if the overall amount of crystalline portion is the same (larger crystallites are stiffer).50 Generally the changes in εB and σM observed either for unfilled PLA/PHB blend or its composite with 1 wt% of carbon black after 120 hours of storage in the dark were not significant.
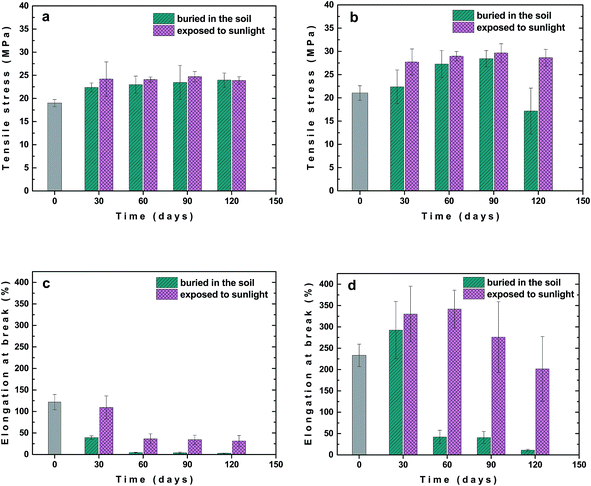
The changes in mechanical properties, σM and εB, for unfilled PLA/PHB blend and its composite with 1 wt% of carbon black after various times of outdoor weathering, i.e. burial in the soil or exposure to the sunlight, are shown in Fig. 7. In the case of unfilled PLA/PHB blend σM increased already after 30 days from 19 up to about 24 MPa and then almost leveled off up to 120 days of ageing regardless whether the sample was buried in the soil or exposed to sunlight (Fig. 7a). The presence of carbon black in PLA/PHB foil led to increase in σM after 30 days from 21 up to about 28 MPa and then stayed almost constant up to 120 days, when the sample was exposed to sunlight (Fig. 7b). However, in the case of sample buried in the soil, slower and progressive increase in σM was observed reaching the value of 28 MPa after 90 days, while prolonged ageing for 120 days led to decrease of σM down to about 17 MPa.
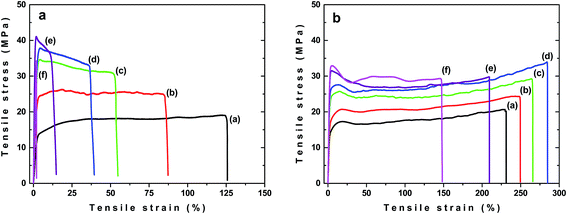
Significant difference between samples buried in the soil and exposed to sunlight were observed during ageing when changes in εB were followed. In the case of unfilled PLA/PHB foils the decrease in εB with ageing time was observed. The εB fell down faster for the samples buried in the soil, reaching the εB values approximately of 5% after 60 days of ageing (Fig. 7c). For samples exposed to sunlight εB decreased slower and after 60 days of ageing it stabilized at value approximately of 30%. Interestingly, for the PLA/PHB foils filled with carbon black, an increase in εB was observed after 30 days of ageing regardless whether the sample was buried in the soil or exposed to the sunlight (Fig. 7d). While prolonged ageing provided significant decrease in εB for samples buried in the soil, in the case of samples exposed to the sunlight the εB stayed almost constant after 60 days and then slowly and progressively decreased reaching εB approximately of 200%, what is value similar to that one for original sample before ageing. Similar results as for samples exposed to the sunlight were obtained also for samples exposed to UV-vis irradiation (Fig. 8) confirming positive effect of carbon black on photochemical ageing of PLA/PHB foils.
The significant difference in the mechanical properties changes between unfilled and carbon black filled PLA/PHB foils after ageing, including the retention of higher elongation at break observed for the foils containing carbon black, can be caused by various factors, such as (a) the nucleation effect of carbon black leading to thinner crystallites formation during both the preparation and ageing of the samples, (b) the protection effect of the carbon black against the irradiation, and (c) slowing down the plasticizer diffusion out of the foil by possible barrier effect of the carbon black and crystallites formed in its vicinity. Probably the final effect is caused by combination of the factors mentioned while possible contribution of other effects cannot be excluded.
3.5. Mineralization of PLA/PHB foils
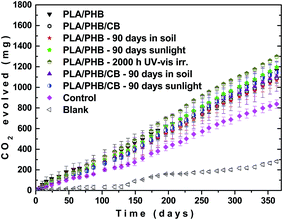
It is well known that the PLA readily degrade under conditions of compost within the range of 40 to 60 days depending on MW, where the temperature of above 50–60 °C (above Tg) allows higher mobility of polymer chains and creates a good conditions for enzymatic degradation by microorganisms.25,51,52 However the (bio)degradation of PLA at room temperature is very slow.53 As mentioned in the first discussion, one borderline case in application of the tested PLA/PHB-based foils can be that some extent of degradation during its application will allow plowing the residues in the soil, where the microbial and hydrolytic degradation would be followed through. Therefore, here we decided to investigate also the effect of pre-ageing of PLA/PHB and PLA/PHB/carbon black foils under various weathering conditions on possible degradation of the foil residues in the soil at 20 °C. In this context, initial pre-degradation by UV-vis irradiation, exposition to the sunlight or outdoor burial in the soil can affect velocity of subsequent mineralization. In mineralization tests the hydrolytic degradation of the PLA/PHB foils could not be excluded, but evolved CO2, measured in these experiments, can be produced exclusively by microorganisms, which use this polymer for feeding. According to the Fig. 9, all polymer samples evolved higher amount of CO2 than the control sample containing only soil without polymer foil. This could indicate increased microbial activity in the presence of the polymer samples. However, this difference was small suggesting that the mineralization was slow for all investigated polymers.The mineralization seemed to be slightly faster for UV-irradiated and the sunlight exposed white samples, but generally for most of the samples the difference could be considered to be within the experimental error. This observation was further confirmed by determination of change in weight of the samples after their withdrawal. All the PLA/PHB and PLA/PHB/carbon black samples lost only approx. 8–12% of its original weight after 1 year of mineralization. It should be noticed that a cellulose sample, commonly used as a standard in mineralization tests, lost after 1 year of its mineralization approx. 50% of its original weight. Thus it can be concluded that the weathering of the PLA/PHB and PLA/PHB/carbon black foils has negligible effect on their subsequent mineralization at ambient temperature.
The photos of both PLA/PHB and PLA/PHB/CB foils after preparation as well as foils after mineralization are shown in Fig. S1.† As it can be seen from the photos the originally transparent PLA/PHB foils became cloudy after mineralization. The PLA/PHB/CB foils contain cracks after mineralization. All the films looked the same after mineralization regardless whether they were pre-aged (90 days by storage in the soil or by exposure to the sunlight) or not.
Scanning electron microscopy was used to compare potential changes in bulk of foils. For that purpose the foils were fractured in liquid nitrogen. It is worth to mention that in the case of all not mineralized samples, it was quite difficult to break the samples because they were elastic even after their immersion to the liquid nitrogen. Contrary that all samples after mineralization were easily fractured shortly after immersion to the liquid nitrogen except the PLA/PHB sample pre-aged for 90 days by light exposure, which still partially retained elastic properties. As seen in Fig. S2,† there were not significant changes in SEM images of fracture of analyzed PLA/PHB foils either before or after mineralization. Contrary to that, slight changes were observed for PLA/PHB/CB foils (see Fig. S3†). The fracture surface of PLA/PHB/CB foils pre-aged by exposure to sunlight, either before or after subsequent mineralization, was smoother than that for not pre-aged foils. No porosity due to potential bio- and/or hydrolytic degradation of foils was observed even after mineralization. That is consistent with minor weight changes determined after mineralization tests.
The samples after mineralization were also characterized by 1H NMR spectroscopy in order to check the changes in PLA/PHB composition (the original composition is protected by PANARA s.r.o. and therefore can not be mentioned here). It was found that mineralization without previous treatment provided only minimal changes in PLA/PHB ratio providing up to 20% increase of the PLA/PHB ratio. Contrary that when the samples were exposed to sunlight and subsequently mineralized, the increase in PLA/PHB ratio was about 130%, indicating that sunlight exposure promoted preferential degradation of PHB. This is in good agreement with UV-vis degradation of PHB observed and described elsewhere.9,54,55
4. Conclusion
PLA/PHB and PLA/PHB/carbon black (1 wt%) foils were prepared and their ageing under various conditions were tested in order to investigate their potential applicability as mulching foils in agriculture. For outdoor weathering, simulating the real application, the samples were either buried in the soil or put on the top of soil and exposed to sunlight. In addition UV-vis irradiation, hydrolytic degradation by exposure to water vapor and mineralization tests were performed under laboratory conditions. GPC spectra showed that while fast and progressive decrease of molar mass was observed under exposure to water vapor at 60 °C, in the case of samples buried in the soil almost no decrease of molar mass was detected after 4 months indicating either slow degradation or preferable hydrolysis through surface erosion mechanism. In addition, higher extent of degradation was observed for PLA/PHB foil than PLA/PHB/carbon black foil under UV-vis irradiation. DSC studies showed formation of additional crystals with higher melting temperatures than the melting temperatures of PLA and PHB in PLA/PHB foils during ageing. Such additional crystals were not observed in PLA/PHB/carbon black foils, where carbon black serves as nucleation agent preventing supplemental crystalization. Both DMTA and tensile tests confirmed that the presence of carbon black in the foil improved significantly the retention of the mechanical properties during ageing. Mineralization tests performed at 20 °C showed that the pre-ageing of the foils had minimal effect on their subsequent enzymatic degradation in the soil. 1H NMR studies showed slight preferential degradation of PHB, which was more pronounced if the samples were sunlight exposed before mineralization, even though the mass decrease after 1 year of mineralization at 20 °C was only in the range of 8–12% for all samples. It can be generally concluded, that the PLA/PHB foil, preferable that containing 1 wt% of carbon black, seems to be a suitable ecological material applicable as mulching foils possessing good retention of properties during one season of application with possibility of subsequent removal from the field for composting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors thank for financial support to European Regional Development Fund through project POLYFRIEND, project no. HUSK 1101/1.2.1/0209, within Hungary-Slovakia Cross-border Co-operation Programme 2007–2013 as well as to projects VEGA 2/0158/17, VEGA 2/0108/14, APVV-15-0528 and APVV-14-0301.References
- J. Lunt, Polym. Degrad. Stab., 1998, 59, 145–152 CrossRef CAS.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, J. Mater. Chem. A, 2013, 1, 13379–13398 CAS.
- W. Pivsa-Art, K. Fujii, K. Nomura, Y. Aso, H. Ohara and H. Yamane, J. Appl. Polym. Sci., 2016, 133, 1–10 CrossRef.
- O. Martin and L. Avérous, Polymer, 2001, 42, 6209–6219 CrossRef CAS.
- J. Yeh, C. Huang, W. Chai and K. Chen, J. Appl. Polym. Sci., 2009, 112, 2757–2763 CrossRef CAS.
- N. Wang, X. Zhang, X. Ma and J. Fang, Polym. Degrad. Stab., 2008, 93, 1044–1052 CrossRef CAS.
- J. Yu, N. Wang and X. Ma, Biomacromolecules, 2008, 9, 1050–1057 CrossRef CAS PubMed.
- K. Sudesh, H. Abe and Y. Doi, Prog. Polym. Sci., 2000, 25, 1503–1555 CrossRef CAS.
- R. K. Sadi, G. J. M. Fechine and N. R. Demarquette, Polym. Degrad. Stab., 2010, 95, 2318–2327 CrossRef CAS.
- S. Wang, P. Ma, R. Wang, S. Wang, Y. Zhang and Y. Zhang, Polym. Degrad. Stab., 2008, 93, 1364–1369 CrossRef CAS.
- R.-J. Mueller, Process Biochem., 2006, 41, 2124–2128 CrossRef CAS.
- Y. Tokiwa and A. Jarerat, Biotechnol. Lett., 2004, 26, 771–777 CrossRef CAS PubMed.
- L. SuPing, J. Schley, B. Loy, D. Lind, C. Hobot, R. Sparer and D. Untereker, Biomacromolecules, 2007, 8, 2301–2310 CrossRef PubMed.
- G. Kale, R. Auras, S. P. Singh and R. Narayan, Polym. Test., 2007, 26, 1049–1061 CrossRef CAS.
- M. Agarwal, K. W. Koelling and J. J. Chalmers, Biotechnol. Prog., 1998, 14, 517–526 CrossRef CAS PubMed.
- Y.-X. Weng, L. Wang, M. Zhang, X.-L. Wang and Y.-Z. Wang, Polym. Test., 2013, 32, 60–70 CrossRef CAS.
- P. Stloukal, A. Kalendova, H. Mattausch, S. Laske, C. Holzer and M. Koutny, Polym. Test., 2015, 41, 124–132 CrossRef CAS.
- P. Sangwan and D. Y. Wu, Macromol. Biosci., 2008, 8, 304–315 CrossRef CAS PubMed.
- M. Hakkarainen, in Degradable Aliphatic Polyesters, Springer Berlin Heidelberg, Berlin, Heidelberg, 2002, pp. 113–138 Search PubMed.
- A. Longieras, J. B. Tanchette, D. Erre, C. Braud and A. Copinet, J. Polym. Environ., 2007, 15, 200–206 CrossRef CAS.
- L. Husárová, S. Pekařová, P. Stloukal, P. Kucharzcyk, V. Verney, S. Commereuc, A. Ramone and M. Koutny, Int. J. Biol. Macromol., 2014, 71, 155–162 CrossRef PubMed.
- Z. Saadi, A. Rasmont, G. Cesar, H. Bewa and L. Benguigui, J. Polym. Environ., 2012, 20, 273–282 CrossRef CAS.
- V. Speranza, A. De Meo and R. Pantani, Polym. Degrad. Stab., 2014, 100, 37–41 CrossRef CAS.
- P. Kucharczyk, E. Hnatkova, Z. Dvorak and V. Sedlarik, Polym. Degrad. Stab., 2013, 98, 150–157 CrossRef CAS.
- M. P. Arrieta, J. López, E. Rayón and A. Jiménez, Polym. Degrad. Stab., 2014, 108, 307–318 CrossRef CAS.
- Y.-X. Weng, X.-L. Wang and Y.-Z. Wang, Polym. Test., 2011, 30, 372–380 CrossRef CAS.
- D. Puglia, E. Fortunati, D. A. D'amico, L. B. Manfredi, V. P. Cyras and J. M. Kenny, Polym. Degrad. Stab., 2014, 99, 127–135 CrossRef CAS.
- W. Sikorska, M. Musiol, B. Nowak, J. Pajak, S. Labuzek, M. Kowalczuk and G. Adamus, Int. Biodeterior. Biodegrad., 2015, 101, 32–41 CrossRef CAS.
- L. M. Oliveira, E. S. Araújo and S. M. L. Guedes, Polym. Degrad. Stab., 2006, 91, 2157–2162 CrossRef CAS.
- G. Gorrasi, C. Milone, E. Piperopoulos, M. Lanza and A. Sorrentino, Appl. Clay Sci., 2013, 71, 49–54 CrossRef CAS.
- H. J. Jeon and N. Kim, Int. Biodeterior. Biodegrad., 2013, 85, 289–293 CrossRef CAS.
- T. Leejarkpai, U. Suwanmanee, Y. Rudeekit and T. Mungcharoen, Waste Manage., 2011, 31, 1153–1161 CrossRef CAS PubMed.
- E. Rudnik and D. Briassoulis, Ind. Crops Prod., 2011, 33, 648–658 CrossRef CAS.
- STN EN ISO 17556, Plastics – Determination of the ultimate aerobic biodegradability of plastic materials in soil by measuring the oxygen demand in a respirometer or the amount of carbon dioxide evolved, 2012, https://www.sutn.sk/eshop/public/standard_detail.aspx?id=116602, accessed March 7, 2017.
- ASTM D5988-12 Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil, 2012, doi:10.1520/D5988-12.
- STN EN ISO 527–3, Plastics. Determination of tensile properties. Part 3: Test conditions for films and sheets, 1997; https://www.sutn.sk/eshop/public/standard_detail.aspx?id=66000, accessed March 8, 2017.
- T. Suyama, Y. Tokiwa, P. Ouichanpagdee, T. Kanagawa and Y. Kamagata, Appl. Environ. Microbiol., 1998, 64, 5008–5011 CAS.
- A. P. Gupta and V. Kumar, Eur. Polym. J., 2007, 43, 4053–4074 CrossRef CAS.
- J. Mosnáček, K. Borská, M. Danko and I. Janigová, Mater. Chem. Phys., 2013, 140, 191–199 CrossRef.
- K. Mosnáčková, A. Šišková, I. Janigová, J. Kollár, M. Šlosár, Š. Chmela, P. Alexy, I. Chodák, J. Bočkaj and J. Mosnáček, Chem. Pap., 2016, 70, 1268–1278 Search PubMed.
- T. Ikehara, H. A. Kimura and Z. Qiu, Macromolecules, 2005, 38, 5104–5108 CrossRef CAS.
- M. P. Arrieta, J. López, A. Hernández and E. Rayón, Eur. Polym. J., 2014, 50, 255–270 CrossRef CAS.
- I. Janigová, I. Lacík and I. Chodák, Polym. Degrad. Stab., 2002, 77, 35–41 CrossRef.
- I. Chodák, in Monomers, Polymers and Composites from Renewable Resources, Elsevier, 2008, pp. 451–477 Search PubMed.
- G. J. M. de Koning, A. H. C. Scheeren, P. J. Lemstra, M. Peeters and H. Reynaers, Polymer, 1994, 35, 4598–4605 CrossRef CAS.
- G. J. M. De Koning and P. J. Lemstra, Polymer, 1993, 34, 4089–4094 CrossRef CAS.
- Z. Špitalský, I. Lacík, E. Lathová, I. Janigová and I. Chodák, Polym. Degrad. Stab., 2006, 91, 856–861 CrossRef.
- I. Chodák, in Degradable Polymers, Springer Netherlands, Dordrecht, 2002, pp. 295–319 Search PubMed.
- Z. Su, A. Weihong, G. Ae, Y. Liu, A. Qiuying, L. Ae and C. Wu, Polym. Bull., 2009, 62, 629–642 CrossRef CAS.
- A. El-Hadi, R. Schnabel, E. Straube, G. Müller and S. Henning, Polym. Test., 2002, 21, 665–674 CrossRef CAS.
- L. Husárová, S. Pekařová, P. Stloukal, P. Kucharzcyk, V. Verney, S. Commereuc, A. Ramone and M. Koutny, Int. J. Biol. Macromol., 2014, 71, 155–162 CrossRef PubMed.
- G. Gorrasi and R. Pantani, Polym. Degrad. Stab., 2013, 98, 1006–1014 CrossRef CAS.
- N. T. Lotto, M. R. Calil, C. G. F. Guedes and D. S. Rosa, Mater. Sci. Eng., C, 2004, 24, 659–662 CrossRef.
- R. M. R. Wellen, E. L. Canedo, M. S. Rabello, G. J. M. Fechine, R. M. R. Wellen, E. L. Canedo, M. S. Rabello and G. J. M. Fechine, Mater. Res., 2016, 19, 759–764 CrossRef.
- J. Lim and J. Kim, Macromol. Res., 2016, 24, 9–13 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08869h |
| This journal is © The Royal Society of Chemistry 2017 |